Transesteri fication of sun flower oil in microchannels with circular obstructions
Harrson S.Santana *,João L.Silva Jr ,Deborah S.Tortola Osvaldir P.Taranto
1 School of Chemical Engineering,University of Campinas,13083-852 Campinas,SP,Brazil
2 Federal Institute of Education,Science and Technology of South of Minas Gerais–IFSULDEMINAS,37560-260 Pouso Alegre,MG,Brazil
1.Introduction
Recently,the scientific community has been accompanied an increment in use of micro fluidic devices,including biological applications[1–3],energy[4,5],liquid–liquid extraction[6],food and agriculturalapplications[7]and microreactors[8–11],among others.The micromixer is a common elementpresentin these devices.Usually,these operations occur under low Reynolds number,in laminar flow regime and requiring long paths or times to promote the fluid mixing.Therefore,several researchers have been engaged to develop different techniques to enhance the mixing efficiency.
Two types of micromixers have been studied:actives and passives[12–19].Active micromixers employ an external energy source(magnetic or electrical)to promote the fluid mixing,while the passive type do not require an external energy source and the flow work provides the energy to blend the fluids.Passive micromixers exhibitas advantage an easy manufacturing and integration with other devices.However,normally this type of micromixer presents longer mixing length and greater Reynolds numbers relatively to the active type.Hence,the development of a micromixer for a high mixing efficiency with smaller channels and lower Reynolds numbers is an essential key.
There are several types of passive micromixers with various geometry configurations.Among these,micromixers with circular obstructions stand out as a device capable to promote fluid mixing at low Reynolds numbers.
Alam et al.[13]studied the mixing between water and ethanol in curved geometry micromixers with different types of obstructions:circular,hexagonal and diamond shapes.Circular and hexagonal obstructions exhibited equivalent mixing efficiency,while diamond shape provides an inferior mixing performance.Alam et al.[13]chose the micromixer with circular obstructions due to simplicity of manufacturing process.The superior mixing performance by micromixer with obstructions is related to the greater interactions among chemical species[20].Therefore,this micromixer configuration can be used in biodieselsynthesis,increasing biofuelproduction yield and contributing for the reduction of diesel use.
Biodieselis a mixing ofalkylesters oflong-chain fatty acids,produced by renewable feedstocks,such as vegetable oils and animal fats[21].Transesteri fication reaction is the most common method to synthesize biodiesel.The synthesis is based in a chemical reaction between an alcohol and a triglyceride(vegetable oil),presenting high reaction rates in presence of acid,alkaline or enzymatic catalysts.This reaction process corresponds to a transformation of an ester in another ester,through the exchanging of an acyl group from ester to other chemical species involved,in order to reduce the vegetable oil viscosity,enabling the use of biodiesel directly as a fuel,without requirement of further modi fications[22].Transesteri fication reaction has been explored successfully in microreactors employing several micromixer configurations[23–26].
Arias et al.[27]used three microchannel configurations(Tesla,Omega and T-shape)made of polydimethylsiloxane(PDMS)in the biodieselsynthesis from canola oilwith ethanoland sodium hydroxide catalyst.Squared cross section microchannels(Width=Height=500μm,Length=1 m)were used.The maximum conversions were 96.7%,95.3%and 93.5%,using Tesla,Omega and T-Shape configurations,respectively,under reaction conditions of 50°C,1%of NaOHcatalyst,alcohol/oil molar ratio of 25:1 and residence time of 10 min.
Zig-zag configuration was used in microchannels by Wen et al.[28]in the transesteri fication of soybean oil and methanol.Nine different configurationsmade ofstainlesssteelwith a length of1.07 m were evaluated.The geometry with 350 curves and hydraulic diameter of 240 μm showed the better performance.The maximum conversion was 99.5%at 56°C,alcohol/oil molar ratio of 9 with a sodium hydroxide concentration of 1.2%for a residence time of 28 s.
Sun et al.[29]studied the transesteri fication of cottonseed oil with methanol and potassium hydroxide as catalyst to produce biodiesel using four micromixer configurations:T-shape;J-shape and multilamination micromixers(rectangular interdigital micromixer e slit interdigital micromixer).The multilamination type exhibited greater efficiency relatively to traditional micromixers(T-and J-shapes).It was obtained a yield of 99.5%,for an alcohol/oil molar ratio of 8:1 at 70°C and residence time of 17 s.
Rahimi et al.[30]carried out the soybean oil transesteri fication with methanol and KOH,with addition of hexane as co-solvent.Three micromixer designs,made on a flat plate by polymethylmethacrylate,were investigated.The configurations consisted in different con fluence angles of three fluid inlets(oil,methanol/KOH and hexane).The con fluence angles were 45°(E1 configuration),90°(E2 configuration)and 135°(E3 configuration).The maximum yield was 98.8%,provided by E1 configuration at 57.2°C,catalyst concentration of 1%,residence time of 9.05 s,oil/methanol volumetric ratio of 3 and hexane/methanol volumetric ratio of 0.45.The authors highlighted an increase on oilmethanol miscibility with addition of the co-solvent,enhancing the reaction rate,due to the mass transfer resistance reduction between the alcohol and the triglyceride.
Although the micromixer development using numerical studies have been employed successfully[13,31,32],there is a lack of research on the using of numerical techniques to evaluate the micromixer ef ficiency and to apply this configurations in the biodiesel synthesis.In this context,the present work studied the biodiesel synthesis of sunflower oil with ethanol using microchannel reactor with circular obstructions.Three different configurations were investigated:T-shape–channel without obstructions;MCO–channel with 3 obstructions ensemble–equally disposed over longitudinal length;MWO–channel with 7 obstructions ensemble.The MCO micromixer was based on work by Alam et al.[13]and MWO is a totally new design.The mixing degree and chemical reaction between the fluids was numerically evaluated,over a range of operating conditions:Reynolds numbers(0.1–100);Temperature(25–75 °C);Molar ratio(6-12)and catalyst concentration(0.75 wt%–1.25 wt%).Experimentalruns were performed in micromixer with circular obstructions over the same operating conditions of temperature,molar ratio and catalyst concentration.
2.Modeling and Simulation
2.1.Geometries and fluid properties
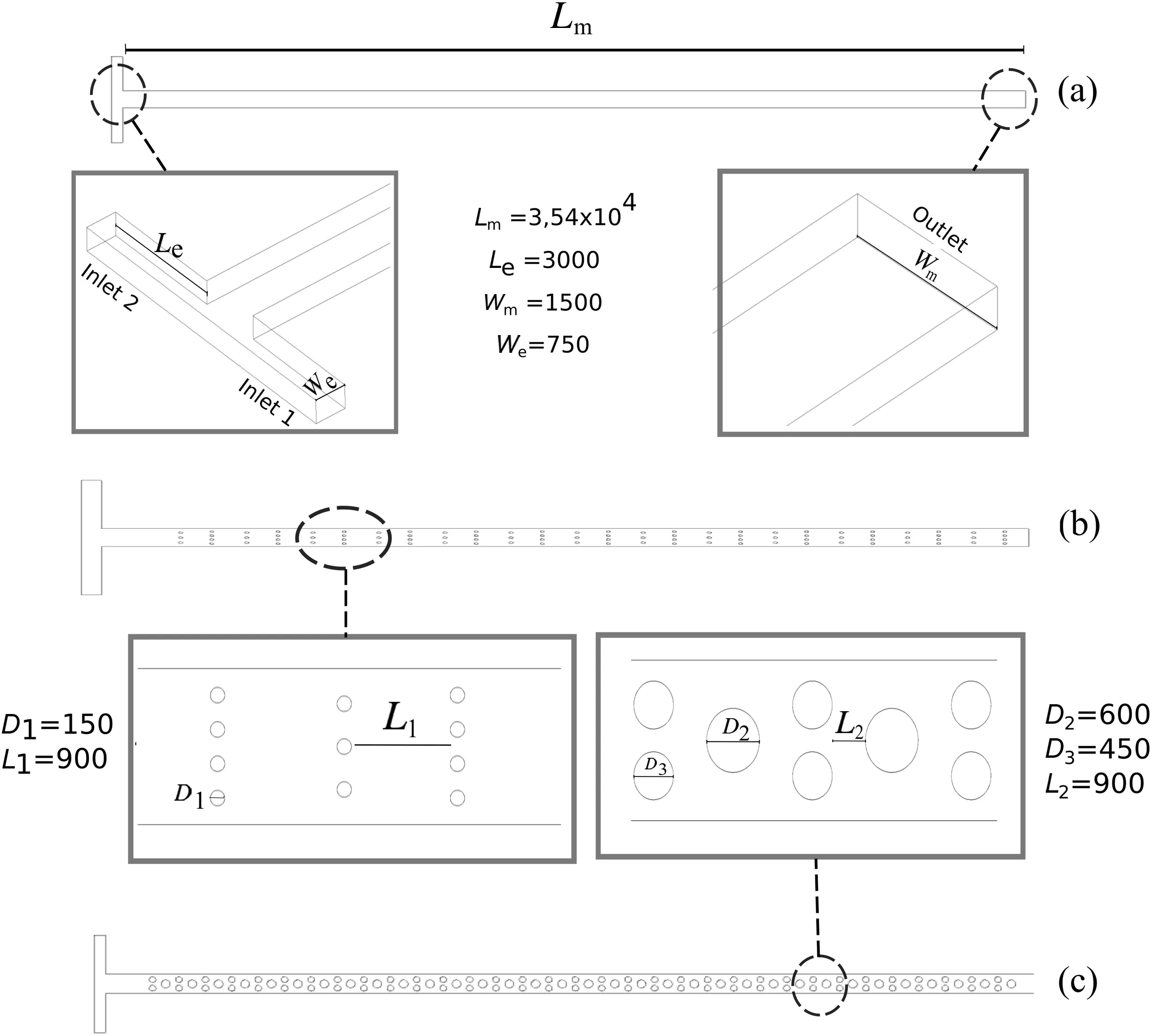
Fig.1.Micromixer configurations:(a)T-shape;(b)MWO;(c)MCO.All units are given in micrometers.
The microchannel configurations used in numerical tests are shown in Fig.1.The T-shape[Fig.1(a)],MWO[Fig.1(b)],and MCO[Fig.1(c)]present rectangular cross section.The inlet length(Le)and inlet width(We)was 3000 μm and 750 μm,respectively.The channel length(Lm)and channel width(Wm)was 3.51 × 104μm and 1500 μm,respectively.The height of micromixers was 200 μm.The obstruction diameter(D1)for MWO configurations was 150 μm,with an interval length(L1)of 900 μm.The obstruction diameters for MCO configuration were 600 μm for the larger obstacles(D2)and 450 μm for the smaller obstacles(D3),with an interval length(L2)of 900 μm.
The geometries were created with Autodesk Inventor2015 software.The discretization processes(subdivision of domain in a finite number of control volumes)were performed using ANSYS ICEM 14.0 software,generating tetrahedral-element unstructured meshes.Before the mixing and chemical reaction studies,an independence mesh test was carried out,in order to minimize the effectofnumericalmesh on the results.The independence mesh test and the study of fluid mixing was performed at 40 °C,with sun flower oil(specific mass=904.3 kg·m-3;dynamic viscosity=0.029 Pa·s)and ethanol(specific mass=785.1 kg·m-3;dynamic viscosity=0.0008 Pa· s).A uniform inlet velocity of 1.741 m·s-1was employed for both inlets.The mixing index parameter(mixing quality based on mass fraction of species)was analyzed.
The chemical composition of fatty acids for the sun flower oil(detailed in Section 3.1)and the mass conservation principle were used to estimate the oil molecular weight,resulting in 876.23 kg·kmol-1for sun flower oil and 305.74 kg·kmol-1for biodiesel.The Appendix A summarizes the physical properties of sun flower oil,ethanol,biodiesel and glycerin.
2.2.Reaction kinetics
The transesteri fication reaction consists in a series of consecutive and reversible reactions,which in alkaline medium,promotes a reaction of triglycerides(TG)with alcohol(A),producing diglycerides(DG)and ethylester(E).The DGreacts with alcohol,yielding esterand monoglyceride,which finally reacts,generating glycerol(GL)and ester(E)[33].The global transesteri fication reaction is given by:

The rate of TG consumption at equilibrium can be expressed as Marjanovic et al.[34]:
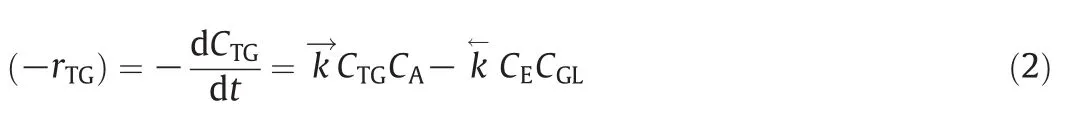
where(-rTG)is the triglyceride rate ofreaction(mol·m-3·s-1),andare the rate of reaction constants(m3·mol-1·s-1)for direct and reverse reactions,respectively,CTG,CA,CEand CGLare the molarconcentrations(mol·m-3)of triglyceride,alcohol,biodiesel and glycerol,respectively.The rates of reaction constants for studied temperature are given in the Appendix B.
2.3.Computational model
The software ANSYS CFX 14.0 was used in the fluid dynamic and chemical reaction studies.The solver is based on the solution of conservation equations for mass[Eq.(3)]and momentum[Eq.(4)]:

where ρ is the specific mass(kg·m-3),U is the velocity vector(m·s-1),p is the pressure(kg·m-1·s-2),μis the dynamic viscosity(kg·m-1·s--1),g is the gravity acceleration(m·s-2).
The mass conservation of chemical species i is given by Eq.(5):

where Yiis the mass fraction of chemical species i,Diis the kinematic diffusivity coefficient of chemical species i,Siis the mass source of chemical species i due to chemical reaction,modeled in accordance with Eq.(6):

where MWiis the molecular weightofchemicalspecies i,ν″andν′are the stoichiometric coefficients of species as products or as reactants,respectively,in the chemical reaction r and rris the rate of reaction r.
Using the kinetic model given by Eq.(2),the mass source for each chemical species is:

where MWTG,MWA,MWGLand MWEare 879.23,46.07,92.08 and 308.45 kg·kmol-1,respectively.The ethanol kinematic diffusivity(1.2 × 10-9m2·s-1)was considered equal to diffusion coefficient of ethanol in oil,estimated employing Wilke-Chang Equation[35].For the other chemical species,the viscosity values of the medium were used to calculate the diffusion coefficient[36].
2.4.Mixing index and reaction efficiency
The mixing degree between sun flower oil and ethanol was based on the oil mass fraction standard deviation at a cross section normal to the flow,according to Eq.(11):

where σ is the mass fraction variance,Yiis the mass fraction at the sample point i,Y is the averaged mass fraction and N is the numberofsample points on the cross section(over 400 points were used).The mixing efficiency,M,was calculated as Eq.(12)[13]:

whereis the maximum variation over the data range at the inlet(the mass fractions range from 0 to 1).The mixing index ranges from 0(total segregation of fluids)to 1(complete fluid mixing).
The oil conversion due to chemical reaction was de fined as Eq.(13):
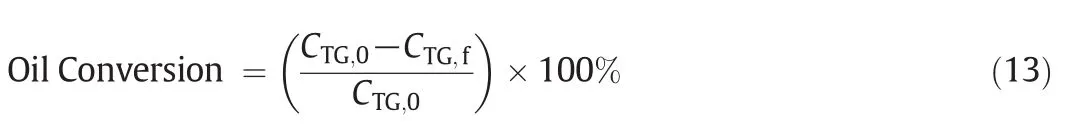
where CTG,0is the initial concentration of sun flower oil(at Inlet 2)and CTG,fis the final concentration of the oil(at Outlet).
2.5.Initial and boundary conditions
The initial and boundary conditions used in simulations were:Inlet 1:pure ethanol feed stream.The mass fraction and inlet velocity(based on Reynolds number and molar ratio ethanol/oil-details are given in Appendix C),were:

Inlet 2:pure sun flower oil feed stream.The mass fraction and inlet velocity were:
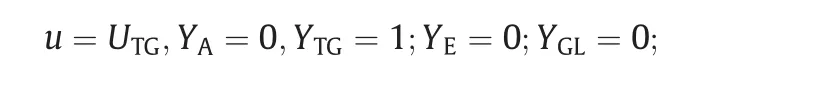
Outlet:relative averaged pressure equal to zero at outlet;
Walls:no-slip conditions applied at solid walls.
For all simulated cases was adopted a single-phase(liquid state),multicomponent system, flowing at steady state and isothermal conditions,under laminar regime flow and at equilibrium state for reaction kinetics.High resolutions schemes were employed in the solver procedure with a convergence criterion of 1×10-5for RMS(Root Mean Square)[37],in a range of 500–5000 iterations.In the reaction system numerical study,an inert oil was added as a constraint,to ensure the mass fraction restriction.The simulations were solved in parallel processing using an eight-core Intel Xeon processor with 3 GHz and 16 GB of RAM,with a Linux Suse 64-bit operating system.
3.Experimental
3.1.Materials and microdevice description
The sun flower oil was obtained from Campestre Indústria e Comércio de Óleos Vegetais LTDA.(São Bernardo do Campo,Brazil).The acidity index,calculated in accordance with Adolfo Lutz Institute methodology[38]was 0.04 mg KOH∙(g oil)-1The saponification and peroxide indexes,determined using standards of American Oil Chemists'Society(Cd 3-25 e Cd 8b-90)[39]were 280 mg KOH∙(g oil)-1and 1.21 mEg∙(kg oil)-1,respectively.The fatty acid composition,carried outatAnalyticalCentre ofChemistry Institute at UNICAMP,showed the presence of the following acids:palmitic(12.44%),stearic(4.51%),oleic(23.30%),linoleic(55.03%)and linolenic(4.75%).The additional materials used were:ethanol(Dinâmica,99.5%),sodium hydroxide(Êxodo Cientí fica,P.A.),chloride acid(Êxodo Cientí fica,P.A.)and hexane(Dinâmica,P.A.).
The experimental tests were performed in microchannels using the MCO[Fig.2.(a)]and MWO[Fig.2.(b)]configurations with width of 1500 μm,height of 200 μm,and longitudinal length of 411 mm,with 13 channels( firstand lastchannels with a length of35 mm,furtherchannels with a length 31 mm).The devices were manufactured in PDMS[40]in the Microfabrication Laboratory(LMF)of Brazilian Nanotechnology National Laboratory(LNNano–Campinas/SP,Brazil).Fig.2(c)and(d)shows the optical microscopy of the microchannels.
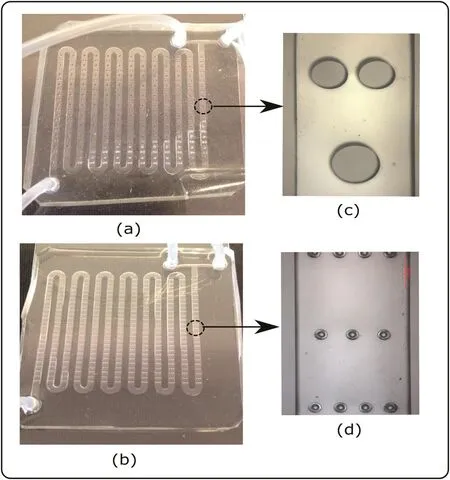
Fig.2.Microreactors based on micromixer configurations:(a)MCO;(b)MWO;and optical microscopy(50 times magnification)of(c)MCO;(d)MWO.
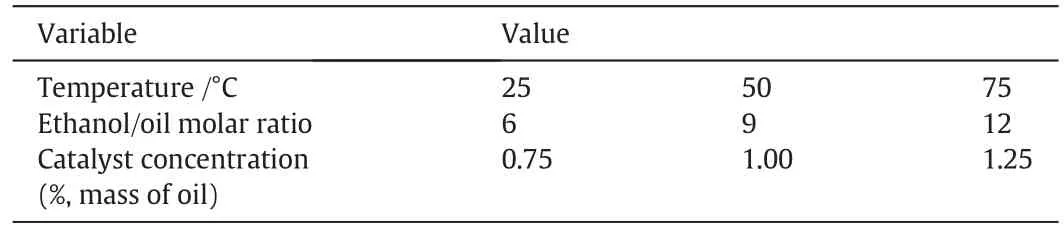
Table 1 Operating conditions range evaluated
3.2.Reaction conditions and experimental procedure
The biodieselsynthesis from transesteri fication ofsun flower oilwith ethanol was performed to evaluate the in fluence of the operating variables:temperature,ethanol/oil molar ratio and catalyst concentration,as summarized in Table 1.The oil and the ethanol solution with sodium hydroxide catalyst were pumped into microreactors using a syringe pump(KDS 100L)at specific flow rates.The temperature was managed using a heat plate(IKA,Model HP7).The microreactors outlets were coupled to a glass bottle with a cooling bath to cease the chemical reaction immediately.Posteriorly,the samples(1 ml)were submitted to an evaporation process(kiln drying at 105°C)to eliminate the excessive alcohol.The samples were centrifuged(1000 r·min-1)for 20 min and stored under refrigeration until analyses.The samples were dilute with hexane in a ratio of 1:10 and filtrated in a PVDF filter,and then analyzed in a gas chromatograph.
Fig.3 presents the experimental apparatus.This system was also used to validate numerical mesh(Section 2.1)by image capture of micromixer flow patterns,adding a blue dye to ethanol.A digitalmicroscope(Dino-Lite Edge AM4115ZT,AnMo Electronics Corporation,Taiwan)aided system were employed to obtain the images.The residence time was approximately 12 s.The ethanol/oil molar ratio was controlled based on oil and ethanol flow rates,as shown in Table 2.
3.3.Gas chromatography identification of esters
The ester characterization was confirmed by gas chromatography with high-precision trace analyzer(Model GC-2010 by Shimatzu),using a capillary column from Stabilwax of 30 m,0.25 mm I.D.,0.25 μm.The electro ionization was performed with 70 eV,using helium gas as moving phase at 15 psi,split 1/50,250 °C at injector and 300 °C at detector.The temperature ramp for the column was 50°C for 2 min,followed by a gradient of 10 °C·min-1until it reaches 180 °C,5 min on hold at 180 °C,a gradient of 5 °C·min-1until it reaches 240°C,ending a 32-minute chromatographic run.For each analysis 1 μl was injected.The results were expressed as ethyl esters percentage(FAEE-Fatty Acid EthylEster),corresponding to the peak area ofthe following acids:palmitic,linoleic and oleic,according to Eq.(14):

Table 2 Flow rates of oil and ethanol(ml·h-1)for evaluated molar ratios

where∑Aiis the summation over all peak areas from fatty acids,and∑A is the summation over all peak areas.
4.Results and Discussion
An independence mesh testwas performed to ensure numericalpredictions with a minimum level of in fluence by mesh discretization.The numerical mesh selection was based on the mesh discretization with few control volumes that exhibited a similar behavior to the most refined mesh,in order to avoid an excessive computational processing cost and ensure a minimization of numerical discretization in fluence on the simulation predictions.The results are shown in Fig.4.It is noticed a decrease in mixing index with an increment of control volumes in the MCO configuration[Fig.4(a)].For MWO configuration[Fig.4(b)],all tested meshes presented a similar trend.The numerical meshes with about 2×106and 3×106control volumes were selected for MCO and MWO configurations,respectively.
A qualitative comparison between experimental and numerical results of flow pattern was made for both configurations at a Reynolds number of 0.1,to validate the numerical meshes selected.The results are shown in Fig.5.Two distinctfully developed flow streams can be noticed for both microchannel configurations.The qualitative flow patterns experimentally observed was captured by numerical predictions.Vegetable oil ful filled a larger portion in the mixing channel.Good agreement of numerical predictions to experimental results can be confirmed with these results in this Reynolds number.
4.1.Mixing analysis
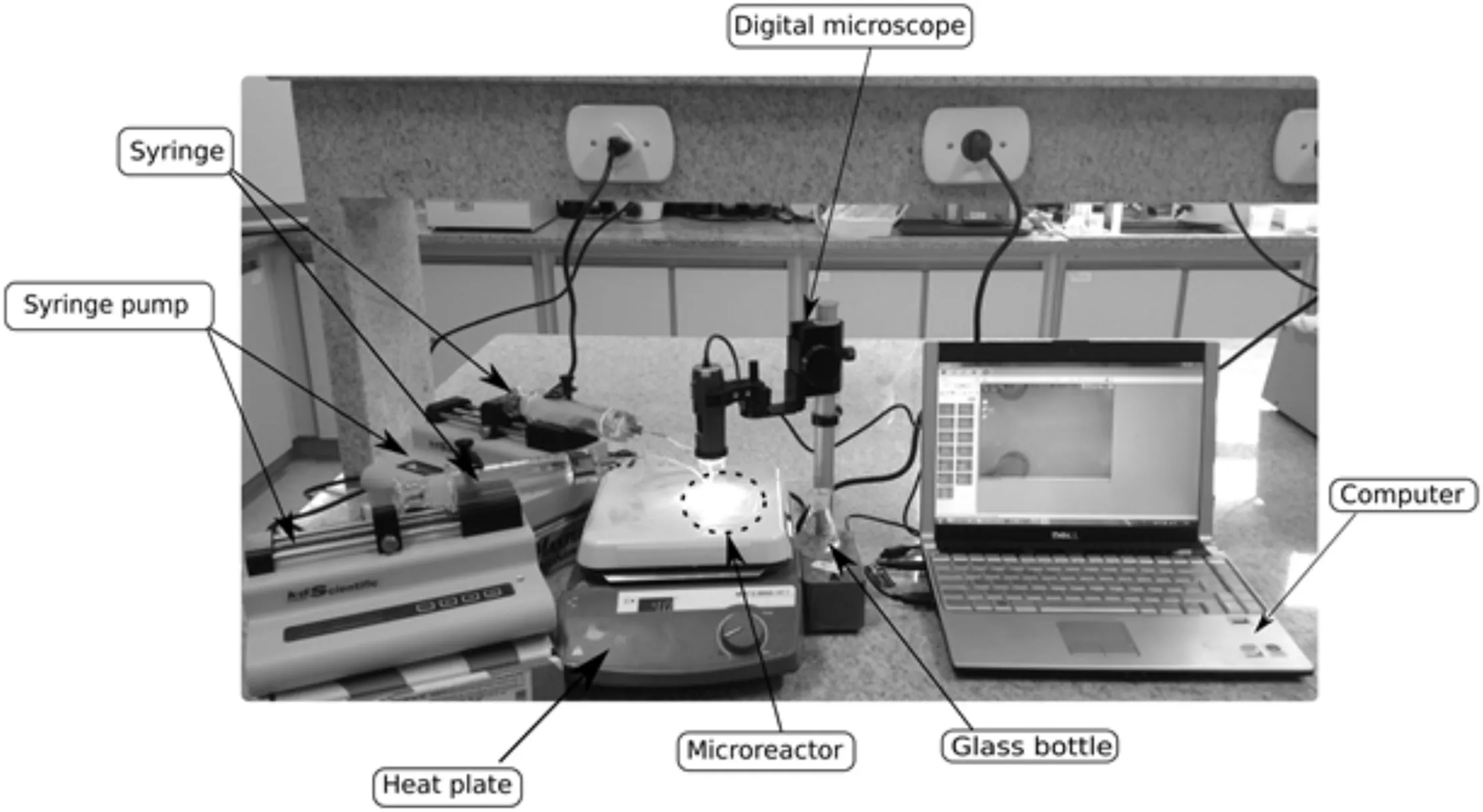
Fig.3.Experimental apparatus scheme.
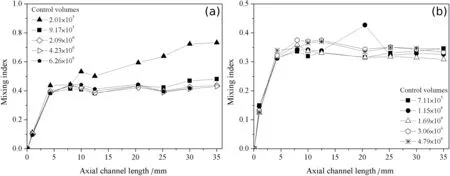
Fig.4.Independence mesh test performed for different control volume numbers:(a)MCO and(b)MWO configurations.
The mixing index at channel outlet obtained for the different microchannel configurations over the Reynolds number from 0.1 to 100 are presented in Fig.6.The highest mixing index was noticed for MCO micromixer,with a maximum value of 0.80.T-shape and MWO configuration resulted in similar mixing index,with maximum of 0.31 and 0.32,respectively.The mixing index for T-shape is virtually independent of Reynolds number.For this geometry configuration,the fluids flow in laminar regime throughout the channel in well-de fined stream zones(Fig.7),where only molecular diffusion plays a role in the fluid mixing.
The MCO configuration enhanced the mixing between vegetable oil and ethanol.For a Reynolds number of 0.1,it was noticed an increment of mixing index for 0.30(T-shape)to 0.40(MCO configuration).At Reynolds number of 100,the mixing index increased of 0.32 for 0.80.
The mixing improvement can be related to two processes:split and recombination of fluid streams and vortex formation[13].The split and recombination process enhances the surface contact area between oil and ethanol(Fig.8),since the obstacles break the parallel flow pattern,splitting the main stream in sub-streams,decreasing the stream width,and consequently,the molecular diffusion path.
The second process involved in the mixing improvement is the vortex formation,which depends on the Reynolds number(Fig.9).The direction changing in the flow stream promoted by the circular obstacles in MCO configuration induces a vortex generation,enhancing the mixing between chemical species[32].For a Reynolds number of 0.1,the fluid flows smoothly around the obstacles(creeping flow),while,at Re=100,fully developed vortex were noticed,acting intensely in fluid mixing process.The mixing index increased from 0.40(Re=0.1)to 0.80(Re=100),highlighting the vortex effect on fluid mixing.
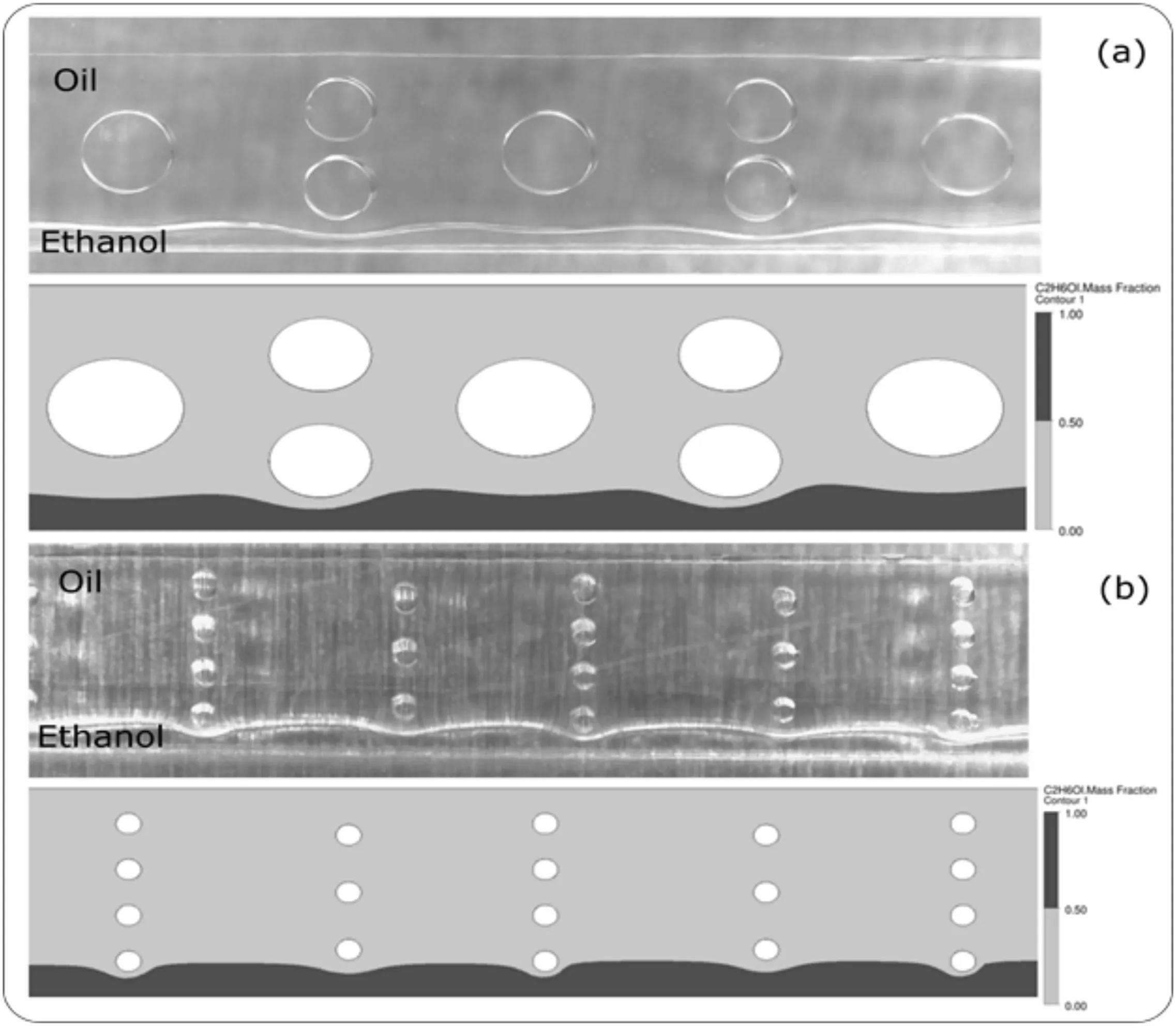
Fig.5.Experimentaland numericalcomparison for micromixers:(a)MCOand(b)MWO.In the experimentaltests,the inlet fluid flow rate was equalto 5.184 ml·h-1 and the temperature was 40 °C.A magnification of 60× was used in the digital microscope.In the numerical tests,a Reynolds number of 0.1 was adopted for the boundary conditions.
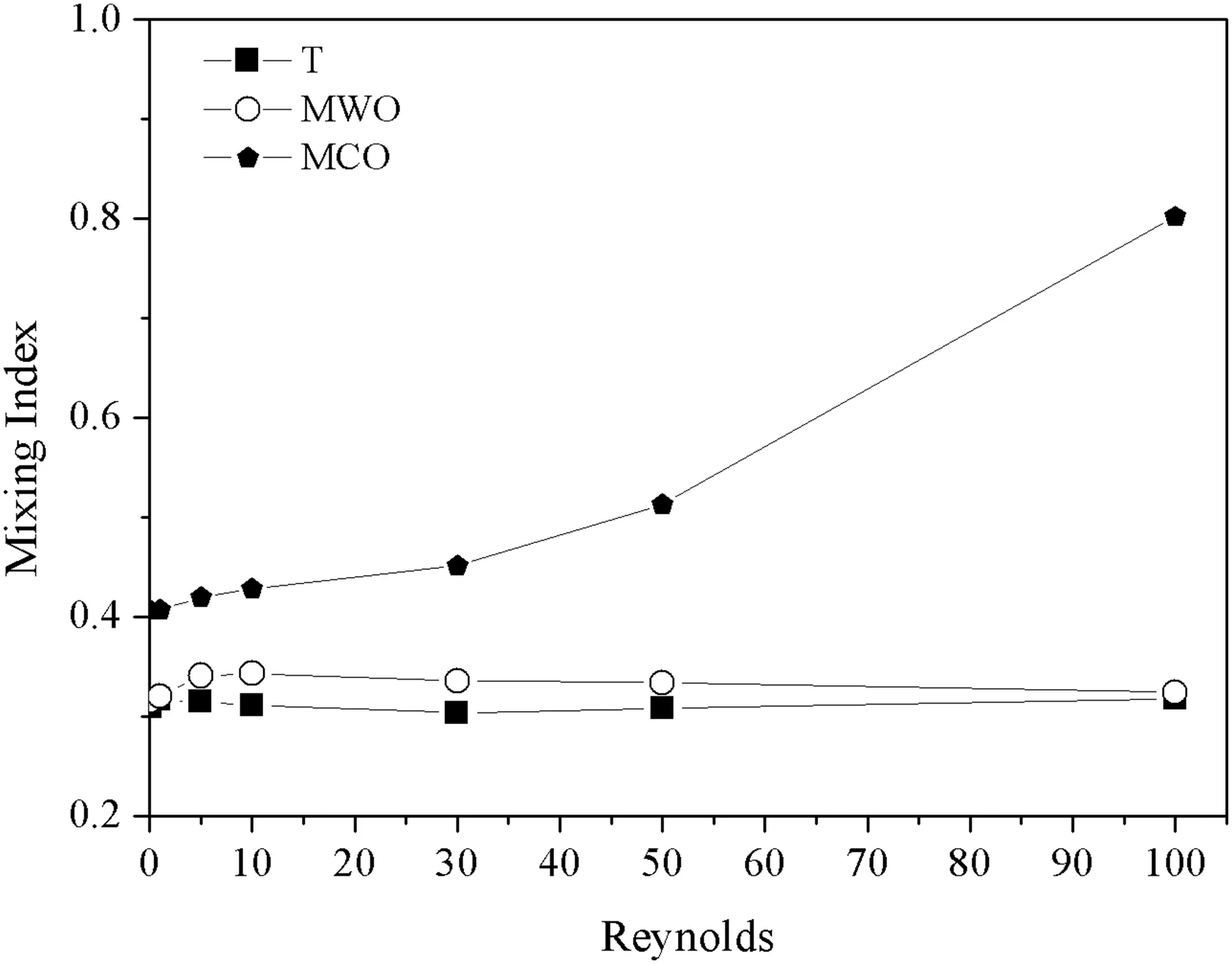
Fig.6.Mixing index as a Reynolds number function.
The MWO configuration did not enhanced fluid mixing,and mixing index as a function ofthe Reynolds numberpresented a similarbehavior to the provided by T-shape.The two mixing enhancement processes(split and recombination of streams and vortex formation)were not induced by this geometry configuration,as observed in Figs.10 and 11.
The MWO obstacles configuration did not affect the flow stream direction inside the channel;consequently,the diffusion path length was not decreased and the mixing index was almost the same predicted by T-shape geometry.In relation to the presence of vortex,Fig.11(a)shows the same behavior of the MCO,with the fluids flowing smoothly through the obstacles.The difference occurs when the Reynolds is equal to 100.While in the MCO the vortices are developed and the mixture of the oil and ethanol is increased,in the MWO the vortices are not suf ficient to favor the mixing of the fluids[Fig.11(b)].
4.2.Numerical simulation of transesteri fication reaction
The chemicalreaction simulation between sun flower oiland ethanol in the presence of sodium hydroxide catalyst was performed in 35-mm longitudinal length microchannel reactors.Oil conversion results are shown in Fig.12.The highest conversion obtained in MCO micromixer configuration was 81.13%at 75°C,molar ratio of 9 and catalyst concentration of1%.At same operating conditions,it was obtained a maximum oil conversion of 76.56%and 76.29%for MWO configuration and T-shape geometry,respectively.The MCO exhibited the highest conversions for all operating variables evaluated.
An increase in temperature caused oil conversion growth for all micromixer configurations.The increment of oil conversion for MCO configuration was from 30.72%at 25 °C to 81.13%at 75 °C,and at the same temperature rise,from 26.58%to 76.56%for MWO configuration,and from 25.51%to 76.29%for T-shape geometry.
