Visual prostheses, optogenetics, stem cell and gene therapies: splitting the cake
The size of the blind population in 2015 was estimated to be ap‐proximately 36 million (Bourne et al., 2017). According to the predictions by Bourne and co‐workers, the number of the visually impaired is expected to reach nearly 100 million by 2050. Although some of these diseases can be treated, to date, some other eye conditions such as retinitis pigmentosa (RP), an inherited degenerative condition of the photoreceptors, have no treatment except electrical stimulation of the surviving neurons of the visual system. This therapy, deliveredviaa visual prosthesis, relies on an electrode array, implanted in close proximity to the target neurons, able to deliver a series of electrical impulses that activate these cells thus eliciting a visual sensation (Lewis et al., 2016). These electrodes can be implanted in the retina (three approaches exist: epiretinal, sub‐retinal and suprachoroidal implants), the optic nerve, the lateral geniculate nucleus or the visual cortex. The medical device industry has spotted the opportunity and several companies have already obtained approval for commercialisation of their devices in the US and the European markets. However, the niche for these technologies may be soon occupied by new promising therapies based on a biological approach.
Optogenetic strategy:An adeno‐associated virus can be engineered to induce light sensitivity in the surviving retinal neurons by altering their genetic information (Gaub et al., 2015). These viral vectors are loaded with genes that codify light‐sensitive proteins and alter the DNA of the retinal neurons to induce their expression. It has been demonstrated that after infection, these neurons exhibit light‐gated ion channels in their cell membranes and therefore become activatedviaincident photons in a similar way to the physiological photoreceptors. While this strategy has been demonstrated effectivein vivousing animal models, its safety is still a question that needs to be further investigated. The main concern of these therapies relies on the potential reaction of the immune system. Although strong immune responses have not been report‐ed in mice or primates in optogenetic experiments involving infection brain and retinal neurons, human immune responses could differ (Busskamp et al., 2012). Another potential limitation relates to the ability of the modified neurons to convey understandable neural messages. The retina codifies visual information in many ways including transition of light through the ON‐ and OFF‐path‐ways. Reactivation of the retinal circuitry is feasible with this technique, but the neural messages elicited by visual scenes may be substantially different compared to those in the physiological retina. In addition, if the aim was to mimic the natural responses of the retina, this approach should target specific cells. However, it is expected the brain plasticity to compensate for inappropriate en‐coding (Busskamp et al., 2012). A third drawback of this approach is the poor light sensitivity imparted to the retinal neurons, but at present, some researchers are already working on this limitation,for example, using native light‐gated G‐protein‐coupled receptors instead of microbial opsins (Gaub et al., 2015).
Therapies based on stem cells:The idea underlying this approach is to regenerate the retinal tissue by transplanting stem cells, a type of cells that have the ability to become, in this case, photoreceptors(Nazari et al., 2015). Brie fly, this technique consists of replacing the unhealthy retinal tissue by a stem cell engineered one. For exam‐ple, a recent study by Shirai and co‐workers (Shirai et al., 2016) has shown, in a primate model, that a layer of photoreceptors obtained from human embryonic stem cells can form synaptic connections with the remaining retinal neurons. These are promising results as optimal host‐graft integration would potentially lead to more natural neural messages being transmitted to higher visual centres in the brain. However, there are relevant technical limitations that need to be addressed before this therapy can reach the bedside,particularly in relation to long‐term safety. Immune responses can occur in some types of implants and there is a potential for these cells to form tumours (Nazari et al., 2015). In these lines, several companies have started clinical trials to test their therapies. For instance, jCyte launched in 2017 a phase IIb clinical trial to test the efficacy of ‘jCell’, an intravitreal injection of allogeneic human retinal progenitor cells able to rescue the degrading photorecep‐tors during progression of RP. Despite the enormous progress in the laboratory, the scientific community is also facing important ethical challenges, for example, in the use of embryonic‐derived stem cells. These concerns may slow down the progression and the development of some of these techniques.
Gene editing therapies:It is now possible to repair the genome of non‐dividing cellsin vivothrough the Clustered Regularly Inter‐spaced Short Palindromic Repeat technique (CRISPR). Using elec‐troporation, an RNA‐guided Cas9 nuclease can cross the cell mem‐brane and edit the DNA of the target cells (Suzuki et al., 2016). This is of particular relevance in the treatment of RP (Bakondi et al.,2016). However, there are other eye problems such as trauma for which this strategy offers no solution. Furthermore, the existence of numerous ethical concerns on the use of this technique may blur the future application of this therapeutic approach. There are in addition strict regulatory requirements that need to be met before these therapies can be approved for the use in humans. Neverthe‐less, CRISPR is making a rapid progress as two clinical trials are scheduled in Europe and the USA in 2018. Although these studies are not related to the treatment of visual impairment, they may facilitate approval of further trials to test its use as a therapy for retinal degenerative diseases.
