When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons?
Circadian rhythm protects neurons:Although the master clock entrains the whole body rhythm, peripheral tissues also express core clock transcription factors Clock and Bmal1, which regulate expression of clock genes in‐cluding Period (Per) and Cryptochrome (Cry) proteins. Complexes of Per and Cry proteins repress Bmal1‐ and Clock‐mediated transcription forming a negative feedback loop, which regulates nearly a 24 hours self‐sustained rhythm including energy metabolism. Circadian rhythm dysfunction is often observed in patients with Alzheimer’s, Parkinson’s and Huntington’s diseases. Clinical studies and experiments in animal models of neurodegen‐erative disorders have revealed the progressive nature of circadian dysfunction throughout the course of neurodegeneration. However, the importance of circadian rhythm in the protection of neurons remains to be elucidated.Recent studies suggest that disruption of the circadian rhythm can impair metabolic cooperation between neurons and astrocytes, and thereby enhance oxidative damages in the brain. Thus, understanding the molecular mechanisms by which endogenous antioxidant defense systems are controlled by the circadian rhythm may inform the design of novel therapeutic strategies to protect against neurodegenerative diseases. We recently proposed that neurotrophins activate the redox sensitive transcription factor nuclear factor(erythroid‐derived 2)‐like 2 (Nrf2), a master regulator of cellular defense against oxidative stress, in a circadian rhythm dependent manner in astro‐cytes to support neurons in the brain (Ishii et al., 2018).
Neurotrophin receptor p75NTR is a clock component:Neurotrophins such as nerve growth factor (NGF) and brain‐derived neurotrophic factor(BDNF) play important roles in energy metabolism, survival and the function of neuronal and non‐neuronal cells. BDNF facilitates the metabolic cooperation between astrocytes and neurons in the central nervous system.Astrocytes store glycogen as an energy reservoir to provide active neurons with the glycolytic metabolite lactate. BDNF transduces signals through high affinity TrkB receptors and the low affinity p75 receptor (p75NTR).p75NTRis the common receptor for all neurotrophins. Notably, p75NTRis a clock component and its gene expression is directly regulated by the tran‐scription factor Clock:Bmal1 complex like Period 2 (Per2) in tissues (Bae‐za‐Raja et al., 2013). Therefore, neurotrophin signaling through p75NTRis influenced by the circadian rhythm. Per2 expression is highly synchronized among neurons and glial cells in local brain regions with its peak around the Zeitgeber time 1 (ZT1, early light/rest phase) and a sharp nadir around ZT13 (early dark/active phase). However, currently there are no precise data showing the daily variation of p75NTRprotein expression levels in brain areas.
P75NTR-mediated signaling induces Nrf2 activation:A notable function of neurotrophins is to activate Nrf2, which upregulates the expression of detoxification enzymes and antioxidants to protect cells from toxic agents and oxidative stress (Itoh et al., 1997; Ishii et al., 2000). Activation of Nrf2 is particularly important in supporting mitochondrial oxidative phosphoryla‐tion. Kosaka et al. (2010) showed that NGF activates Nrf2 in rat pheochro‐mocytomous PC12 cells and that Nrf2 upregulates gene expression of NGF,thereby forming a positive feedback loop to stably enhance Nrf2‐mediated cell protection by NGF. We recently proposed that neurotrophins may ac‐tivate Nrf2viap75NTR‐mediated generation of the lipid signal mediator cer‐amide (Ishii et al., 2018). Dobrowsky et al. (1994) first showed the stimulation of p75NTRactivates neutral sphingomyelinase to generate ceramide, but its physiological effect other than apoptosis was not clear. As low levels of ceramide can activate atypical protein kinase Cζ (PKCζ), which phosphor‐ylates and activates casein kinase 2 (CK2), we proposed that neurotrophins activate p75NTR‐ceramide‐PKCζ‐CK2 signaling pathway. Subsequently, CK2 directly phosphorylates and stabilizes/activates Nrf2 (Figure 1A). As p75NTRexpression is controlled by the cell clock, this signaling pathway must be altered with time.