The ethanol/oilmolar ratio effectresulted in a differentbehavior.For MCO,oilconversion raised from52.87%to 66.80%when molar ratio was elevated from 6 to 9,decreasing to 65.73%at a molar ratio of 12.The T-shape and MWO configuration geometries present an analogous behavior.Oil conversion increased from 45%to 58%(T-shape)and from 46.70%to 59.51%(MWOconfiguration),raising the molarratio from 6 to 9.For an ethanol/oil molar ratio of 12,the oil conversion decreased to 57%(T-shape)and 58.82%(MWO configuration).
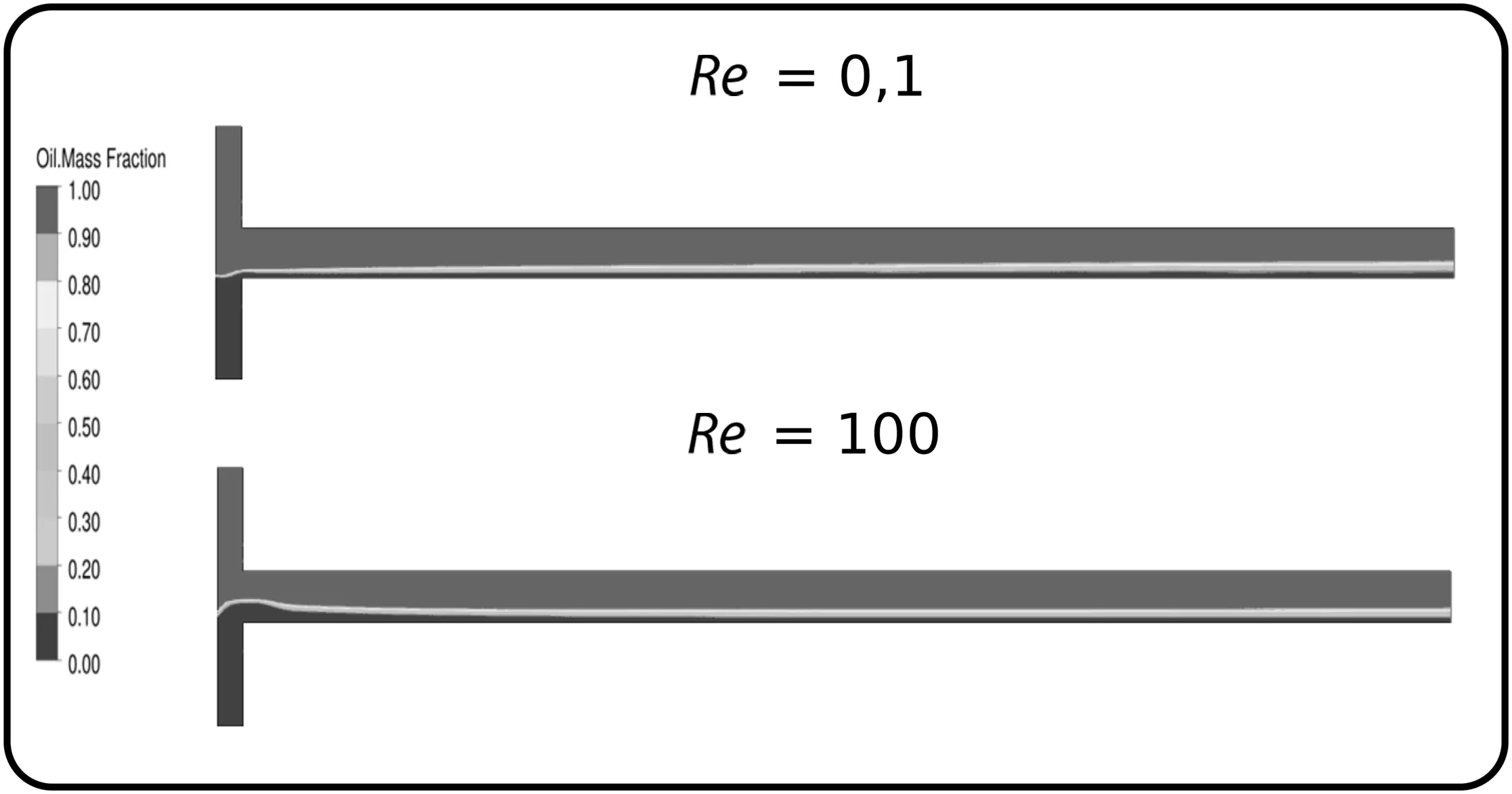
Fig.7.Sun flower oil mass fraction distribution for T-shape geometry at Reynolds number of 0.1 and 100.
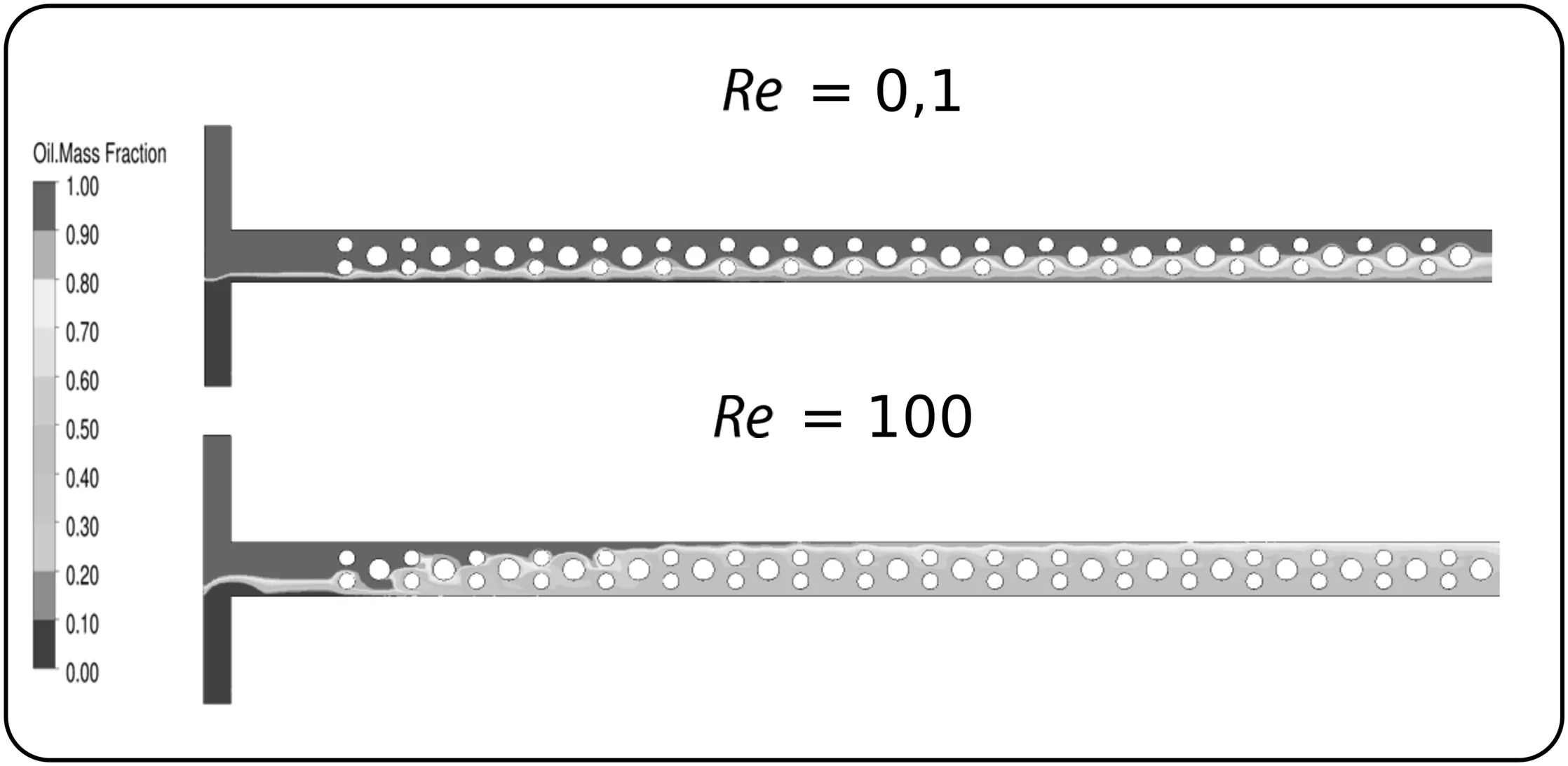
Fig.8.Sun flower oil mass fraction distribution for MCO configuration at Reynolds number of 0.1 and 100.
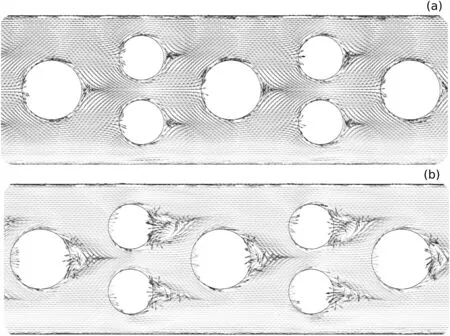
Fig.9.Velocity vector field predicted by MCO configuration in Reynolds of:(a)0.1 and(b)100.
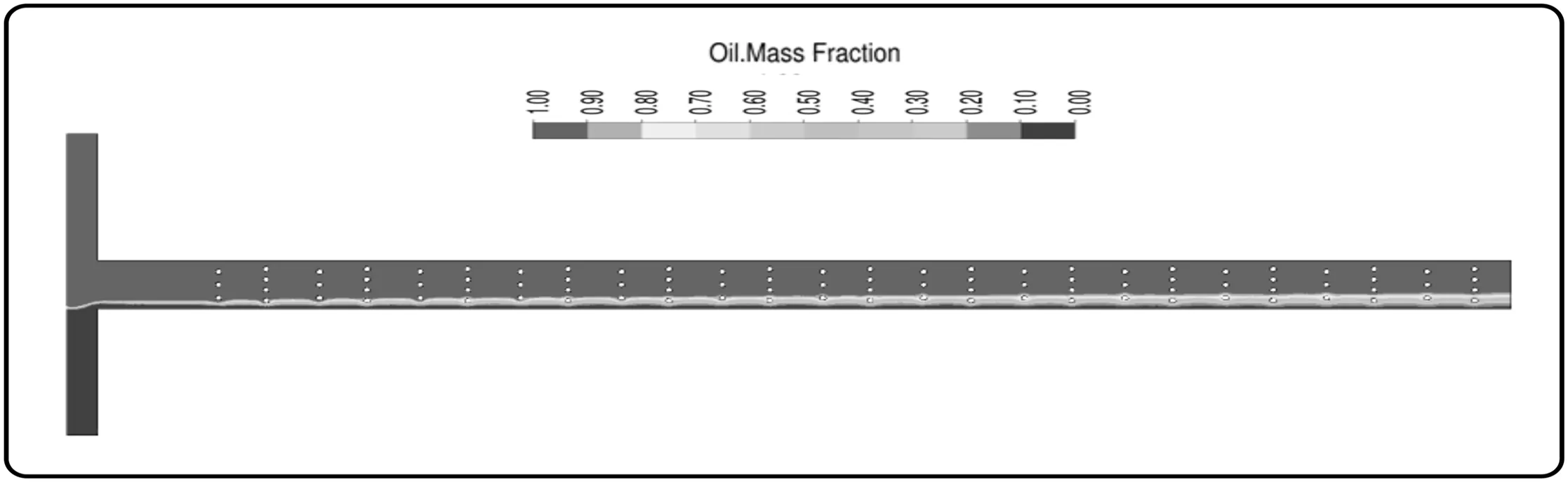
Fig.10.Sun flower oil mass fraction distribution for MWO configuration at a Reynolds number of 0.1.
The catalyst concentration in fluenced the oil conversion likewise to the temperature effect.The increment of catalyst concentration promoted an enhancement on the oil conversion:from 52.69%(0.75%)to 71.56%(1.25%),for MCO configuration,46.52%(0.75%)to 64.25%(1.25%)for MWO configuration,and from 45%(0.75%)to 62%(1.25%)for T-shape geometry.
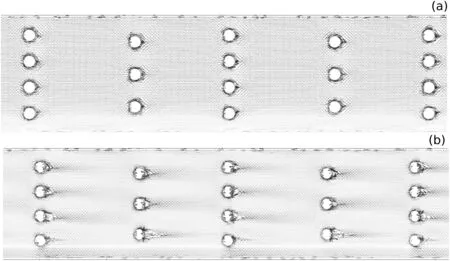
Fig.11.Velocity vector field predicted by MWO configuration in Reynolds number of:(a)0.1 and(b)100.
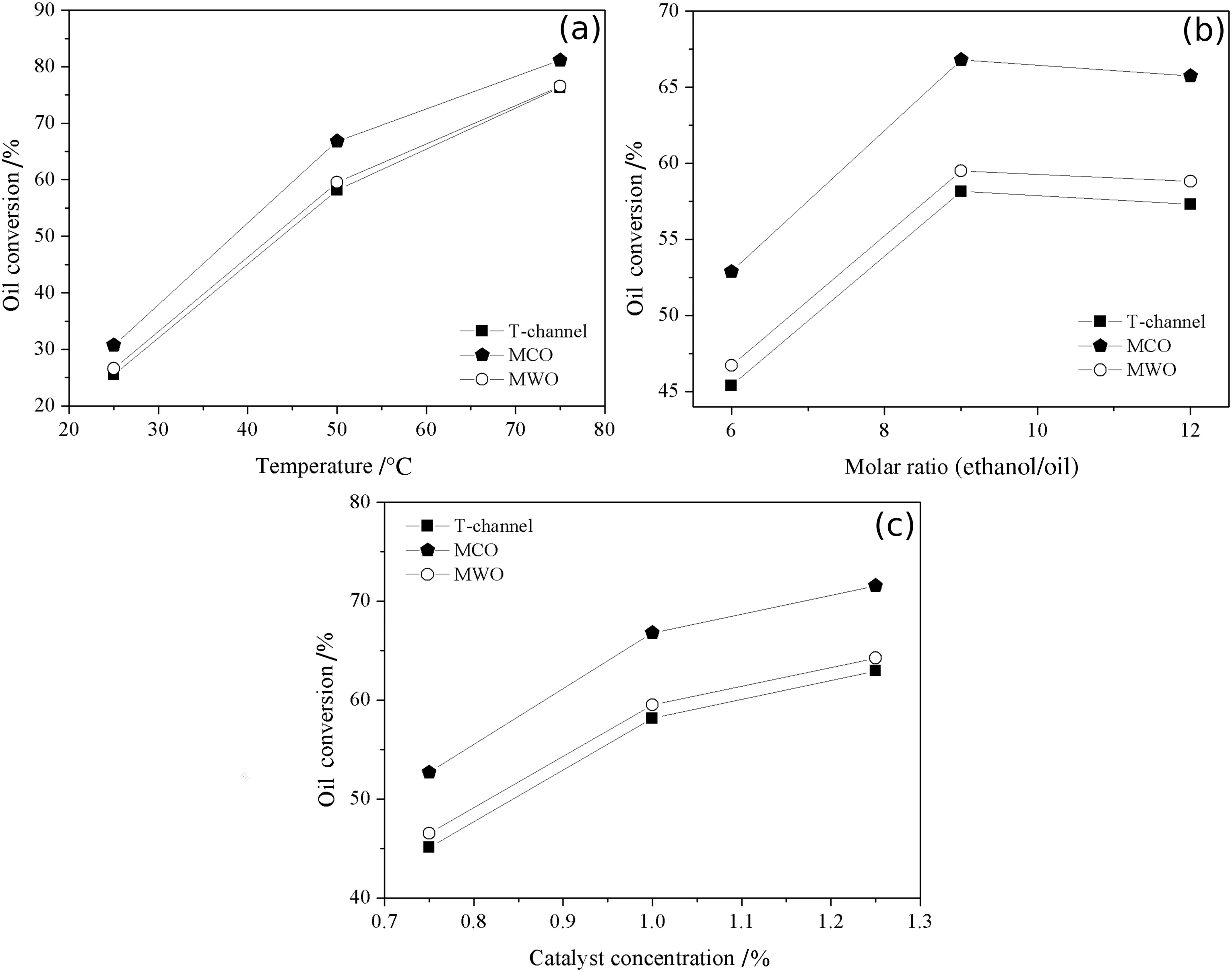
Fig.12.Effects ofoperating variables on the oilconversion(%):(a)Temperature(Ethanol/oilmolarratio=9;Catalystconcentration=1%);(b)Ethanol/oilmolar ratio(Temperature=50°C;Catalyst concentration=1%);(c)Catalyst concentration(Temperature=50 °C;Ethanol/oil molar ratio=9).
The MCO micromixer configuration exhibited the best results for the operating variables evaluated,considering the sun flower oil conversion results presented over the different conditions(temperature,ethanol/oil molar ratio and catalyst concentration).This superior performance can be related to the quality of mixing provided by MCO configuration.Since the chemical reaction conditions and the initial and boundary conditions employed for the different geometry configurations were the same,the performance of oil conversion is attributed to the fluid mixing pattern.A factor in favor of this hypothesis is the conversion of vegetable oilto the micromixers T and MWO.As shown in Fig.6,the average mixing index was slightly higher in MWO than in T.The same behavior was observed in the analysis of oil conversion in these micromixers(Fig.12).
4.3.Experimental tests of the transesteri fication reaction
The biodiesel synthesis from sun flower oil and ethanol was carried out using micromixer with circular obstructions:3 obstructions(MCO configuration)and 7 obstructions(MWO configuration),disposed equally throughout the mixing channel.Fig.13 presents the results.The highest Fatty Acid Ethyl Ester(FAEE)percentages obtained for both micromixers,MCO and MWO,was 99.99%.
The temperature rising from 25 °C to 50 °C presented an insignificant effecton FAEE[Fig.13(a)]for allmicroreactors.Apositive relationship between temperature and chemical reaction yield was related[41–44],showing an increment in oil conversion with temperature.This behavior seems to be contradictory as observed in Fig.13(a).However,the ethanol/oil molar ratio plays an important role on the temperature in fluence[44].For an ethanol/sun flower oil molar ratio of 9,Velickovic et al.[44]related that the reaction temperature unaffected oil conversion,showing an interaction dependence between the other operating variables[44].This behavior explains why FAEE was insensitive with temperature variation from 25 °C to 50 °C for both micromixer configurations.
At 75°C,a decrease in FAEE percentage was observed,resulting in 97.67%for MCO configuration and 88.3%for MWO configuration.This decrease with incrementoftemperature from 50 °C to 75 °C could be attributed to ethanol evaporation,reducing the amount of reactant available.This argument is based on other researches[24,43,45].In these studies,the transesteri fication reaction was done with different vegetable oils with methanol,and a common point highlighted was a decrease in reaction yield in conditions near or above the methanol boiling point(64.7°C).Since ethanol boiling point(78.4 °C)is close to 75 °C,and in microdevices the heat transfer between reactor wall and reactants are highly uniform,the same behavior could be occurred in the presentstudy.
The ethanol/oil molar ratio was evaluated and the results are shown in Fig.13(b).For the MWO configuration,an increment in FAEE was noticed for a molar ratio variation from 6(98.10%)to 9(99.99%),decreasing for a 12:1 molar ratio(98.66%).A similar behavior was observed for MCO configuration increasing the molar ratio from 6(91.65%)to 9(99.99%);however,for a molar ratio of 12,the FAEE remained at 99.99%.Since the transesteri fication reaction is a reversible reaction occurring with alcohol excess,this supports the products generation(biodiesel).It was observed for the ethanol/oil molar ratio ranging from 6 to 9.
The catalyst concentration effect on FAEE is showed in Fig.13(c).Increasing the catalyst concentration from 0.75%to 1.00%enhanced FAEE for both micromixer designs:93.24%to 99.99%(MCO configuration)and 98.18%to 99.99%(MWO configuration).An increment of catalyst concentration to 1.25%promoted a decrease in FAEE percentage in both micromixers,being more pronounced for MCO configuration.

Fig.13.Effects ofoperating variables on FAEE(%):(a)Temperature(Ethanol/oilmolar ratio=9;Catalyst concentration=1%);(b)Ethanol/oilmolar ratio(Temperature=50°C;Catalyst concentration=1%)e(c)Catalyst concentration(Temperature=50°C;Ethanol/oil molar ratio=9).
The catalyst concentration effect on FAEE yield can be associated with the parallel reaction occurrence involving and consuming the catalyst,decreasing the reaction yield.Anastopoulos et al.[42]related an ethyl esters yield increment for ethanol/sun flower oil reactive system,when increasing NaOH catalyst concentration from 0.25%to 0.75%,however,a decreasing on oil conversion was noted with an increment of NaOH concentration above 0.75%.Mansourpoor and Shariat[43]investigated biodiesel production from sun flower oil with methanol and potassium hydroxide as catalyst.They observed a decrease on biodiesel yield for catalyst concentrations above 0.68%.Both researches[42,43]related the biodiesel yield reduction above a critical catalyst concentration to the occurrence of saponification reaction,an unwanted secondary chemical reaction that consumes the alkaline catalyst,decreasing the amount of biodiesel produced.These observations explain the FAEE decrease noticed in the two micromixer configurations.
Based on these results,both micromixer designs evaluated presented high ethyl esters percentage values,with a maximum of 99.99%.The lower observed FAEE yield was 88.30%(MWO configuration)and 91.65%(MCO configuration).The experimental results showed insignificant differences between the two configurations,as was observed numerically.This deviation between experimental and numerical results can be related to the reactants mixing in microreactors.For a Reynolds number of 0.1,the fluids were well mixed at first channel end of MCO configuration,rather than in MWO configuration(Fig.14).However,atgeometry outlet,the fluids were totally mixed for both micromixer designs.This flow pattern could have in fluenced the noticed difference between numerical and experimental approaches.A smaller longitudinal path required to complete the fluid mixing were observed for MCO configuration[Fig.14(a)],since this geometry exhibited a better mixing performance in the numerical study.

Fig.14.Sun flower oil mass fraction distribution at a Reynolds number of 0.1 for:(a)MCO and(b)MWO configurations.
5.Conclusions
High chemical reaction yield can be reached using microreactors with circular obstructions.Three micromixer design configurations were numerically investigated:T-shape–channel without obstructions;MCO–channel with 3 obstructions and MWO–channel with 7 obstructions.Simulation results showed a superior performance of MCO configuration on fluid mixing degree,due to the presence of stream split and recombination process and vortex formation,promoting consequently,higher vegetable oil conversions in biodiesel synthesis.The oil conversion increased with the increment of temperature and catalyst concentration,and for alcohol/oil molar ratio below 9.Experimental data showed high ethyl esters(FAEE)yield for both micromixer designs tested(MCO and MWO configurations),with a maximum value of 99.99%.The FAEE yield decreased in operating temperature near to the ethanol boiling point.For MCO configuration,the FAEE yield enhanced with an increment of ethanol/oil molar ratio from 6 to 9,however,for higher molar ratios,FAEE decreased.The increase ofcatalystconcentration from 0.75%to 1.00%also promoted a superior FAEE yield.Catalyst concentrations above 1.00%reduced the ethyl esters production.Therefore,it was demonstrated the use of micromixers with circular obstructions in biodiesel synthesis in order to improve the biofuel production efficiency.
Acknowledgements
Acknowledgments to the Unicamp Scholarship Program,CAPES and the financial support provided by FAPESP(São Paulo Research Foundation,Process 2013/25850-7).The authors would like to thank Prof.Dr.Milton Mori for giving space to perform the simulations and the Microfabrication Laboratory(Proposal LMF-19844).
Appendix A.Physical properties of chemical species
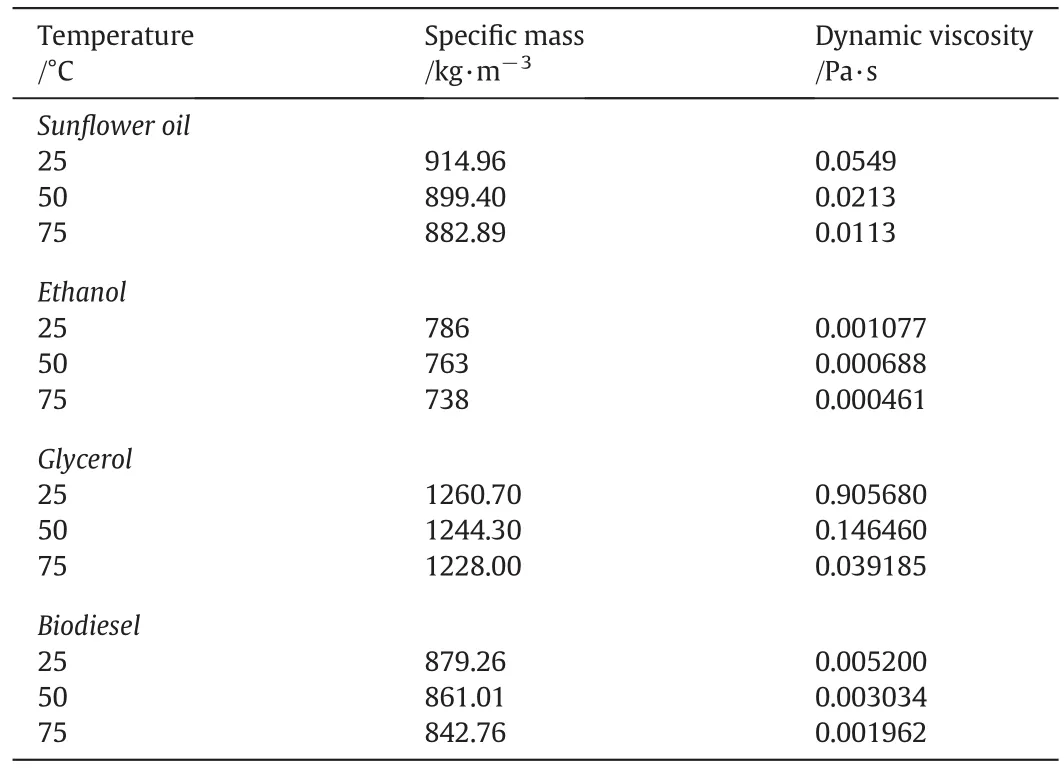
Temperature/°C Dynamic viscosity/Pa·s Sun flower oil 25 914.96 0.0549 50 899.40 0.0213 75 882.89 0.0113 Ethanol 25 786 0.001077 50 763 0.000688 75 738 0.000461 Glycerol 25 1260.70 0.905680 50 1244.30 0.146460 75 1228.00 0.039185 Biodiesel 25 879.26 0.005200 50 861.01 0.003034 75 842.76 0.001962 Specific mass/kg·m-3
Appendix B.Evaluated variables values and rate of reaction constants
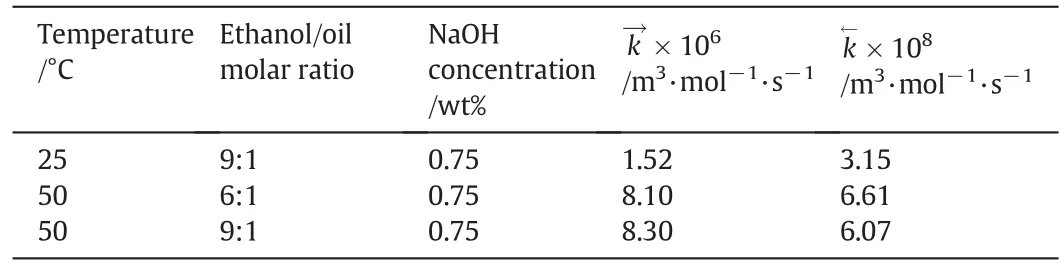
Temperature/°C Ethanol/oil molar ratio NaOH concentration/wt%k→×106/m3·mol-1·s-1 k←×108/m3·mol-1·s-1 25 9:1 0.75 1.52 3.15 50 6:1 0.75 8.10 6.61 50 9:1 0.75 8.30 6.07
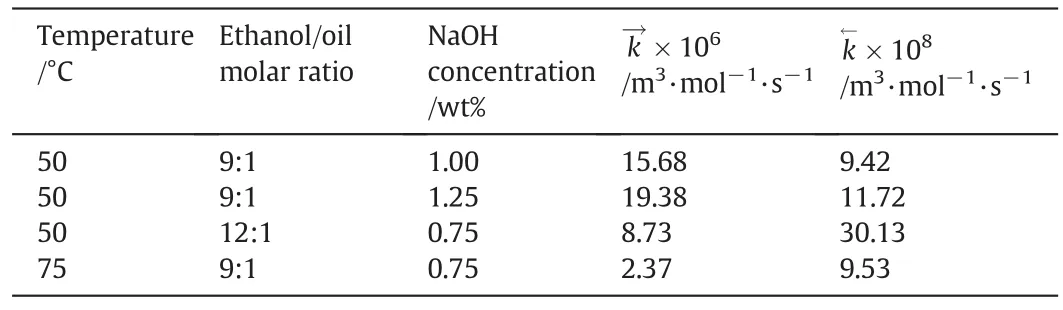
Temperature/°C Ethanol/oil molar ratio NaOH concentration/wt%k→×106/m3·mol-1·s-1 k←×108/m3·mol-1·s-1 50 9:1 1.00 15.68 9.42 50 9:1 1.25 19.38 11.72 50 12:1 0.75 8.73 30.13 75 9:1 0.75 2.37 9.53
Appendix C.Boundary conditions ofinletvelocities(m·s-1)for sunflower oil and ethanol.The inlet velocities for sun flower oil and ethanol were identical in the mixing study

Reynolds number Velocity/m·s-1 Ethanol/oil molar ratio Inlet velocity×103/m·s-1 U Oil U Ethanol 0.1 0.0096 6 8.88 2.96 1 0.096 9 7.40 4.44 5 0.479 12 6.66 5.18 10 0.96 30 2.873 50 4.788 100 9.6
References
[1]C.M.B.Ho,S.H.Ng,K.H.H.Li,Y.-J.Yoon,3D printed micro fluidics for biological applications,Lab Chip 15(2015)3627–3637.
[2]J.Shi,T.Cha,J.C.Claussen,A.R.Diggs,J.H.Choi,D.M.Porter field,Microbiosensors based on DNA modi fied single-walled carbon nanotube and Pt black nanocomposites,Analyst 136(2011)4916–4924.
[3]I.K.Dimov,J.L.Garcia-Cordero,J.O'Grady,C.R.Poulsen,C.Viguier,L.Kent,P.Daly,B.Lincoln,M.Maher,R.O'Kennedy,T.J.Smith,A.J.Ricco,L.P.Lee,Integrated micro fluidic tmRNA puri fication and real-time NASBA device for molecular diagnostics,Lab Chip 8(2008)2071–2078.
[4]T.Jiwanuruk,S.Putivisutisak,P.Ponpesh,C.Kositanont,Tagawa,H.Yamada,C.Fukuhara,S.Assabumrungrat,Comparison between parallel and checked arrangements of micro reformer for H2production from methane,Chem.Eng.J.268(2015)135–143.
[5]G.Kolb,Review:microstructured reactors for distributed and renewable production of fuels and electrical energy,Chem.Eng.Process.65(2013)1–44.
[6]G.Hellé,C.Mariet,G.Cote,Liquid-liquid extraction of uranium(VI)with Aliquat 336 from HCl media in micro fluidic devices:combination of micro-unit operations and online ICP-MS determination,Talanta 139(2015)123–131.
[7]S.Neethirajan,I.Kobayashi,M.Nakajima,D.Wu,S.Nandagopal,F.Lin,Micro fluidics for food,agriculture and biosystems industries,Lab Chip 11(2011)1574–1586.
[8]X.Yao,Y.Zhang,L.Du,J.Liu,J.Yao,Review of the applications of microreactors,Renew.Sust.Energ.Rev.47(2015)519–539.
[9]S.K.R.Cherlo,K.Sreenath,S.Pushpavanam,Screening,selecting and designing microreactors,Ind.Eng.Chem.Res.48(2009)8678–8684.
[10]O.Flögel,J.D.C.Codée,D.Seebach,P.H.Seeberger,Microreactor synthesis of β-peptides,Angew.Chem.Int.Ed.45(2006)7000–7003.
[11]E.Corner,M.G.Organ,A microreactor for microwave-assisted capillary(continuous flow)organic synthesis,J.Am.Chem.Soc.127(2005)8160–8167.
[12]H.Bockelmann,D.P.J.Barz,Optimised active flow control for micromixers and other fluid applications:sensitivity-vs.adjoint-based strategies,Comput.Fluids 106(2015)93–107.
[13]A.Alam,K.-Y.Kim,Mixing performance of a planar micromixer with circular obstructions in a curved microchannel,Chem.Eng.Res.Des.92(2014)423–434.
[14]N.Tran-Minh,T.Dong,F.Karlsen,An efficient passive planar micromixer with ellipse-like micropillars for continuous mixing of human blood,Comput.Methods Prog.Biomed.117(2014)20–29.
[15]Y.Su,A.Lautenschleger,G.Chen,E.Y.Kenig,A numerical study on liquid mixing in multichannel micromixers,Ind.Eng.Chem.Res.53(2014)390–401.
[16]J.Ortega-Casanova,Enhancing mixing at a very low Reynolds number by a heaving square cylinder,J.Fluids Struct.65(2016)1–20.
[17]G.Xia,J.Li,X.Tian,M.Zhou,Analysis of flow and mixing characteristics of planar Asysmmetric split-and-recombine(P-SAR)micromixers with fan-shaped cavities,Ind.Eng.Chem.Res.51(2012)7816–7827.
[18]N.-T.Nguyen,S.T.Wereley,Fundamentals and Applications of Micro fluidics,Publishing House Artech House,Boston/London,2006.
[19]V.Hessel,H.Löwe,F.Schönfeld,Micromixers– a review on passive and active mixing principles,Chem.Eng.Sci.60(2005)2479–2501.
[20]A.A.S.Bhagat,E.T.K.Peterson,I.Papautsky,A passive planar micromixer with obstructions for mixing at low Reynolds numbers,J.Micromech.Microeng.17(2007)1017–1024.
[21]ASTM D6751–15,Standard Specification for Biodiesel Fuel Blend Stock(B100)for Middle Distillate Fuels,ASTM,Internacional,West Conshohocken,PA,2015(www.astm.org).
[22]U.Schuchardt,R.Sercheli,R.M.Vargas,Transesteri fication of vegetable oils:a review,J.Braz.Chem.Soc.9(1)(1998)199–210.
[23]R.Bhoi,N.Sen,K.K.Singh,S.M.Mahajani,K.T.Shenoy,H.Rao,S.K.Ghosh,Transesteri fication of sun flower oil in microreactors,Int.J.Chem.React.Eng.12(2014)47–62.
[24]M.Rahimi,B.Aghel,M.Alitabar,A.Sepahvand,H.R.Ghasempour,Optimization of biodiesel production from soybean oil in a microreactor,Energy Convers.Manag.79(2014)599–605.
[25]E.Santacesaria,M.Di Serio,R.Tesser,R.Turco,M.Tortorelli,V.Russo,Biodiesel process intensi fication in a very simple microchannel device,Chem.Eng.Process.52(2012)47–54.
[26]R.Jachuck,G.Pherwani,S.M.Gorton,Green engineering continuous production of biodiesel using an alkaline catalyst in an intensi fied narrow channel reactor,J.Environ.Monit.11(2009)642–647.
[27]E.L.Martínez Arias,P.F.Martins,A.L.Jardini Munhoz,L.Gutierrez-Rivera,R.Maciel Filho,Continuous synthesis and in situ monitoring of Biodesel production in different micro fluidic devices,Ind.Eng.Chem.Res.51(2012)10755–10767.
[28]Z.Wen,X.Yu,S.-T.Tu,J.Yan,E.Dahlquist,Intensi fication of biodiesel synthesis using zigzag micro-channel reactors,Bioresour.Technol.100(2009)3054–3060.
[29]P.Sun,B.Wang,J.Z.L.Yao,N.Xu,Fast synthesis of biodiesel at high throughput in microstructured reactors,Ind.Eng.Chem.Res.49(2010)1259–1264.
[30]M.Rahimi,F.Mohammadi,M.Basiri,M.A.Parsamoghadam,M.M.Masahi,Transesteri fication of soybean oil in four-way micromixers for biodiesel production using a cosolvent,J.Taiwan Inst.Chem.Eng.64(2016)203–210.
[31]N.Solehati,J.Bae,A.P.Sasmito,Numerical investigation of mixing performance in microchannel T-junction with wavy structure,Comput.Fluids 96(2014)10–19.
[32]A.Alam,K.-Y.Kim,Analysis of mixing in a curved microchannel with rectangular grooves,Chem.Eng.J.181-182(2012)708–716.
[33]B.Freedman,E.H.Pryde,T.L.Mounts,Variables affecting the yields of fatty esters from transesteri fied vegetable oils,J.Am.Oil Chem.Soc.61(1984)138–1643.
[34]A.V.Marjanovic,O.S.Stamenkovic,Z.B.Todorovic,M.L.Lazic,V.B.Veljkovic,Kinetics of the base-catalyzed sun flower oil ethanolysis,Fuel 89(2010)665–671.
[35]R.Byron Bird,W.E.Stewart,E.N.Lightfoot,Transport Phenomena,2ndedition John Wiley&Sons,New York,2002.
[36]Ansys Inc.,Ansys CFX-Pre User's Guide Release 14.0,Ansys Inc.,U.S.A,2011.
[37]C.A.Cortes-Quiroz,A.Azarbadegan,M.Zangeneh,Evaluation of flow characteristics that give higher mixing performance in the 3-D T-mixer versus the typical T-mixer,Sensors Actuators B Chem.202(2014)1209–1219.
[38]IAL-Instituto Adolfo Lutz(São Paulo),Métodos Físico-químicos para Análise de Alimentos/coordenadores Odair Zenebon,Neus Sadocco Pascuet e Paulo Tiglea-São Paulo:Instituto Adolfo Lutz,2008.
[39]American Oil Chemists's Society,Of ficial Methods and Recommended Practices of the AOCS,5thed.AOCS Press,2004.
[40]H.S.Santana,S.Tortola,É.M.Reis,J.L.Silva,O.P.Taranto,Transesteri fication reaction of sun flower oil and etanol for biodiesel synthesis in microchannel reactor:experimental and simulation studies,Chem.Eng.J.302(2016)752–762.
[41]A.Bouaid,M.Martinez,J.Aracil,A comparative study of the production of ethyl esters from vegetable oils as a biodiesel fuel optimization by factorial design,Chem.Eng.J.134(2007)93–99.
[42]G.Anastopoulos,Y.Zannikou,S.Stournas,S.Kalligeros,Transesteri fication of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters,Energies 2(2)(2009)362–373.
[43]M.Mansourpoor,A.Shariati,Optimization of biodiesel production from sun flower oil using surface methodology,J.Chem.Eng.Process Technol.3(2012)3–5.
[44]A.Velickovic,O.S.Stamenkovic,Z.B.Todorovic,V.B.Veljkovic,Application of the full factorial design to optimization of base-catalyzed sun flower oil ethanolysis,Fuel 104(2013)433–442.
[45]B.Aghel,M.Rahimi,A.Sepahvand,M.Alitabar,H.R.Ghasempour,Using a wire coil insert for biodiesel production enhancement in a microreactor,Energy Convers.Manag.84(2014)541–549.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
- 17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
- The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
- Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination☆