The three emerging therapies described here are promising a different scenario in the treatment of some types of visual impair‐ments, and may replace, in some cases, the use of visual prostheses.However, with a number of challenges yet to be overcome, these biological approaches may not become the mainstream for number of years, and a generation, or perhaps two, of blind people may miss the opportunity of being sighted again. Hence, at present those patients currently suffering from vision loss have no other alternative but visual prosthetics. For those, there are two approved retinal implants, the Argus®II (Second Sight Medical Products,Sylmar, CA, USA) and the Alpha IMS (Retina Implant AG, Reut‐lingen, Germany) (Lewis et al., 2016); the first, accounting a total of 60 electrodes, is an epiretinal device that relies on an external cam‐era to bypass the degenerated photoreceptors, and the second is a subretinal implant that uses an array of 1,500 microphotodiodes to elicit visual perception. Several implants still remain on the bench but are making important progress towards the bedside, and some other devices such as the epiretinal IRIS®II (Pixium Vision, Paris,France) or the cortical Orion (Second Sight Medical Products) are currently undergoing clinical trials. Although the second type of prostheses requires brain surgery to implant the electrode array on the visual cortex, they can target a wider spectrum of pathologies and therefore may be able to compete with the emerging biological approaches when they reach maturity. However, these devices have some limitations as well and can only provide a rudimentary functional visual perception. Bionic vision is mainly limited by the electrochemical reactions that can occur at the electrode‐tissue inter‐face during electrical stimulation (Barriga‐Rivera et al., 2017a) and by the interferences created between neighbouring electrode sites(Matteucci et al., 2016). In fact, these interferences, known as cross‐talk, can lead to inhibition of the neural activity due to summation of the overlapping electric fields produced when several electrodes are activated concomitantly, as in the case of bright visual scenes(Barriga‐Rivera et al., 2017b). Retinal implants have also a limited capacity to elicit physiological neural messages. For example, when a stimulus is delivered, both ON‐ and OFF‐pathways are activated simultaneously resulting in confusing information being sent to the brain. To address these limitations, researchers are directing their efforts in different ways (Barriga‐Rivera et al., 2017a): (1) the use of new biomaterials such as conducting polymers or carbon nano‐tubes among others may help reducing the electrochemical burden of conventional metallic electrodes, (2) by growing neurons on the surface of the electrodes as shown in Figure 1, the development of living electrodes may provide an optimal electrode‐neuron inter‐face, and (3) the development of new stimulation strategies, particularly those relying on the use of high frequency neurostimulation,can provide a method to selectively activate different cell types.
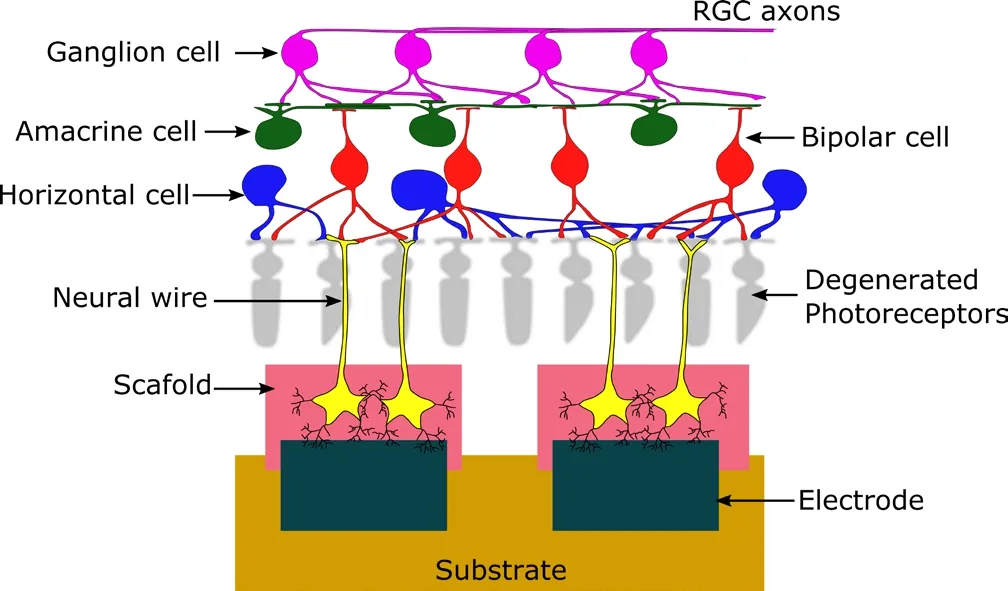
Figure 1 Subretinal electrode array consisting of a group of metallic electrodes coated with a cell-laden material.
In a scenario of rapid development and intense competition for restoring sight to the blind the question on whether bionic vision will remain as the main therapy is under debate. The biological approaches are in a strong position to become the gold standard in the treatment of some eye diseases. This would leave a reduced spectrum available for the application of bionic vision technologies. A recent example of success of the gene therapies is Luxturna(Spark Therapeutics, Philadelphia, PA, USA), the first gene therapy approved by the US Food and Drug Administration (FDA) to treat blindness. In particular this solution targets patients with mutations in theRPE65gene. This is quite an expensive therapy that has a great potential for causing dangerous side effects, but it also shows clearly the potential of the biological approaches. Among all visual prosthetic devices, cortical implants may have a more exclusive niche as stimulation of the visual cortex can be used to treat almost any type of blindness. Despite the fierce competitors of visual prosthetics, progress in the delivery of bionic vision must continue not only because it will benefit a number of patients with no current alternative, but also because advances in this field can be easily adopted by other forms of neuromodulation therapies, or perhaps, because the combination of visual implants and biological techniques may exploit new synergies, as in the case of organic electrodes (Aregueta‐Robles et al., 2014).
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sk-lodowska-Curie grant agreement No 746526 and from the National Health and Medical Research Council (RG1063046).
Alejandro Barriga-Rivera*, Gregg J. Suaning
Division of Neuroscience, University Pablo de Olavide, Sevilla,Spain (Barriga‐Rivera A)
Faculty of Engineering and Information Technologies, The University of Sydney, Sydney, Australia(Barriga‐Rivera A, Suaning GJ)
*Correspondence to:Alejandro Barriga-Rivera, Ph.D.,alejandro.barriga-rivera@sydney.edu.au.
orcid:0000-0001-9474-4905 (Alejandro Barriga-Rivera)
Accepted:2018-03-19
doi:10.4103/1673-5374.232469
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer:Fei Gao, West Virginia University, USA.
Comments to authors:In this manuscript, the author described four potential approaches currently to treat vision impairments, diseases or blindness,and carefully compared their applications and limitations. The authors did a very good job in organizing and writing this manuscript.
Aregueta‐Robles UA, Woolley AJ, Poole‐Warren LA, Lovell NH, Green RA(2014) Organic electrode coatings for next‐generation neural interfaces.Front Neuroeng 7:15.
Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA,Breunig JJ, Svendsen CN (2016) In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter‐3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther 24:556‐563.
Barriga‐Rivera A, Bareket L, Goding J, Aregueta‐Robles UA, Suaning GJ(2017a) Visual prosthesis: interfacing stimulating electrodes with retinal neurons to restore vision. Front Neurosci 11:620.
Barriga‐Rivera A, Guo T, Yang CY, Al Abed A, Dokos S, Lovell NH,Morley JW, Suaning GJ (2017b) High‐amplitude electrical stimulation can reduce elicited neuronal activity in visual prosthesis. Sci Rep 7:42682.
Bourne RR, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB,Keeffe J, Kempen JH, Leasher J, Limburg H (2017) Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta‐analysis. Lan‐cet Glob Health 5:e888‐e897.
Busskamp V, Picaud S, Sahel J, Roska B (2012) Optogenetic therapy for ret‐initis pigmentosa. Gene Ther 19:169‐175.
Gaub BM, Berry MH, Holt AE, Isacoff EY, Flannery JG (2015) Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol Ther 23:1562‐1571.
Lewis PM, Ayton LN, Guymer RH, Lowery AJ, Blamey PJ, Allen PJ, Luu CD, Rosenfeld JV (2016) Advances in implantable bionic devices for blindness: a review. ANZ J Surg 86:654‐659.
Matteucci PB, Barriga‐Rivera A, Eiber CD, Lovell NH, Morley JW, Suaning GJ (2016) The effect of electric cross‐talk in retinal neurostimulation.Invest Ophthalmol Vis Sci 57:1031‐1037.
Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T,Clegg DO, Kashani AH, Hinton DR (2015) Stem cell based therapies for age‐related macular degeneration: the promises and the challenges. Prog Retin Eye Res 48:1‐39.
Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T,Assawachananont J, Kimura T, Saito K, Terasaki H (2016) Transplanta‐tion of human embryonic stem cell‐derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A 113:E81‐E90.
Suzuki K, Tsunekawa Y, Hernandez‐Benitez R, Wu J, Zhu J, Kim EJ,Hatanaka F, Yamamoto M, Araoka T, Li Z, Kurita M, Hishida T, Li M,Aizawa E, Guo S, Chen S, Goebl A, Soligalla RD, Qu J, Jiang T, et al. (2016)In vivo genome editing via CRISPR/Cas9 mediated homology‐indepen‐dent targeted integration. Nature 540:144‐149.
- 中国神经再生研究(英文版)的其它文章
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations