A novel process integrating vacuum distillation with atmospheric chlorination reaction for flexible production of tetrachloroethane and pentachloroethane☆
Xian Chen ,Yunpeng Li,Ge Xu ,Jihai Tang ,2,*,Zhuxiu Zhang ,Ming Chen ,Zhaoyang Fei,Mifen Cui,Xu Qiao ,2,*
1 State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Jiangsu National Synergetic Innovation Center for Advanced Materials(SICAM),Nanjing 210009,China
1.Introduction
Reactive distillation(RD)that integrates reaction and separation process in a single unit is a process intensi fication approach with green engineering attributes that can facilitate high reaction conversion with low energy cost in the real compact plants[1–5].It has been utilized commercially in several reaction systems[6–9],but specific requirements on the agreement of temperature/pressure between reaction and distillation section[10,11]limit its extensive industrial application[12–14].Side reactor configuration(SRC)that enables the coupling of a nonreactive distillation column with side reactors are widely believed to circumvent above-mentioned drawbacks[15].For example,SRC can provide better economics than conventional reactive distillation in the direct hydration of cyclohexene to cyclohexanol when there is a temperature mismatch between reaction and distillation[16].In addition,atmospheric reaction and vacuum distillation have been fused together for the multistep consecutive chlorination reactions thanks to the SRC[17,18].It is of note that both equipment and process operating parameters should be optimized in order to take full advantages of SRC process[19].
Tetrachloroethane(TeCA,including 1-TeCA and 2-TeCA)and pentachloroethane(PCA)are significantchemicalintermediates in the refrigerant and cleaner industry.Although trichloroethylene(TCE)and perchloroethylene(PCE)have been used as raw materials for the production of TeCA/PCA[20],the high capital cost of raw materials and the complicated process for the production have provided impetus for finding alternative synthetic approaches,as exempli fied by the direct chlorination of 1,2-dichloroethane(DCA).The key issue for the practical application of chlorination of DCA resides with controlling the chlorination depth and reducing the energy cost associated with the separation of different chlorinated products in the distillation column.However,traditional RD process is considered ineffective for such reaction because high boiling point of TeCA and PCA will result in the high temperature in both the column and reactor,which may cause the low solubility of chlorine in the reaction mixture and subsequent low chlorination depth.
It occurred to us that the process integrating vacuum distillation with atmospheric chlorination reaction(VD-ACR),an SRC technology,could facilitate the flexible production of TeCA/PCA from DCA.In the real process,rigid production is not amenable to changes of the market especially when the economic environment is uncertain.In contrast,flexible production has certain advantages ifit can be realized with similarprocesses between productions ofdifferentsingle-componentproducts[21].Reaction process can stay at any stage in this multistep consecutive chlorination so thatthe ratio ofTeCA/PCAproductis adjustable.The optimal design parameters of VD-ACR were initially obtained when TeCA or PCA was set as the only target product.Then the molar ratio of TeCA/PCA in the product was adjusted by varying the amount of chlorine pumped into side reactors.Meanwhile,the distribution of chlorine to each side reactor and boilup ratio(BR)were optimized for each molar ratio of TeCA/PCA in the simulation.According to the results of simulation and optimization,the pilot-scale experiment was carried out in order to verify the feasibility of the VD-ACR process for the flexible production of TeCA/PCA.
2.Process Design and Description
Fig.1 presents the process flow diagram of the VD-ACR for the flexible production of TeCA and PCA.It contains a distillation column,a pre-chlorination reactor,two side reactors,a condenser,a reboiler and several pumps.In order to reduce reaction loads,operation costs and operation time,DCA was pre-chlorinated to various extents in a pre-reactor depending on different target products before flowing into the column.Then the mixture was pumped into the column.The feed location of pre-chlorination stream was the stage lower than the feedback stream from the bottom side reactor.The column was divided into a distillation zone with two external reactors and a stripping zone.The continuous flow of chlorine was divided into multiple streams and then introduced into all side reactors.Every reactor was assumed as a stirred tank reactor and chlorination reaction only occurs in the external reactors rather than in the column.
Totalliquid was transferred fromdistillation tray into the externalside reactors for chlorination.Reactors liquid effluents were pumped back to the column on the tray below the trap-out tray from which they were withdrawn.Ef fluent from the total condenser flowed into side reactor numbered 1#,and the stream was fed back to the first tray in the column for recycle.Similar to the above,the trays of trap-out and feedback in the column corresponding to the side reactor numbered 2#were the third and fourth tray respectively.Notably,the column and side reactors were operated at vacuum and atmospheric pressure respectively.The generated hydrogen chloride escaped fromthe side reactors to hydrochloric absorption system directly.
3.Method and Properties
3.1.Simulation method
Distillation simulation was conducted with the steady-state fractionation model RADFRAC from Aspen Plus,and it is based on an equilibrium stage model for solving the MESH equations(material balances,equilibrium relationships,summation equations,and heat balances).RCSTR model was chosen to simulate the side chlorination reactor.Liquid phase compositions of the reactor outlet were calculated by programming according to existing reaction kinetics and the consumption of chlorine set before using FORTRAN program embedded in the software.The calculation of the volume of the reactors was completed in accordance with the liquid phase composition of the reactor outlet and the consumption of chlorine.
3.2.Reaction kinetics
The chlorination of DCA is a photocatalytic gas–liquid substitution process shown in Fig.2.
Chen[22]studied the kinetics of this multistep photo-chlorination process at the temperature ranging from 343 to 383 K under atmospheric pressure in an internal loop reactor.Relevant kinetic data were obtained and the kinetic model which can exactly describe the behavior of the reaction kinetics of DCA chlorination was presented.With the process carried through,the reaction amount of chlorine was always changing and each component competed for the chlorine to complete chlorination reaction.Thus correction factors marked as Xmiwere introduced to estimate the kinetic parameters and X denotes the degree of chlorination.The detailed reaction kinetics in Eqs.(1)–(6),and X was described as below[22]:


Fig.1.Process flow diagram of the VD-ACR process for the flexible production of TeCA and PCA.

Fig.2.The chlorination reaction pathways of 1,2-dichloroethane.

As listed above,r1,r2,r3,r4,r5and r6are reaction rate and as well as the consumption rate of the chlorine at each step.CDCA,CTCA,C1-TeCA,C2-TeCAand CPCAare the mole fractions of DCA,TCA,1-TeCA,2-TeCA and PCA,respectively.PCl2is the partial pressure of chlorine in the system.Values of miin Xmiwere listed in Table A1.The suitable reaction temperature was thereby set around 353 K and corresponding reaction rate constants(ki)were also given in Table A1 as well.
3.3.Thermodynamic characteristics
The property database in Aspen Plus was used for the calculation of the thermodynamic properties and phase behavior in this studied system.The UNIQUAC(universal quasi-chemical)activity coefficient model was used to predict the liquid phase non-ideal behavior[23],and binary interaction parameters were taken from the database(in Table A2)or estimated using the UNIFAC(universal functional activity coefficient)model(in Table A3).
At normal pressure,bubble points of 1-TeCA,2-TeCA,PCA and HCA are 411 K,418 K,433 K and 458 K respectively.The high temperature of these bubble points will lead to high temperature of column bottom,inducing carbonization of coking easily.Besides,it needs to heat reboiler with the high grade heat source giving rise to larger energy consumption.The temperature of column bottom will decrease if the operation pressure is reduced.The relationship of bubble points and pressures was calculated by Aspen Plus using UNIQUAC(universal quasi-chemical)activity coefficient mode and shown in Fig.3.The suitable operating pressure of the column was if xed at 20 kPa.

Fig.3.Relationships between bubble point temperatures and pressures.
4.Results and Discussion of Simulation
4.1.Production of TeCA
The parameters of simulated base case and the composition of prechlorination stream are listed in Tables 1 and A4.

Table 1 The parameters of simulated base case
Firstly,the simulation of production of TeCA was carried out.When TeCAis the desired product,DCAshould be pre-chloridized to the extent before the generation of PCA and the composition of pre-chlorination solution is shown in Table A4 at 353 K.There are many structural and operating design variables in this VD-ACR process.The effects of three configuration variables were studied as follows:the numberofstripping stages in the stripping zone(NS),the number of stages between each reactor(NRS)and the numberof external side reactors(NR).The effects of two operating design variables were studied as follows,the boilup ratio(BR)and side stream flow rate(SFR).The feed rate of DCA was setto 5 kmol·h-1.The mole fraction ofthe targetproductin the bottom stream of the column was set as the investigation target.
4.1.1.Effects of BR

Fig.4.Effects of BR on product compositions(NS=13,NRS=1,NR=3,SFR=70 kmol·h-1).
Fig.4 shows the effects of boilup ratio on product composition.It is observed that as BR increases from 5 to 15,the mole fraction of TeCA in the product slightly increases while the mole fraction of TCA and PCA both decreases.The reason is that more DCA and TCA were gathered in the upper area of the column with an increase of the BR,and then,they further react with chlorine in the side reactors to produce more TeCA.PCA and HCA were produced because TeCA is hardly to move up.With a further increase in BR,the mole fraction of TeCA in the product almost remains the same.Therefore,the optimum BR to attain the desired purity of TeCA in this system is 15.It is concluded that the increasing boilup ratio not only enhances the separation of the components,but also improves the reaction performance by recycling the unconverted reactants to the reaction zone.However,optimal BR should be selected considering the negative effectthatmore energy costis needed with too high value of BR.
4.1.2.Effects of NS
The total number of stages can be calculated once the number of stages in the distillation zone with side reactors and stripping zone are determined.Fig.5 shows the effects of the number of stripping stages on product composition.As NS increases from 2 to 13,the mole fraction of TeCA rises from 76%to 94%and the reason of this situation is similar to that of effects of BR.However,the composition of TeCA remains unchanged basically when NS increases to the value over 13.In a multicomponent system,the changes in the separation efficiency tend to adjust composition profiles rather than produce more clearly de fined product splits[24].As NS increases from 2 to 13,the capability of removing TeCA from the reaction zone has been strengthened,and this is effective in improving the reaction performance.The optimum number of stripping stages is 13 because it can provide enough separation driving force,and it may result in difficulties in operation and higher capital investment with more stripping stages.

Fig.5.Effects of NS on products composition(BR=15,NRS=1,NR=3,SFR=70 kmol·h-1).
4.1.3.Effects of NRS
In this simulation,the distributed ratio of chlorine into two side reactors was set as 0.6:0.4.The effects of NRS on product composition are shown in Fig.6.It reveals that with the increase of NRS,the product composition of each component remains almost the same as before.In order to explain the problem in detail,liquid composition distribution of TCA and TeCA in the column is shown in Fig.7 when the NRS were set as 1,5 and 9 respectively.Apparently,the increasing trays in distillation zone with side reactors caused by the increasing NRS are of no importance to the separation effect.Besides,compositions of liquid into the adjacent side reactors were almost the same.On the contrary,the rising of NRS might cause the negative effects such as difficulty in operation and higher capital investment.Therefore,only one intermediate stage between each reactor is suf ficientto achieve the mostappropriate separation performance.

Fig.6.Effects of NRS on product composition(BR=15,NS=13,NR=3,SFR=70 kmol·h-1).
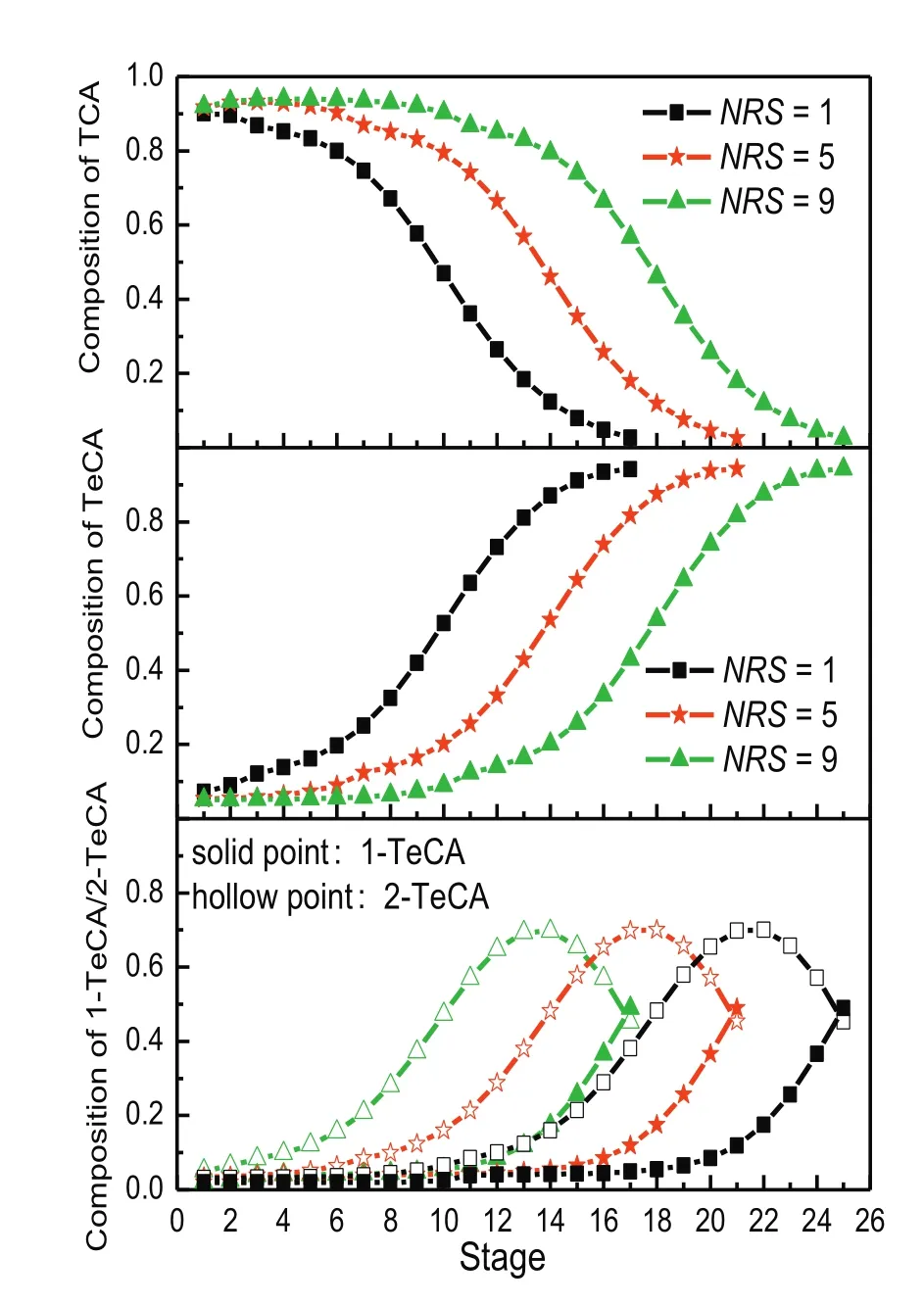
Fig.7.Liquid composition distribution of TCA and TeCA when NRS was set as 1,5,9 respectively(BR=15,NS=13,NR=3,SFR=70 kmol·h-1).
4.1.4.Effects of NR

Fig.8.Effects of NR on product composition(BR=15,NS=13,NRS=1,SFR=70 kmol·h-1).
The number of side reactors has vital impacts on selectivity of target product.Fig.8 reveals the effects of the number of side reactors on product composition.The increasing number of side reactors from 1 to 5 improves the purity of TeCA in the products due to the higher dispersion of chlorine,larger reaction space and enhanced distillation effect resulted from the increased total number of stages.It's worth noting that the mole fraction of TeCA increases by 1.2%when NR rising from1 to 3 while itonly has a slightincrease(only 0.4%)when NR rising from 3 to 5.Accordingly,the optimum distribution ratio of chlorine to the third reactoris only 12%when NR is 3,and the newly added reactors are useless in improving the purity of TeCA as shown in Fig.A1.Meanwhile,the negative effects such as difficulties in operation and control,higher capital investment and more complex process can be endowed with superabundant side reactors.Therefore the optimum number of side reactors is 3.
4.1.5.Effects of SFR
The side stream flow rate directly determines the amount of reaction capability in the side reactors.Under the optimal distributed ratio of chlorine to each reactor(f bom),the side stream flow rate has significant in fluence on the system performance.Fig.9 shows the effects of side stream flow rate on the mole fraction of TeCA in product.The mole fraction of TeCA in the bottom increases linearly from 0.946 to 0.951 as SFR rising from 30 kmol·h-1to 70 kmol·h-1.This is due to that the increasing of SFR leads to the improvement of the chlorination reaction selectivity under the fixed amount of consumption of chlorine.The larger side stream flow rate,the higher the mole fraction of TeCA in product so that the optimum operation mode is total withdrawal.

Fig.9.Effects of SFR on product composition(BR=15,NS=13,NRS=1,NR=3).
4.2.Flexible production of TeCA/PCA
4.2.1.The relationship between the production composition and total chlorine amount
With similar research process described in the previous Section 4.1,the simulation in the production of HCA using VD-ACR process was carried out.It is obvious that most optimal configuration and operating parameters in the production of PCA are the same as those in the production of TeCA as displayed in Table A5 except NS,SFR and f bom.Besides,the heat duty of reboiler in the production of PCA is a bit higher than that in the production of TeCA.In view of the analogous integrated configuration in the production of TeCA and PCA,the method of the flexible production of TeCA and PCA was explored through adjusting the amount of chlorine pumped into side reactors.
The simulated base case and composition of pre-chlorination stream was the same as those in production of TeCA in Section 4.1.A relationship between the composition in the bottom product and the total flowrate of chlorine to the side reactors(FCl2)was achieved,and it was shown in Fig.10.The composition of TeCA in the product increases as the feed rate of chlorine to the side reactor rises,and this is bene ficial at the beginning.The reason is that the light components of DCA and TCA can easily move to the top of the column to react with chlorine to produce TeCA,while the heavy component of TeCA is hardly to move up.Hence,the mole fractions of PCA and HCA are almost zero.However when FCl2is larger than 5 kmol·h-1,the selectivity of TeCA begins to decrease and the composition of PCA rises rapidly.A reasonable explanation for this phenomenon is that DCA and TCA are consumed completely and TeCA which is the light component under this condition moves to the top area to react with chlorine,so the highest mole fraction of TeCA appears when FCl2is 5 kmol·h-1.Thus,changing the amount of feed flow rate of chlorine can adjust the ratio of TeCA/PCA in the product at a fixed integrated configuration of VD-ACR.

Fig.10.Relationship between composition and reaction amount at the bottom.
4.2.2.Flexible production of TeCA and PCA
The total stage number of the column and NR were fixed at 19 and 3 respectively.The feed stage of pre-chlorination mixture is the sixth stage.The flow rate and composition of pre-chlorination mixture,the operation pressures of column and reactors were the same as those in Table 1.The molar ratio of TeCA/PCA was adjusted from 0.9:0.1 to 0.1:0.9 through changing FCl2and the minimum BR and reboiler heat duty to meet the purity of the product that are sought at the same time as shown in Fig.11.As we can see,the required amount of Cl2is proportional to the increase while the BR and reboiler heat duty both increase faster and faster,as molar ratio of TeCA/PCA was adjusted from 0.9:0.1 to 0.1:0.9.In addition,the amount of Cl2assigned in the second and third reactors slightly increases as the BR increases on accountofincreased liquid flow rate into them resulting from increased rising steam from reboiler.

Fig.11.Matching relationship between reaction and separation capacity at three product ratios.
Fig.A2 shows the variation trend of the volume of side reactors at various molar ratio of TeCA/PCA.It is obvious that each side reactor volume and the total volume of all reactors both show a trend of increase molar ratio of TeCA/PCA that was adjusted from 0.9:0.1 to 0.1:0.9.Considering the resultsfrom both Figs.11 and A2,itis concluded that more energy consumption and equipment costs are needed when the mole fraction of PCA in the TeCA/PCA product is added in the same integrated configuration.
4.2.3.Effects of pre-chlorination reaction extent
The effects of three different compositions of pre-chlorination liquid on the process were studied with the molar ratio of TeCA/PCA fixed at 0.5:0.5.As shown in Table A6,pre-chlorination liquid 1#,2#and 3#are the products of which DCA is pre-chloridized to the degree before TeCA,PCA and HCA generation respectively.Table A7 presents the calculated parameters of column with three different pre-chlorination liquids.Apparently,parameters of column such as temperatures and heat duty of both condenser and reboiler,tower diameter almost keep unchanged in Table A7 as well as the liquid composition distribution in the column revealed in Fig.A3.In other words,the degree of pre-chlorination has little impact on separation of components.
The calculated volumes of each side reactor were shown in Fig.A4.It can be found that the volume of each corresponding side reactor all decreases with higher degree of pre-chlorination.Above all,the effects of the degree of pre-chlorination are mainly manifested in the changes of side reactor volumes.Appropriate improvement of pre-chlorination extent contributes to reducing the volumes of side reactors.
5.Pilot Plant Test of VD-ACR
5.1.Design basis and process parameters
In order to validate the process model used to analyze the process,a pilot-scale distillation column with side reactors was conducted.The process was designed according to some conditions which we call them design basis.The molar ratio of TeCA/PCA in the products was set up at 0.5:0.5,and the mole fraction of PCA was controlled less than 2%.The mole fractions of the components in pre-chlorination liquid were the same as that in pre-chlorination liquid 3#which is shown in Table A6 for the sake of reduced volumes of side reactors on the basis of previous research in the Section 4.2.3.The optimized simulation parameters based on design basis were list in Table 2.The distillation column used in the pilot experiment was the plate column,of which plate efficiency was set as 0.72.According to optimized simulation parameters and the established process were summarized in Table 2 as well.

Table 2 Values of Parameters for Simulation and Pilot Test
5.2.Pilot-scale experiment
The whole process is divided into two workshop sections including pre-chlorination and VD-ACR.
5.2.1.Pre-chlorination workshop section
Two cubic meters of DCA was pumped into the pre-chlorination reactor,and then,chlorine was introduced in the flow rate at 45 m3·h-1.The flow rate of chlorine was raised to 90 m3·h-1and 183 m3·h-1respectively after having reacted for half an hour and 1 h.The change of temperature in the pre-chlorination reactor was observed simultaneously,and the temperature should be controlled within 333 K and 353 K.Samples were taken to analyze the composition of reaction solutions in the reactor every half hour during the process of prechlorination.When the composition was close to the values of prechlorination liquid 3#listed in Table A6,pre-chlorination was stopped and the reaction solutions remained was pumped into storage tank.
5.2.2.VD-ACR workshop section
One and a half cubic meters of the pre-chlorination mixture was pumped into each side reactor with volume of 2 m3.Pre-chlorination liquid was infused into the column through a flow controller to twothirds ofthe liquid levelmeter location.Meanwhile,the vacuum system ofcolumn was launched,and the operation pressure was keptin 20 kPa.Steam was piped into reboiler and then reaction solutions of two side reactors were pumped into distillation column to establish total re flux system after the appearance of re flux in the top of the column.At the same time,changes of material composition and temperature in the side reactors were monitored and analyzed.Chlorine was piped intoside reactors after thatthe composition keptunchanged.The flow rate of cooling water was set up according to the temperature display of reaction kettle to ensure that the temperature is not higher than 383 K.When there was no TCA and mole fraction of HCA was about 0.5%in the bottom of the column,Pre-chlorination liquid was pumped into the column continuously and the flow rate was controlled in 0.15 m3·h-1.
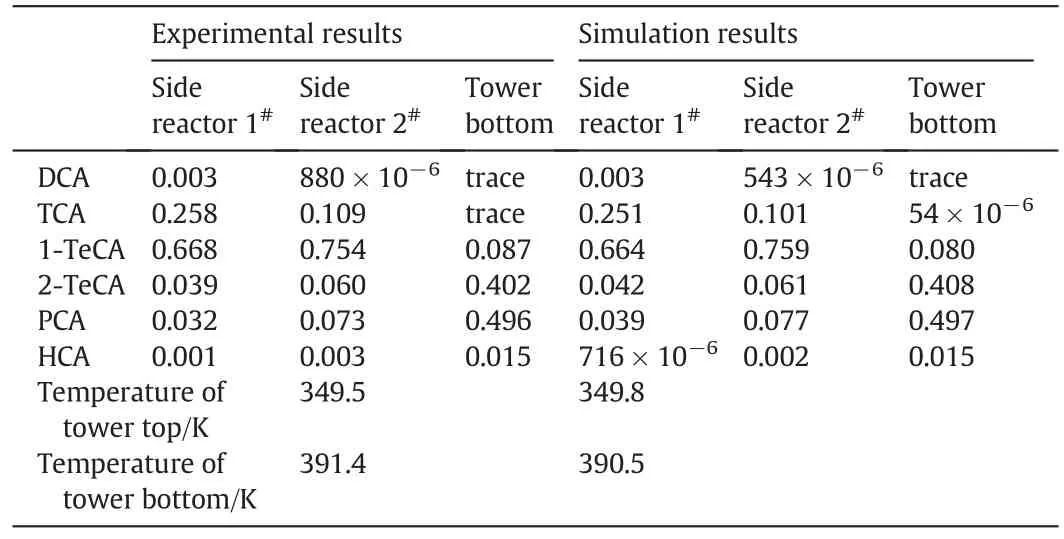
Table 3 Experimental and simulation results for pilot-scale flexible production
5.3.Experimental versus simulation results
The simulation and pilot test run of the production process was carried outunder the operating conditions in Table 2.The simulation results are given in Table 3 together with the experimentalresults.Table 3 shows that the simulation results agree well with those of experiment.Therefore,it can be deduced that the simulation and optimization method is reliable and as well as the simulation results of the flowsheet in the above section.
6.Conclusions
In this study,we reported a computational study of a novel process integrating vacuum distillation with atmospheric chlorination reaction(VD-ACR)in the context of the flexible production of TeCA/PCA from DCA.Optimal configuration and operating parameters were obtained based upon the sensitivity analysis when setting TeCA or PCA as the target product.The molar ratio of TeCA/PCA in products is alterable by varying the boilup ratio and amount/distribution of chlorine pumped into side reactors because the integrated configuration parameters of producing TeCA and PCA are similar.Increasing the mole fraction of PCA in the TeCA/PCA product will generate higher energy consumption and equipment costs.The simulation results presented in this paper were also validated by an experiment of distillation with side reactors in a pilot-scale column.Therefore,the VD-ACR is an efficient model for designing flexible production of TeCA/PCA from DCA in an industrial scale.
Nomenclature
CDCAmolar concentration of 1,2-dichloroethane,mol·L-1
CTCAmolar concentration of 1,1,2-trichloroethane,mol·L-1
CPCAmolar concentration of pentachloroethane,mol·L-1
C1-TeCAmolar concentration of 1,1,1,2-tetrachloroethane,mol·L-1
C2-TeCAmolar concentration of 1,1,2,2-tetrachloroethane,mol·L-1
FCl2feed rate of chlorine to the side reactor,kmol·h-1
f bomoptimum distributed ratio of chlorine to the m th reactor
ki first-order reaction rate constants,h-1·kPa-1
PCl2chlorine partial pressure,kPa
rireaction rate,mol·L-1·h-1.
w mass fraction
X the degree of chlorination
Xmicorrection factor
Appendix

Table A1 Reaction rate constants at 353 K and power series of competition factors①

Table A2 Values of binary interaction parameters taken from the database using UNIQUAC model

Table A3 Values of binary interaction parameters estimated by the UNIFAC model using UNIQUAC model

Table A4 Composition of pre-chlorination stream when setting TeCA as target product

Fig.A1.Optimal distribution ratios of chlorine in side reactors with different values of NR(BR=15,NS=13,NRS=1,SFR=70 kmol·h-1).

Table A5 Comparison between optimization results of two processes

Fig.A2.Volumes of side reactors at various product ratios.

Table A6 Compositions of pre-chlorination liquid of three different extents
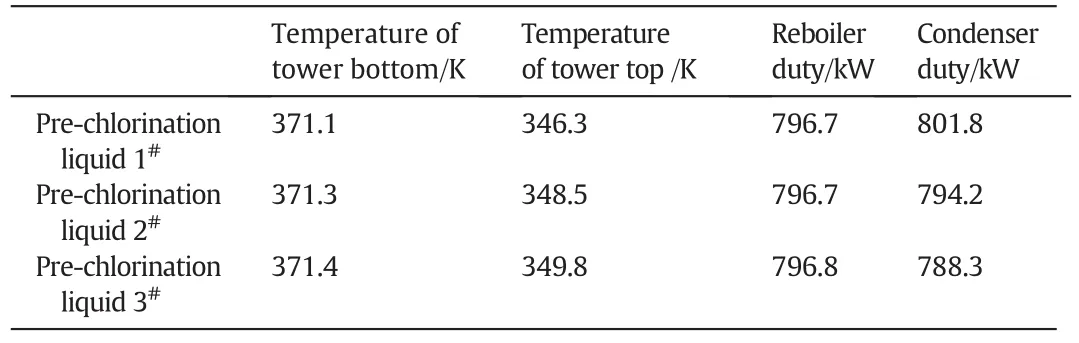
Table A7 Results of parameters of column at three pre-chlorination extents

Fig.A3.Liquid composition distributions of TCA and TeCA at three pre-chlorination extents.
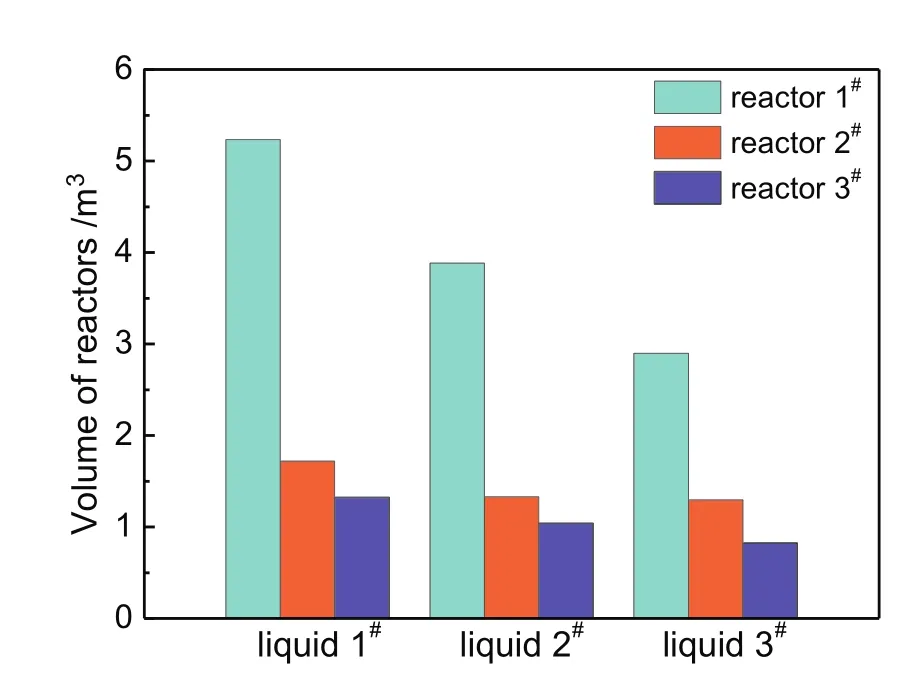
Fig.A4.Volumes of side reactors at three pre-chlorination extents.
References
[1]M.F.Malone,R.S.Huss,M.F.Doherty,Green chemical engineering aspects of reactive distillation,Environ.Sci.Technol.37(2003)5325–5329.
[2]J.L.DeGarmo,V.N.Parulekar,V.Pinjala,Consider reactive distillation,Chem.Eng.Prog.88(1992)43–50.
[3]J.J.Siirola,Industrial applications of chemical process synthesis,Adv.Chem.Eng.23(1996)1–62.
[4]D.B.Kaymak,W.L.Luyben,Quantitative comparison of reactive distillation with conventional multiunit reactor/column/recycle systems for different chemical equilibrium constants,Ind.Eng.Chem.Res.43(2004)2493–2507.
[5]H.Tian,S.Y.Zhao,H.D.Zheng,Z.X.Huang,Optimization of coproduction of ethyl acetate and n-butyl acetate by reactive distillation,Chin.J.Chem.Eng.23(2015)667–674.
[6]C.L.Cheng,Y.H.Chung,H.Y.Lee,Design and control of reactive distillation process for the production of methyl valerate,Ind.Eng.Chem.Res.55(2016)1347–1360.
[7]U.Sahapatsombud,A.Arpornwichanop,S.Assabumrungrat,Simulation studies on reactive distillation for synthesis of tert-amyl ethyl ether,Korean J.Chem.Eng.22(2005)387–392.
[8]S.J.Wang,S.H.Cheng,P.H.Chiu,Design and control of a thermally coupled reactive distillation process synthesizing diethyl carbonate,Ind.Eng.Chem.Res.53(2014)5982–5995.
[9]Z.Lei,C.Dai,Y.Wang,Process optimization on alkylation of benzene with propylene,Energy Fuel 23(2009)3159–3166.
[10]D.B.Kaymak,W.L.Luyben,O.J.Smith,Effect of relative volatility on the quantitative comparison of reactive distillation and conventional multi-unit systems,Ind.Eng.Chem.Res.43(2004)3151–3162.
[11]D.B.Kaymak,W.L.Luyben,Optimum design of a column/side reactor process,Ind.Eng.Chem.Res.46(2007)5175–5185.
[12]R.Knishna,Reactive separations:more ways to skin a cat,Chem.Eng.Sci.57(2002)1491–1504.
[13]C.Noeres,E.Y.Kenid,A.Gorak,Modeling of reactive separation process:reactive absorption and reactive distillation,Chem.Eng.Process.42(2003)157–178.
[14]R.Thery,X.M.Meyer,X.Joulia,Analysis of the feasibility synthesis and conception of processes of reactive distillation:state of the art and critical analysis,Can.J.Chem.Eng.83(2005)242–266.
[15]S.B.Gadewar,L.Tao,M.F.Malone,Process alternatives for coupling reaction and distillation,Chem.Eng.Res.Des.82(2004)140–147.
[16]J.Ye,J.Li,Y.Sha,Evaluation of reactive distillation and side reactor configuration for direct hydration of cyclohexene to cyclohexanol,Ind.Eng.Chem.Res.53(2014)1461–1469.
[17]L.H.Ding,J.H.Tang,M.F.Cui,X.Chen,C.M.Bo,X.Qiao,Optimum design of coupled atmospheric reaction-vaccum distillation technology for benzyl chloride production,CIESC J.62(2011)2323–2327(in Chinese).
[18]C.M.Bo,J.H.Tang,Y.J.Bo,X.Qiao,L.H.Ding,S.Zhang,The design and control of distillation column with side reactors for chlorobenzene production,Chin.J.Chem.Eng.20(2012)1113–1120.
[19]T.Ouni,K.Jakobsson,A.Pyhälathi,Enhancing productivity of side reactor configuration through optimizing the reaction conditions,Chem.Eng.Res.Des.82(2004)167–174.
[20]Y.Correia,J.C.Stini,Process for Obtaining 1,1,1,2-tetrachloroethane,USA,1975 235777.
[21]H.J.Boonman,V.Hagspiel,P.M.Kort,Dedicated vs product flexible production technology:strategic capacity investment choice,Eur.J.Oper.Res.244(2015)141–152.
[22]W.Chen,Study on Reaction Kinetics and Reactive Distillation of Consecutive Multi-Step Photo-Chlorination of Dichloroethane,M.S.Thesis Nanjing Tech Univ.,Nanjing 2014(in Chinese).
[23]Z.X.Huang,J.L.Li,L.Y.Wang,H.M.Jiang,T.Qiu,Novel procedure for the synthesis of dimethyl carbonate by reactive distillation,Ind.Eng.Chem.Res.53(2014)3321–3328.
[24]M.G.Sneesby,M.O.Tade,R.Datta,T.N.Smith,ETBE synthesis via reactive distillation.1.Steady-state simulation and design aspects,Ind.Eng.Chem.Res.36(1997)1855–1869.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
- 17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
- Transesteri fication of sun flower oil in microchannels with circular obstructions
- The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