BDNF activates Nrf2 in a TrkB.T1-p75NTR-dependent manner:Blöchl and Sirrenberg (1996) measured ceramide generation in rat mesencephal‐ic neurons in culture following stimulation either with NGF or BDNF.Treatment of neurons with NGF (100 ng/mL) for 15 minutes generated ceramide levels about 3‐fold higher than the control. As neurons hardly ex‐press TrkA, these authors concluded that NGF generated ceramide through direct interaction with p75NTR. In contrast, BDNF (100 ng/mL) only marginally increased ceramides under similar culture conditions irrespective of the fact that the neurons express full length TrkB (TrkB.FL). However,pretreatment of neurons with the tyrosine kinase inhibitor K252a increased ceramide generation, suggesting tyrosine kinase activity of TrkB.FL in‐hibits p75NTR‐mediated activation of neural sphingomyelinase. Their study indicates that the BDNF‐TrkB.FL‐p75NTRaxis does not induce ceramide‐de‐pendent Nrf2 activation in neurons. However, this study suggested that the truncated form of TrkB (TrkB.T1), which lacks the intracellular receptor tyrosine kinase domain, does not inhibit ceramide generationviap75NTR(Figure 1A). As previous studies established that BDNF can activate CK2 in a p75NTRdependent manner and that astrocytes predominantly express TrkB.T1, we proposed that BDNF‐TrkB.T1‐p75NTR‐mediated generation of ceramide triggers the PKCζ‐CK2‐Nrf2 signaling pathway (Ishii et al., 2018).When neuronal activity remains relatively low during the rest/light phase,astrocytes preferentially store energy through these p75NTR‐dependent signaling pathways. As Clock:Bmal1 regulated Per2 protein peaks in the early light/rest phase and sharply decreases in the early dark/active phase in rodent local brain regions, we speculate the p75NTR‐mediated signaling is relatively high during the light phase in astrocytes, when neuronal activity remains low.When neuronal activity increases in the active/dark phase, astrocytes in turn reduce oxygen consumption and increase glycogen hydrolysis and glycolysis through cAMP/PKA‐dependent signaling pathways to support neuronal activity. As p75NTRlevels decreases, TrkB.T1 changes its functional partner from p75NTRto the adenosine 2A receptor (A2AR) to support cAMP/PKA signaling to facilitate lactate production (Ishii et al., 2018).
Does BDNF activate Nrf2 in neurons?Our model raises the question whether or not BDNF can regulate Nrf2 activation in neurons. Since BD‐NF‐TrkB.FL‐p75NTRsignaling does not support ceramide‐CK2 dependent Nrf2 activation, p75NTRexpression may be down‐regulated in neurons in the active/dark phase. Notably, neurons require Nrf2‐regulated antioxidant defenses during the active/dark phase rather than the rest/light phase. Is it possible that BDNF can upregulate Nrf2 in different time phases in neurons? We here discuss the possibility of such a mechanism by citing two key studies. Firstly, neurons express both TrkB.FL and TrkB.T1 receptors but the ratio of these receptor levels changes depends on neural activity(Gomes et al., 2012). Gomes et al. (2012) observed excitotoxic stimulation of cultured rat hippocampal or striatal neurons downregulated TrkB.FL,while upregulation of TrkB.T1 expression levels caused a significant change in the ratio of the two receptors within a few hours. TrkB.FL downregula‐tion was mediated through Ca2+‐sensitive protease calpain and an increase in TrkB.T1 was dependent on transcription and translation. These authors further showed that TrkB.T1 acts as dominant‐negative inhibiting TrkB.FL tyrosine kinase‐dependent signaling by forming a hetero‐dimer, with an increase in the ratio of TrkB.T1 to TrkB.FL protecting neurons from damage during excitotoxic stimulation with glutamate. Their study suggests activated neurons increase the ratio of TrkB.T1 to TrkB.FL, allowing BDNF to induce Nrf2 activation if sufficient levels of p75NTRare expressed in the membrane.
Secondly, another study shows that p75NTRis relatively stable in neurons and can be released from neurons in exosomes (Escudero et al., 2014).Transfer of small vesicles, called exosomes, has been implicated in astro‐cyte‐to‐neuron signaling. Exosomes carrying microRNA, mRNA, proteins and lipids generated from endosomal membranes have the potential to modulate the function of the recipient cells. Escudero et al. (2014) charac‐terized the internalization and post‐endocytic trafficking of p75NTRin neu‐ronal cells. The authors found that p75NTRevades the lysosomal route and accumulates in two different organelles, Rab11‐positive endosomes for re‐cycling back to membrane and CD63‐positive multivesicular bodies (MVBs)for exosomes. After stimulation of PC12 cells with NGF for 2 hours, these authors found 43% of p75NTRmoved to MVBs different from Rab7‐posi‐tive MVBs that mature into lysosomes. Similar accumulation of p75NTRin the MVBs was also observed in sympathetic neurons following treatment with BDNF. Notably, release of exosomes can be induced by membrane depolarization. After treatment with KCl, they found full‐length p75NTRwas enriched in the exosomal fraction released from both cell types. These results indicate BDNF/NGF treatment induces accumulation of p75NTRin the exosome‐related MVBs without rapid degradation in neurons, leading to release from cells in exosomes when neuronal activity increases. Neurons may reabsorb exosomes to increase p75NTRlevels in the plasma membrane.Astrocytes also secrete exosomes to communicate with other cells including neurons. As exosomes contain sortilin which could act as co‐receptor for p75NTRin the proNGF‐ and proBDNF‐induced cell death, and glioblastoma cells release TrkB‐containing exosomes (Pinet et al., 2016), it is possible that astrocytes provide p75NTRto neuronsviaexosomes. This possibility would?be of interest to verify in future studies (Figure 1B). Taken together, it is possible that activated neurons retain an ability to activate the BDNF‐TrkB.T1‐p75NTR‐Nrf2 signaling pathway.
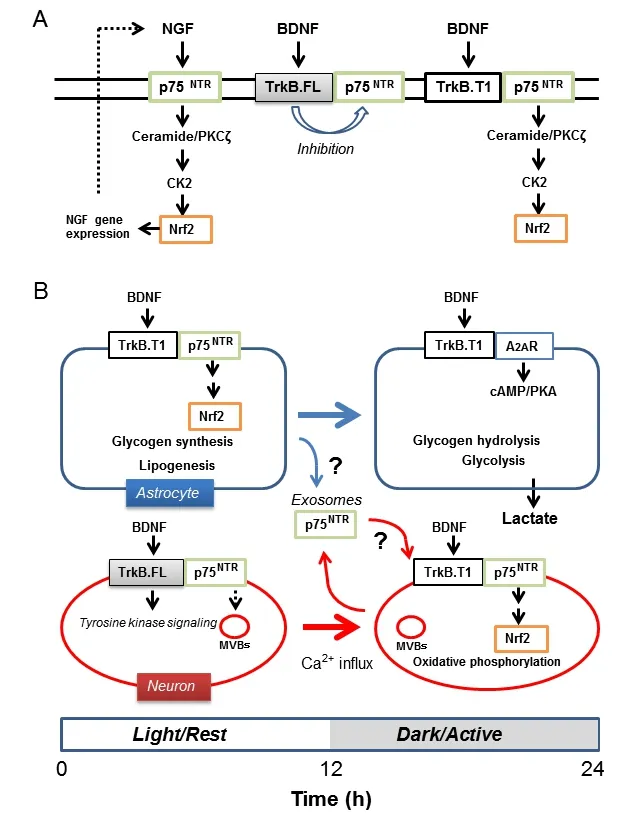
Figure 1 Differential time-dependent regulation of nuclear factor (erythroidderived 2)-like 2 (Nrf2) through brain-derived neurotrophic factor (BDNF) and its receptors in astrocytes and neurons.
Conclusion and future perspective:Ceramide generation may act as a double‐edged sword. Overstimulation of p75NTRinduces apoptosis due to over‐generation of ceramide. TrkB.FL receptor tyrosine kinase activity inhibits neutral sphingomyelinase protecting cells from ceramide toxicity and at the same time restricts p75NTR‐mediated signaling. In contrast, the BDNF‐TrkB.T1‐p75NTRsignaling complex is the machinery to generate physiological concentrations of ceramide to activate PKCζ leading to activation of CK2 and Nrf2, thereby modulating energy metabolism and antioxidant capacity. We refer readers to our recent review, which discusses the importance of BDNF receptor‐mediated cell shape changeviaregulation of Rho GTPases (Ishii et al., 2018). We suggest that the BDNF‐TrkB.T1‐p75NTR‐ceramide‐PKCζ signaling could modify voltage‐activated potas‐sium (Kv)1.5 type channels causing membrane depolarization and influx of Ca2+leading to activation of ras homolog gene family member A (RhoA)in astrocytes (Ishii et al., 2018). As many neurons express other types of Kvchannels, BDNF‐TrkB.T1‐p75NTR‐ceramide‐PKCζ signaling may not always induce membrane depolarization and RhoA activation in neurons (Ishii et al., 2013). Differential regulation of the actin cytoskeleton in astrocytes and neurons through BDNF receptors is another important aspect of metabolic cooperation between these two cell types. In summary, in addition to main‐taining normal expression of BDNF, preserving circadian control of p75NTRexpression and its dynamics in both astrocytes and neurons may serve as therapeutic targets to ameliorate neurodegeneration.
A part of the work (Ishii et al., 2018) was presented at the annual meeting of the Society for the Free Radical Biology&Medicine, on November 30th, 2017 at Baltimore, MD, USA, poster number 91.
We acknowledge the support of JSPS KAKENHI Grant Number 21500386 (TI)and British Heart Foundation (GEM, FS/15/31298; FS/16/67/32548).
Tetsuro Ishii*, Giovanni E. Mann
University of Tsukuba, Tsukuba, Japan (Ishii T)School of Cardiovascular Medicine and Sciences, King’s British Heart Foundation Centre of Excellence, Faculty of Life Sciences and Medicine, King’s College London, London, UK (Mann GE)
*Correspondence to:Tetsuro Ishii, Ph.D., ishiitetsuro305@gmail.com.
orcid:0000-0002-1402-9918 (Tetsuro Ishii)
Accepted:2018-03-19
doi:10.4103/1673-5374.232468
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Agnès Rioux Bilan, Universite de Poitiers, France.
Comments to authors:This manuscript describing when and how does BDNF activate Nrf2 in astrocytes and neurons is interesting and well documented. Figure 1 allows a better comprehension and appropriately completes the manuscript.
Reviewer 2:Supriya D. Mahajan, State University of New York, USA.
Comments to authors:The current manuscript is an interesting perspective on the role of BDNF and Nrf2 in the interplay between circadian rhythms and cellular redox metabolism. Manuscript is concise and well written and highlights the time dependent regulation of Nrf2 through BDNF and its receptors. The interesting concept is the transcriptional activation of p75NTR is under circadian regulation and also its effects on metabolism. Figure 1 is great and highlights the cross talk between the astrocytes and neurons.
Baeza‐Raja B, Eckel‐Mahan K, Zhang L, Vagena E, Tsigelny IF, Sassone‐Corsi P,Ptacek LJ, Akassoglou K (2013) p75 neurotrophin receptor is a clock gene that regulates oscillatory components of circadian and metabolic networks. J Neurosci 33:10221‐10234.
Blöchl A, Sirrenberg C (1996) Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem 271:21100‐21107.
Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA (1994) Activation of the sphingomyelin cycle through the low‐affinity neurotrophin receptor.Science 265:1596‐1599.
Escudero CA, Lazo OM, Galleguillos C, Parraguez JI, Lopez‐Verrilli MA, Cabeza C,Leon L, Saeed U, Retamal C, Gonzalez A, Marzolo MP, Carter BD, Court FA, Bron‐fman FC (2014) The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J Cell Sci 127:1966‐1979.
Gomes JR, Costa JT, Melo CV, Felizzi F, Monteiro P, Pinto MJ, Inacio AR, Wieloch T, Almeida RD, Graos M, Duarte CB (2012) Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci 32:4610‐4622.
Ishii T, Warabi E, Mann GE (2018) Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c‐Rel to reset energy metabolism in as‐trocytes via brain‐derived neurotrophic factor. Free Radic Biol Meddoi:10.1016/j.freeradbiomed.2018.01.026.
Ishii T, Warabi E, Siow RC, Mann GE (2013) Sequestosome1/p62: a regulator of re‐dox‐sensitive voltage‐activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic Biol Med 65:102‐116.
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress‐inducible genes in macrophages. J Biol Chem 275:16023‐16029.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K,Hatayama I, Yamamoto M, Nabeshima Y (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313‐322.
Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, Kitajima C, Maruyama A, Yama‐moto M, Shirasawa T (2010) Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem 147:73‐81.
Pinet S, Bessette B, Vedrenne N, Lacroix A, Richard L, Jauberteau MO, Battu S, Lal‐loué F (2016) TrkB‐containing exosomes promote the transfer of glioblastoma aggressiveness to YKL‐40‐inactivated glioblastoma cells. Oncotarget 7:50349‐50364.
- 中国神经再生研究(英文版)的其它文章
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations