From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
Syieluing Wong ,Nurul Atiqah Najlaa Yac'cob ,Norzita Ngadi,*,Onn Hassan ,Ibrahim M.Inuwa
1 Faculty of Chemical and Energy Engineering,Universiti Teknologi Malaysia,81310 Skudai,Johor,Malaysia
2 Department of Industrial Chemistry,Kaduna State University,Kaduna,Nigeria
1.Introduction
Remediation of industrial wastewater has become an important issue in scholar research,as the pollutant contained in the wastewater,when flow into river bodies,creates negative impacts on the ecosystem and human health,which are irreversible in some cases.The current strategy applied in wastewater treatmentfocused on removal of pollutants through various means prior to its discharge.Despite the advancement of latest technologies in wastewater treatment(e.g.,membrane technology[1],advanced oxidation process[2]),adsorption remains a popular method,due to its simplicity in operation and process control,high efficiency,as well as low material and operational costs when compared to other methods[3].Following the establishment of wastewater treatment using commercial activated carbon,the current research interested lies in the development of activated carbons(AC)from cheap sources,especially waste materials.Conversion to AC also adds values to such waste,which otherwise requires extra costs for disposal.Therefore,there is an increasing demand from different industries to seek the possibility to convert their waste to ACs,which can then be sold to wastewater treatment companies,while reducing the cost of waste disposal.
Despite the contribution of the textile industry as an important income source in many countries,the environmental impacts brought by such industry cannot be overlooked.The textile industry has been recognized as the most significant source of wastewater pollution[4],mostly related to the discharge of dyes that are mostly highly recalcitrant and toxic[5].When discharged into water bodies,dye molecules persist for a long time as their large and complex molecule structures resist degradation by natural means,such as light and microorganism actions.These dye molecules hinder the penetration of sunlight into the water,hence reduce the photosynthesis activities of aquatic plants,the producer in aquatic food web.This is especially true for methylene blue(MB)and Reactive Black 5(RB5),which are dark in colour at high concentration.This could lead to disastrous effects to the aquatic ecosystem.In addition,the safety of seafood is compromised due to biomagnification and bioaccumulation effects of dye molecules in the marine ecosystem.Transfer of these compounds into human body through ingestion of seafood could lead to undesirable and irreversible effects to human nervous systems[6].
In view of the hazards brought by dye molecules to human health,dye removal from textile wastewater via a great number of adsorbents,especially those synthesized from agricultural waste has been investigated.Pradhananga et al.[7]successfully synthesize AC from chemical activation of bamboo cane powder using H3PO4,which possesses high adsorption capacity on Lanasyn orange(2600 mg·g-1)and Lanasyn grey(3040 mg·g-1)respectively.AC produced from cashew nut shell via ZnCl2activation by Spagnoli et al.[8]recorded adsorption capacity of 476 mg·g-1for methylene blue.Orlandi et al.[9]also explored the potential of AC from residual sludge in the cellulose and paper industry as adsorbent of methylene blue.
Nevertheless,little attention is given to textile sludge(TS),which is another source of pollution generated from the textile industry.The sludge contains high amount of metal ions(especially aluminium and iron that originate from coagulant and flocculants depend on the treatment process)as well as phosphorus,nitrogen and potassium originating from the chemicals used in various steps of textile dying and processing[10].Therefore,unregulated disposal of TS at dumpsites and land fills may cause land and water pollution.In Malaysia,TS is considered as scheduled waste,therefore treatment of such waste through on-site facilities or licensed waste treatmentcompany incurs costto the textile manufacturers.In view of such scenario,it is necessary to seek a better alternative to disposal of TS.There are a very limited number of studies in literature that report synthesis of AC from TS for removal of strontium[11],oil[12]and dyes[13]from wastewater.This paper presents the adsorption behaviour ofmethylene blue(MB)and Reactive Black 5(RB5)onto activated carbon synthesized from textile sludge(TSAC),which is not reported previously.Preliminary study was conducted to compare the performance ofadsorbents activated by different reagents(H2SO4,KCl,ZnCl2),and the TSAC with the best performance was selected for characterizations as well as isotherm,kinetic and thermodynamic studies.
2.Materials and Methods
2.1.Materials
TS was obtained from the textile industry in Batu Pahat,Johor,Malaysia.The sludge was dried at 105°C for 24 h,then ground and sieved to obtain particles with an average size of 0.5 mm.RB5 and MB were purchased from Sigma-Aldrich and HmbG Chemicals respectively.
2.2.Preparation of TSAC
The procedure for TSAC synthesis was adapted from the work by Rozada et al.[14].Firstly,TS was chemically activated by impregnation with sulfuric acid(H2SO4)as activating agent.The mass ratio of sulfuric acid with respect to TS was 1:1.The chemical activation lasted for 48 h in a shaker at room temperature(30°C).Samples were then dried in an oven at105°C for 24 h to a constantweight.Next,the samples were carbonized in a furnace with nitrogen flow at a heating rate of 5 °C·min-1to 650°C,then hold for 30 min.After that,the carbonized product was rinsed with 10 wt%solution of HCl to eliminate excess dehydrating agents and soluble ash.Next,the sample was rinsed with distilled water,and dried in an oven at 80°C for 24 h.ACs were also prepared using ZnCl2and KCl(mass ratio of 1:1)as activating agents for comparison purpose in terms of adsorption performance.
2.3.Preparation of RB5 and MB dye solutions
Aqueous RB5 and MB stock solutions with concentrations of 150 mg·L-1were prepared by mixing the dyes with distilled water.To prevent decolourization by direct sunlight,the stock solutions were stored in a dark place before being used.According to the Beer–Lambert Law[15],a linear relationship can be observed between FTIR absorbance and adsorbate concentrations,provided that low concentration of adsorbate is used.Such law is widely applied to quantify concentration of a solute in solution[16].By utilizing such concept,measurement of absorbance of RB5(at 598 nm)and MB(at 630 nm)at different concentrations was performed by an Aquamate v4.60 UV–Vis Spectrophotometer,followed by generation of calibration curves that relate absorbance values of MB and RB5 dye solutions to their concentrations.Fig.1 indicates the calibration curves for the dye solutions.
2.4.Screening of ACs treated with different activating reagents
Removal of RB5 and MB onto ACs treated with different reagents was studied in a batch study with the method adapted from literature[17,18].For each AC,0.1 g of AC was mixed with 50 ml dye solution(100 mg·L-1)in 125 ml conical flasks.The mixtures were agitated for 60 min at 200 r·min-1.After 60 min,the adsorbent was filtered and the concentrations for residual dye in the filtered solutions were determined using a UV–VIS Spectrophotometer with the help of a calibration curve(Fig.1).Percentage removals ofRB5 and MB in each solution were then calculated using Eq.(1).AC with the highest removal performance was selected for further adsorption study and characterizations.
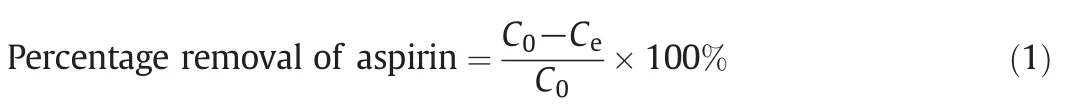
where C0and Cedenote the initial and equilibrium concentration(mg·L-1)of MB and RB5 dyes respectively.
2.5.Adsorbent characterization
The functional groups of TS and TSAC were characterized using Fourier Transform Infrared(FTIR)Spectroscopy(IRTracer-100 Spectrophotometer,Shimadzu Cooperation,Japan).The spectra were recorded from 400 to 4000 cm-1.The surface morphologies of TS and TSAC were observed using Field Emission Scanning Electron Microscopy(FESEM)(Hitachi SU8020).The surface area and pore size of TS and TSAC were measured by a Nitrogen Adsorption–Desorption Isotherm Analyser(NAD)model Micromeritics 3 Flex Surface Characterization Analyser.Samples were degassed at 77 K under nitrogen gas flow for 4 h prior to analysis.

Fig.1.Calibration curves for(a)RB5 and(b)MB.
2.6.Batch mode adsorption study
In order to determine the effectof reaction time on adsorption equilibrium,a study on batch mode adsorption was carried out by agitating TSAC in 20 ml of solutions containing RB5 and MB aqueous dyes with a concentration of 50 mg·L-1at room temperature(30 °C)for 200 min.After the adsorption process,the adsorbent was filtered from the aqueous dye solution.The finalconcentrations ofRB5 and MB in aqueous dye solutions were calculated based on measurement result from the UV–Vis Spectrophotometer.The experiment was also performed to study the effect of initial dye concentration(10–200 mg·L-1)and temperature(30–60 °C).
The adsorption capacity of RB5 and MB dyes in the adsorption system,qewas calculated by Eq.(2):

where m is the mass of the adsorbent(g);and V is the volume of the solution(L).
2.7.Adsorption isotherm
Adsorption isotherms at 30,50 and 60°C were studied respectively with RB5 and MB dye concentrations varying from 10 to 200 mg·L-1.The equilibrium data were fitted to Langmuir and Freundlich isotherm models.The Langmuir equation could be described as shown below:

where qeis the amount of adsorbate adsorbed per unit mass of adsorbent(mg·g-1),Q0and KLare Langmuir constants related to adsorption capacity and the rate of adsorption,respectively.
Based on the analysis of the Langmuir model,the essential characteristics of the Langmuir equation can be expressed in terms of a dimensionless separation factor,RLas de fined in Eq.(4).

The value ofthe separation factor,RLindicates whether the nature of the adsorption process is irreversible(RL=0),favourable(0<RL<1),linear(RL=1)or unfavourable(RL>1).
The Freundlich isotherm model is de fined as

Both KFand n represent Freundlich constants,where n indicates the favourability of the adsorption process and KF((mg·g-1)·(mg-1)1/n)indicates the adsorption capacity of the adsorbent.
Generally,the value of n should be between 1 and 10 to show a favourable adsorption process.The higher value of n(smaller value of 1/n)illustrates the stronger adsorption intensity[19].Linear adsorption that leads to identical adsorption energies for all sites exists when the value of 1/n equals to 1[20].
2.8.Kinetic studies
Sorption kinetic studies were conducted at30°C using dye solutions with an initialconcentration of50 mg·L-1using pseudo- first-order and pseudo-second-order kinetic models.Additionally,the intraparticle diffusion model was used to study the diffusion mechanism of dyes through the porous adsorbent.
The pseudo- first-order kinetic model is described by the formula

where qt(mg·g-1)are the amounts of adsorbate adsorbed at any time,t(min),respectively.The constant k1(min-1)is the adsorption rate constant of the first order adsorption.
The pseudo-second-order kinetic model is de fined by the formula

where k2(g·mg-1·min-1)is the rate constant of the second-order adsorption.
The intraparticle diffusion model is expressed by the equation

where t1/2(min1/2)is the half-life time in second,and kpis the intraparticle diffusion rate constant(mg·g-1·min-1/2).
3.Results and Discussion
3.1.Removal performances of ACs from different activating agents
Fig.2 shows the performances of three different ACs prepared on the removal of anionic RB5 and cationic MB dyes.From the graph,it is clearly showed that the AC with H2SO4activation possesses the highest percentage removal for both RB5 and MB.The superior effect of AC via H2SO4activation is attributed to the formation of stable C–O complexes during activation,which promotes development of the internal porosities ofAC.Besides that,integration ofsulfuric acid into the carbonaceous TS during activation retards the formation of tars and promotes the introduction of oxygen functionalities according to the reaction[21].Improvementof adsorption capacity of dyes on AC modi fied with sulfuric acid was also reported by Martin-Lara et al.[22].Such improvement is the result of oxygenated groups'introduction onto the AC surface by H2SO4,which is reported in several studies[23,24].Furthermore,the use of H2SO4as a chemical activating agent is more attractive as it gives less impact to the environment[25].Therefore,TSAC via H2SO4activation was used in the following part of this study.
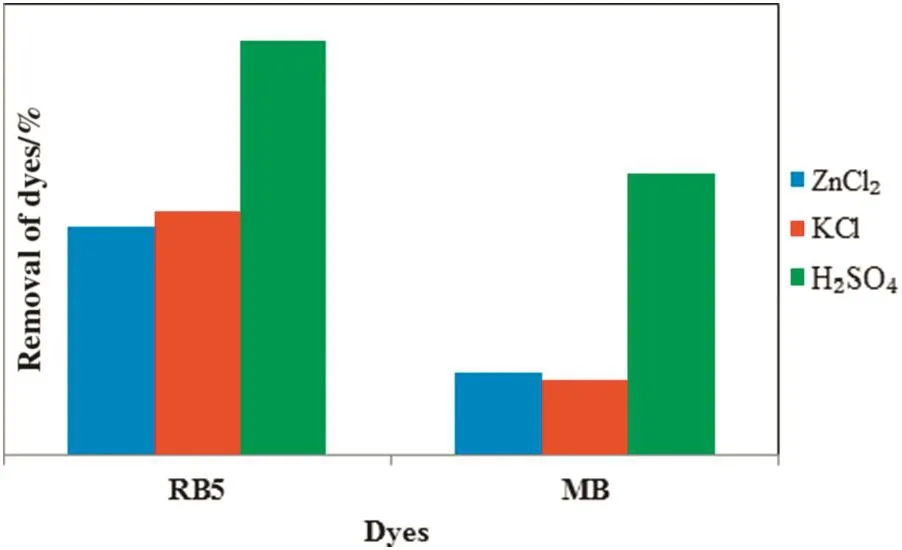
Fig.2.Removal of anionic RB5 and cationic MB dyes by three different ACs.
3.2.Adsorbent characterizations
3.2.1.FTIR
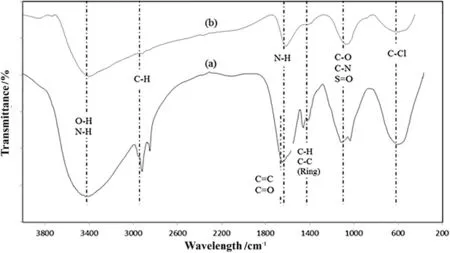
Fig.3.FTIR spectra of(a)TS and(b)TSAC.
The spectra for TS and TSAC showed a similar pattern of bands(Fig.3).However,several differences were observed in the TSAC spectrum compared to the TS spectrum by the decrease of the peak intensities.These may be ascribed to the stretching vibration from the activation process of the TS.Both TS and TSAC samples showed strong and broad bands at 3434–3400 cm-1.These may be assigned to the inter/intra-hydrogen bonding(O--H)stretching which is related to hydroxyl groups that can be found in phenols,alcohols and carboxylic structures[12].The spectra also illustrate the presence of N--H stretches[26].A strong band observed at 2921 cm-1and 2850 cm-1for TS spectrum,and 2918 cm-1for TSAC spectrum was assigned to the asymmetric C--H band,which is present in alkyl groups[16].Stretching adsorption bands at 1651 cm-1for TS spectrum may correspond to carboxyl group C=O present in esters,aldehydes,ketone groups and acetyl derivatives,and C=C stretching that is attributed to the aromatic C--C(1458 cm-1;1409 cm-1)bands and COO--asymmetric stretching[26–28].TSAC spectrum also showed the C--C stretching(in-ring)at 1403 cm-1.The band at 1116 cm-1and 1035 cm-1(TS spectrum)and 1068 cm-1(TSAC spectrum)is ascribed to the strong band of C--O and C--N stretching[28,29]and S=O stretching[30].The presence of S=O group shows that surface chemistry of the TSAC was modi fied during activation of biomass precursor with H2SO4.Furthermore,broad peak of C--Cl stretching showed in both TS and TSAC spectra at 616 cm-1and 617 cm-1respectively[31].The presence of chlorine in the AC is probably due to the use of dyes and bleaching agents in the textile manufacturing process.
3.2.2.Surface morphology
FESEM micrographs(Fig.4)convey different morphology of TS and TSAC surfaces.The examination of the initial raw TS showed an uneven surface with no pores.On the other hand,an uneven surface with pore openings was observed on the surface of TSAC.Such observations indicate that chemical activation by H2SO4and the carbonization processes athigh temperature led to the developmentofporosity on TSACsurface.This surface characteristic of TSAC would de finitely substantiate to higher adsorption capacity of RB5 and MB dyes onto the TSAC.
3.2.3.Nitrogen adsorption–desorption isotherm
The nitrogen adsorption–desorption curve(Fig.5(a)and(b))provides qualitative information on the TS and TSAC samples.From the figure,the best- fit for nitrogen adsorption–desorption curve for TS is type V isotherm,which refers to small adsorbate–absorbent interaction potentials and is associated with pores in the range of 1.5 to 100 nm.Similar to type III,the adsorption oftype V adsorbentproceeds,as the adsorbate's interaction with an adsorbed layer is greater than the interaction with the adsorbent surface.For TSAC,large amount of nitrogen was adsorbed at relatively higher pressure.This implies isotherm type IV for TSAC adsorbent,whereas the slope showed increased uptake of adsorbate at a higher pressure as pores are filled.The in flection point reached near completion of the first monolayer.Type V isotherm also signified the porous structure of TSAC in the range of 1.5 to 100 nm.

Fig.4.FESEM images of(a)TS and(b)TSAC.
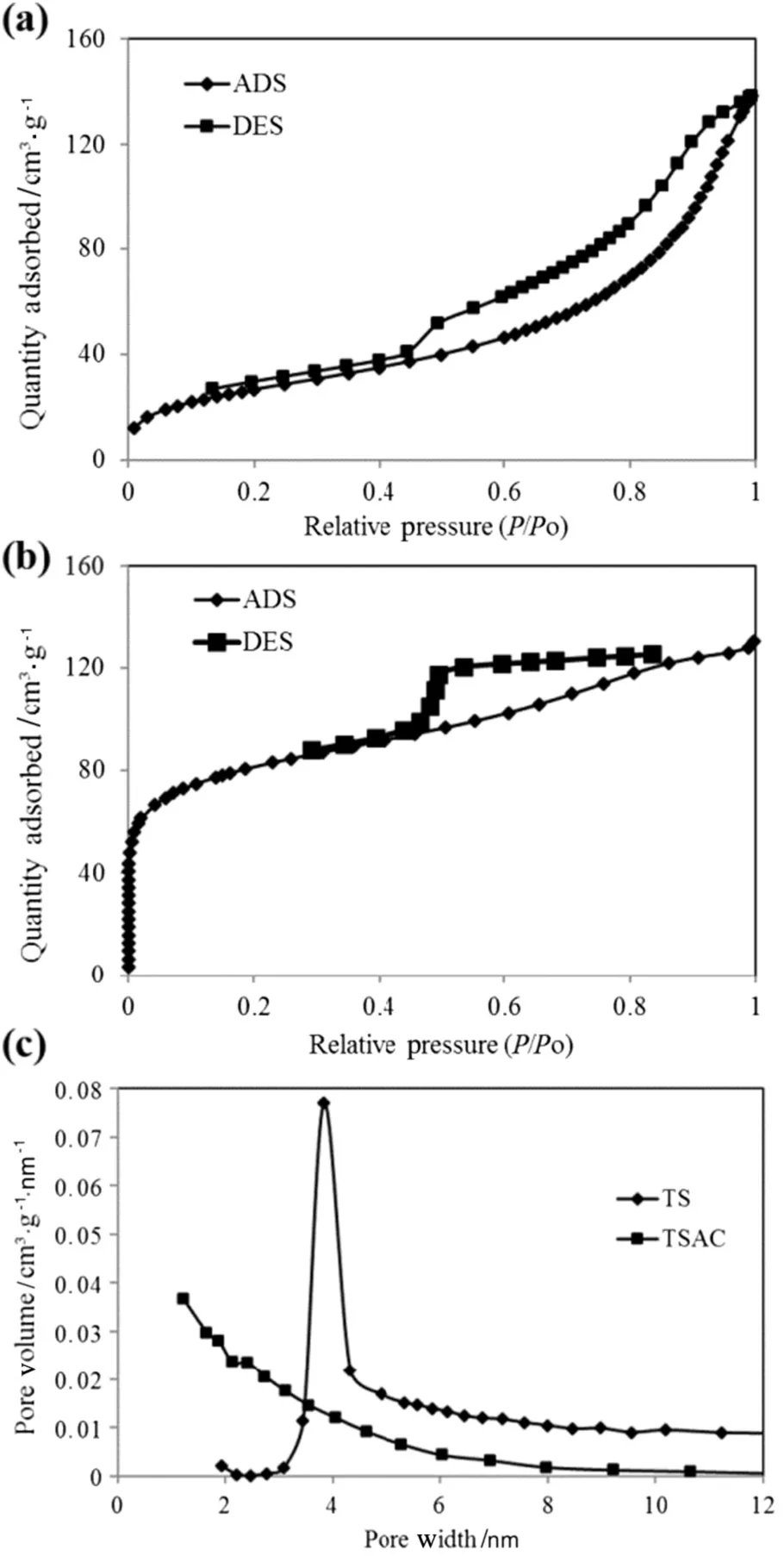
Fig.5.(a)Nitrogen adsorption/desorption isotherms for(a)TS and(b)TSAC,as well as(c)pore size distribution of TS and TSAC.
The surface physical parameters obtained from the N2adsorption–desorption isotherms are summarized in Table 1.From the data,it is marked that the BET surface area of TSAC was greatly improved(from 90.65 to 221.52 m2·g-1),implying pore developmentduring the chemical activation with H2SO4and carbonization at 650°C.According to Smith et al.[25],high carbonization temperature can maximize the BET surface area as a consequence of increased aromatization,whichoccurred at high temperatures.HCl washing at the end of the synthesis of TSAC also resulted in the increase of TSAC's surface area as it lowers down the inorganic contents of the carbonaceous material through realizing the partial dissolution of the inorganic fraction[25].

Table 1 Characteristic of samples
The structural heterogeneity is generally characterized in terms of pore size distribution.According to the classi fication of IUPAC-pore dimensions,the pores of adsorbents were grouped into micropore(d<2 nm),mesopore(d=2–50 nm)and macropore(d>50 nm).The pore distribution data were determined by BJH method for both samples(Fig.5(c)).From the plotted curves,the pore size distribution curves showed the highest peaks at pore widths of 3.8205 nm for TS sample and 1.2154 nm for TSAC sample.Thus,the TS was identified as a mesoporous material,whereas the TSAC as a microporous material.The expansion ofpores in the activation process showed positive results of the micropore growth in the TSAC adsorbent.
3.3.Effect of reaction time on adsorption equilibrium
Adsorption of RB5 from its aqueous solution(50 mg·L-1,20 ml)at room temperature(30°C)by 0.2 g of TSAC was analysed as a function of reaction time(Fig.6(a)).It was observed that the amount of RB5 dyes adsorbed increased with reaction time until the equilibrium was reached.Initially,the rate of adsorption increased rapidly before showing a constant line(qe=5.00 mg·g-1)at a reaction time of 90 min onward.The rapid increase of adsorption was due to higher vacant surface sites available during the initial stage of the adsorption process.A similar pattern is shown in the adsorption of MB onto TSAC as a function of adsorption time(Fig.6(b)).The rate of adsorption was initially increasing rapidly before it became constant(qe=3.65 mg·g-1)when the equilibrium was reached.The equilibrium time showed that the TSAC adsorbent reached saturation in approximately 120 min.Therefore,equilibrium time was set conservatively at 120 min for further experiments in adsorption of both MB dyes;the equilibrium time for adsorption of RB5 was prolonged to 120 min(2 h)to standardize the experimental condition.
Effects ofiron-and aluminium-loaded adsorbents on performance of adsorbents were investigated in several works[32,33].Thus,it is reasonable to believe that the performance of dye adsorption onto TSAC is affected by the presence of impurities,including metal ions contained in TS(due to use of chemicals during textile dyeing and processing as mentioned in the Introduction section).This is the most possible reason that leads to deviation of data points from the fitted lines as shown in Fig.6(b),although some other possible factors,including precision of spectrophotometer readings may also contribute to the deviation.Nevertheless,more studies are needed to draw conclusive links between the presence of multiple metals on TSAC and the adsorption performance.
3.4.Effect of initial dye concentration on the adsorption equilibrium
The effects of initial concentration of aqueous dye solution on the amount adsorbed(qe)of RB5 and MB dyes on TSAC are shown in Fig.7.The curve indicates thatthe qevalues increase with the increment of the initial dye concentration.A rapid increase in the amount of dyes absorbed onto TSAC adsorbent was spotted at the initial concentration of dye solution.The adsorption processes then showed only a slight change at a higher initial concentration of dye solution.These results were attributed to the increase of driving force of dye molecule due to concentration gradient[34],which resulted in a higher number of collisions between RB5 and MB dye molecules with TSAC adsorbent surface.Thus,the adsorption capacities of both dyes were increased.
3.5.Adsorption equilibrium isotherms
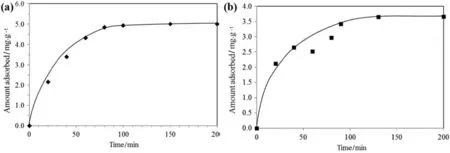
Fig.6.Effectofadsorption time on adsorption of(a)RB5;(b)MB(0.2 g TSAC;20 mlaqueous solution;room temperature;pH 6.5(originalpHofRB5 aqueous solution)and pH 6.2(original pH of MB aqueous solution)).
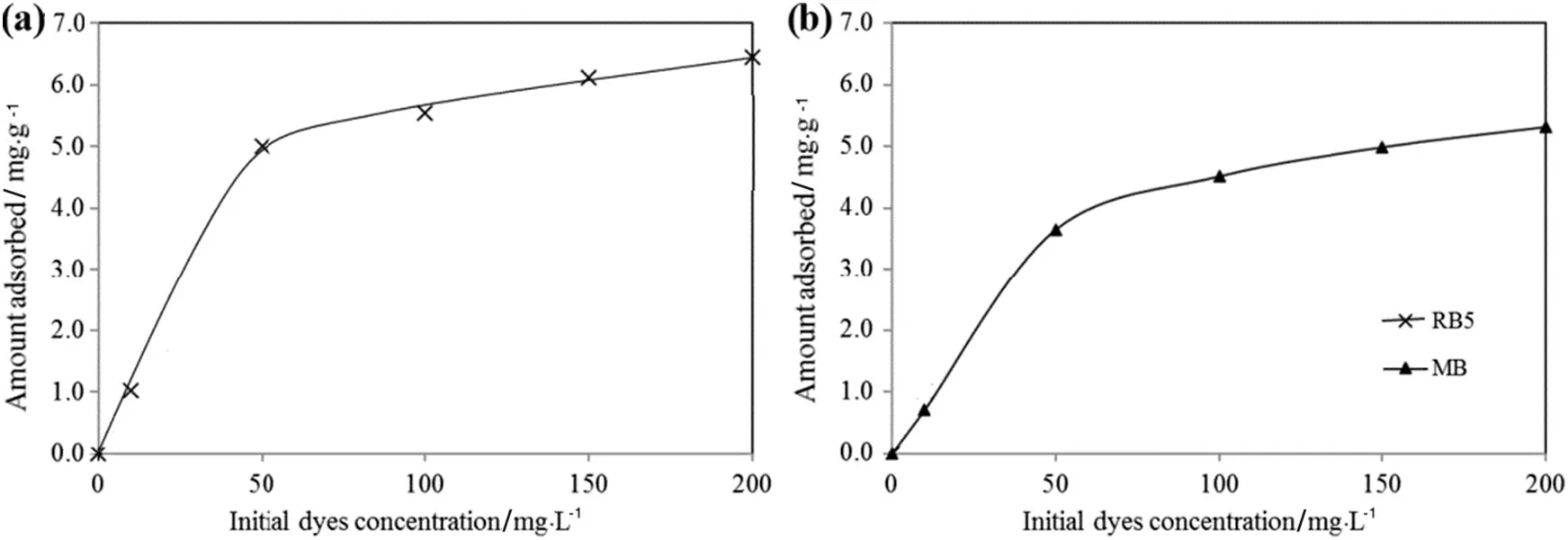
Fig.7.Effectofinitialdye concentration on adsorption of(a)RB5 and(b)MB(0.2 g TSAC;2 h adsorption time;20 mlaqueous solution;pH 6.5(originalpHofRB5 aqueous solution)and 6.2(original pH of MB aqueous solution)).
Fig.8 shows that the resulting isotherms at three different temperatures are positive,both linear and curved plots in relation to the concentration axis,which indicates complete monolayer saturation of the dye covering the surfaces of TSAC at all examined temperatures.The adsorption isotherms were also characterized by a very favourable shape,which the initial slope of the curve was inclined,indicating a great affinity of the material for the dye molecules[35].
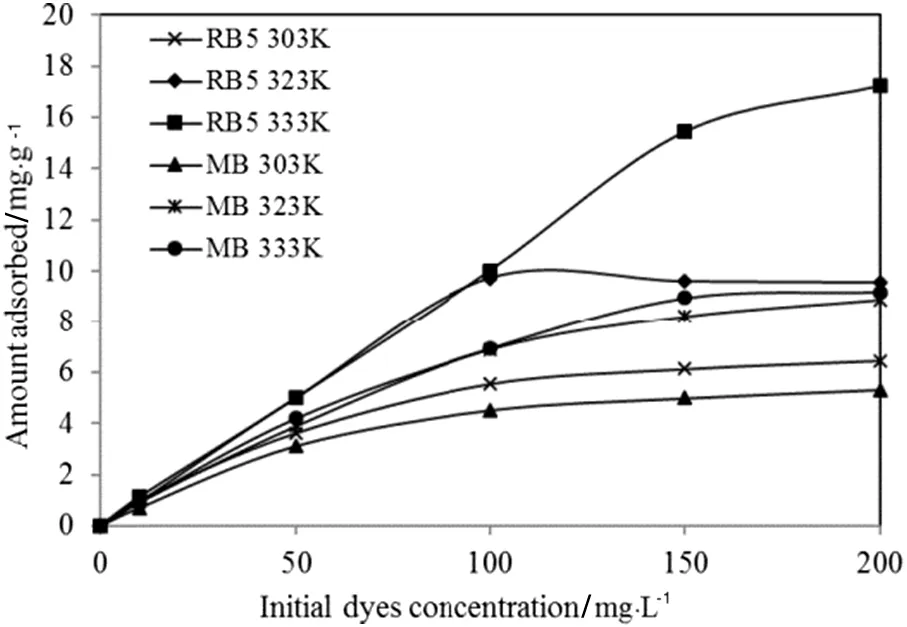
Fig.8.Adsorption isotherms of RB5 and MB by TSAC adsorbent obtained at different temperatures.
The linearized Langmuir and Freundlich isotherm plots for both RB5 and MB dye adsorption onto TSAC are shown in Fig.9.Table 2 summarizes the Langmuir and Freundlich isotherm parameters calculated from the adsorption process.High correlation coefficients,R2for the Freundlich isotherm model at various temperatures were observed for both dyes.These high correlation coefficients strongly support the fact that the cationic and anionic dye adsorption onto TSAC closely followed the Freundlich isotherm model,which explains the heterogeneous nature of the TSAC surface.Moreover,the Q0values obtained from the Langmuir plot were higher than the value of qmaxin experimental values(9.017 and 10.320 mg·g-1for RB5 and MB,respectively).Such observation indicated suitability of the Freundlich isotherm model to represent the adsorption process of RB5 and MB onto TSAC,which can further be examined from the Freundlich constant of the equation.The Freundlich constant KFshowed the adsorption capacity of the adsorbent,which are 0.260,0.168 and 0.134 for adsorption of RB5,and 0.141,0.165 and 0.163 for the adsorption of MB.Higher values of KFsuggest the highest adsorption capacity.The value of the Freundlich constant n also gives a measure of favourability of adsorption[36].The n value between 1 and 10 showed the favourability of the adsorption process.The adsorption is more favourable at the smaller value of n with n value between 1 and 10[37].The n values were found to be 1.573,1.224 and 1.073 for the adsorption of RB5,and 1.331,1.280 and 1.259 for the adsorption ofMB.These values indicate favourable adsorption processes for the adsorption of both dyes onto TSAC governed by physisorption.Similar results were reported for the adsorption of MB and Reactive Red 222(RR222)dyes onto composite beads,activated clay,and chitosan beads[38].
3.6.Adsorption kinetic study
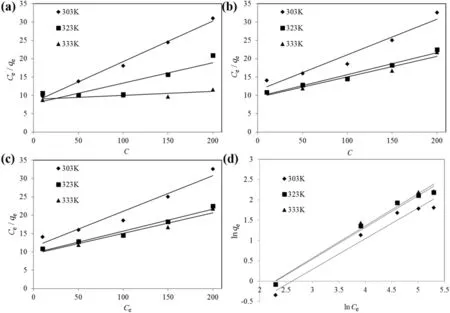
Fig.9.Linearized(a)Langmuir and(b)Freundlich isotherm plots for anionic RB5 adsorption by TSAC;and linearized(c)Langmuir and(d)Freundlich isotherm plots for anionic MB adsorption by TSAC.
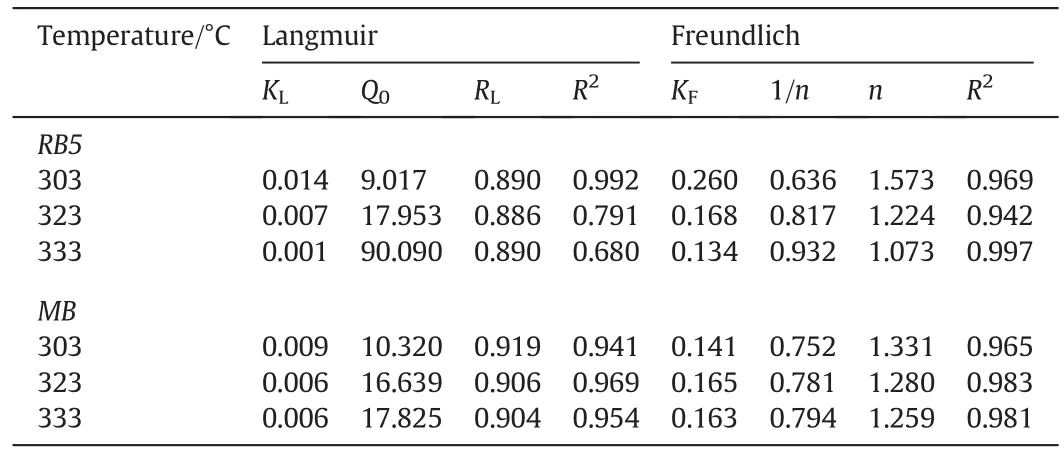
Table 2 The Langmuir and Freundlich isotherm parameters for the adsorption of RB5 dye onto TSAC
The kinetics of adsorption describes the rate of adsorption at the solid–liquid interface.The kinetics of RB5 and MB adsorption onto TSAC adsorbent was analysed using two kinetic models,namely pseudo- first order model and pseudo-second order model(Fig.10).The value of correlation coefficient,R2as well as slope and intercept obtained from the plots are tabulated in Table 3.The comparison of qedata shows that the calculated qevalues of the pseudo-second order are closer to the experimental values as compared to those from the pseudo- first order.The agreement on experimental and calculated qevalues indicates that the adsorption system follows the pseudo-second order kinetic model.Moreover,the correlation coefficient,R2of the pseudo-second order model showed higher values for both dyes which indicate that the adsorption of the dyes onto TSAC was classi fied as chemisorption as the rate-limiting step,which involves valence forces of electrons between adsorbate and adsorbent[39].
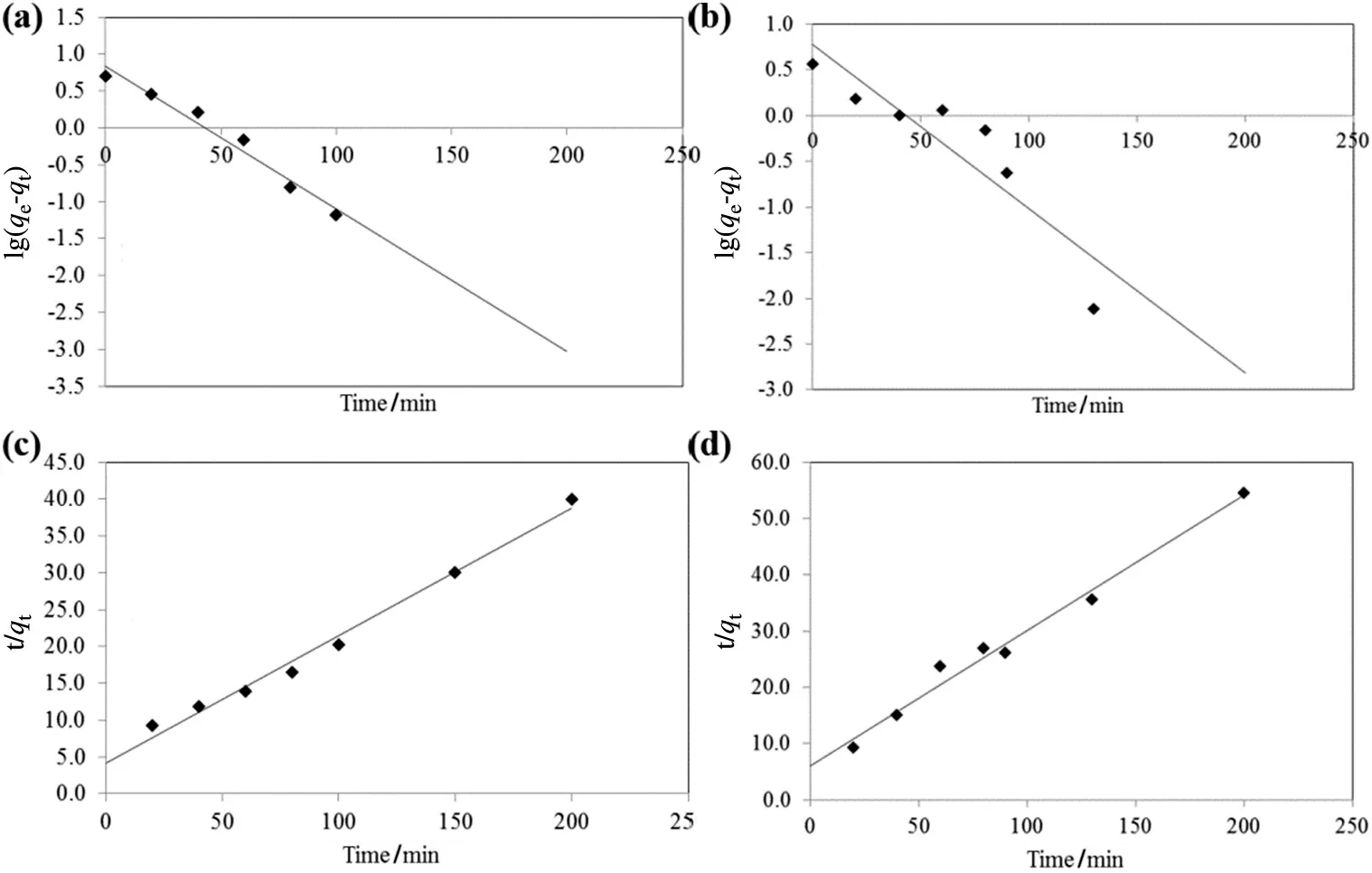
Fig.10.Plots of pseudo- first order kinetic model for adsorption of(a)RB5 and(b)MB,as well as pseudo-second order kinetic model for adsorption of(c)RB5 and(d)MB by TSAC.
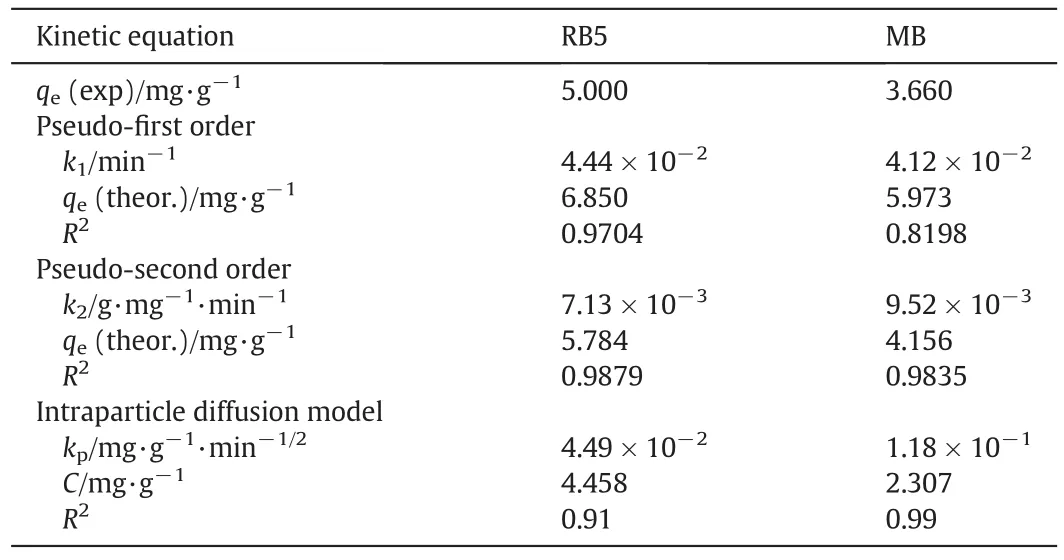
Table 3 Kinetic parameter of the pseudo- first order,pseudo-second order and intraparticle diffusion for the adsorption of RB5 and MB dyes
The solute transfer in a solid/liquid adsorption process is generally divided into three categories:external mass transfer(boundary layer diffusion),intraparticle diffusion or both[40].Three consecutive steps involved in the adsorption diffusion include,the external mass transfer as the first step,where the diffusion occurs between the liquid films to the adsorbent surface.The second step is the intraparticle transport,which involves the diffusion of the liquid contained in the pores and/or along the pore walls.The third step is the reaction at phase boundaries,where the adsorption and desorption between the adsorbate and active sites are generally controlled by the kinetics of bond formation[41].The intraparticle diffusion model(Fig.11)represents the diffusion mechanism of RB5 and MB dyes adsorbed through the TSAC adsorbent.The figure contains linear plots with different slopes in three regions,indicating three steps in the adsorption process.The initial slope represents the film diffusion,the second slope represents intraparticle as the rate-limiting step,whereas the last section is the final equilibrium stage.The sorption of RB5 and MB dyes onto TSAC adsorbent may be controlled by film diffusion at earlier stages and as the TSAC particles were loaded with RB5 and MB dye ions,the sorption process may be controlled by intraparticle diffusion[40].Thus,the intraparticle diffusion was not the only rate-determining factor.The intercept values represent the thickness of the boundary layer.Larger intercept values propose that the surface diffusion has a larger contribution as the rate-limiting step.
Table 4 shows the comparison of the maximum adsorption capacity of dyes onto various adsorbents.The TSAC prepared in this study provided a relatively low RB5 and MB dye adsorption capacity,whereas the value is comparable to other adsorbents reported in literature.Nevertheless,application of TSAC is an interesting option for the textile industry when considering the elimination of treatment cost of TS as scheduled waste.Moreover,previous research on the use of AC for the adsorption of strontium by Kacan and Kutahyali[11]shows the BET surface area(SBET)of 135 m2·g-1and the adsorption capacity was reported to be 12.11 mg·g-1.Thus,itshows thatthe TSACprepared in this study was improved from the previous research with a higher SBETof 221.52 m2·g-1.
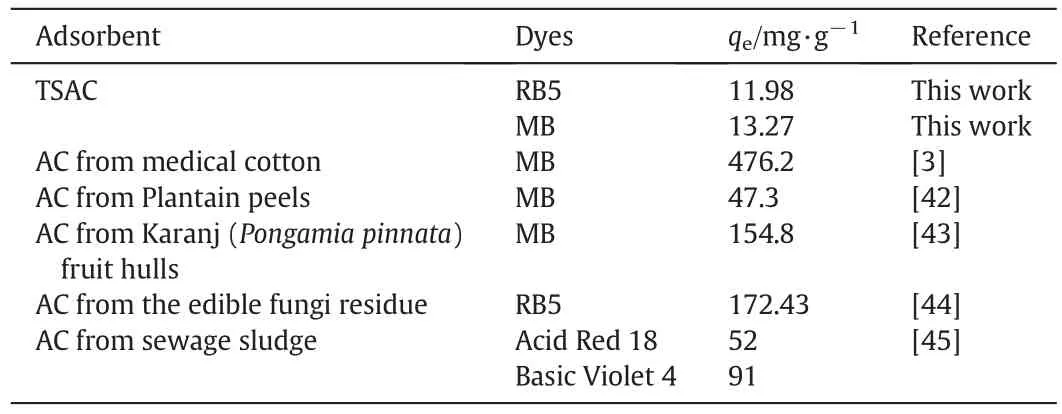
Table 4 Comparison of the maximum monolayer adsorption capacity of dyes onto various adsorbents
4.Conclusions
TSAC was successfully synthesized from TS as an alternative for low-cost adsorbent.TSAC prepared via H2SO4treatment exhibited higher adsorption performance than TSAC treated with KCl and ZnCl2.FTIR result confirmed the existence of important hydroxyl and carboxyl functional groups in TSAC which are absent in TS.Increased porosity on the surface of TSAC by 144%from TS surface was indicated from BET analysis result.The microporous character of TSAC was attributed to the activation process during the synthesis process.Comparison of surface morphology through FESEM indicates development of porosity on TSAC during chemical activation.Adsorption study results showed thatoptimumconditionsforequilibrium adsorption time were different for RB5 and MB dyes.Similar adsorption behaviour was observed for both anionic RB5 and cationic MB dyes as a response to initial dye concentration.The adsorption isotherms showed that the adsorption of RB5 and MB dyes onto TSAC fits well to the Freundlich model while kinetic studies showed that the adsorption of both dyes onto TSAC is best described by pseudo-second order model.In conclusion,this study demonstrates the possibility to transform TS as a source of pollution to adsorbents of dye from wastewater,and more studies are needed to improve the adsorption performance of TSAC.

Fig.11.Intraparticle diffusion plots for(a)RB5 and(b)MB adsorption by TSAC.
Acknowledgments
The authors extend their sincere gratitude to the Ministry of Higher Education,Malaysia(MOHE)for the financial supports received under University Grant(08H05)and Fundamental Research Grant Scheme(4F872),as well as Universiti Teknologi Malaysia for the GUP grant No.17H65 and the support to the main author,Wong Syie Luing,in the form of Post-Doctoral Fellowship Scheme for the project"Catalytic Cracking of Low Density Polyethylene Waste to Liquid Fuels in Fixed Bed Reactor”.
References
[1]N.Remmas,P.Melidis,G.Paschos,E.Statiris,S.Ntougias,Protozoan indicators and extracellular polymeric substances alterations in an intermittently aerated membrane bioreactor treating mature land fill leachate,Environ.Technol.38(1)(2017)53–64.
[2]R.Poblete,I.Oller,M.I.Maldonado,Y.Luna,E.Cortes,Cost estimation of COD and color removal from land fill leachate using combined coffee-waste based activated carbon with advanced oxidation processes,J.Environ.Chem.Eng.5(1)(2017)114–121.
[3]X.H.Duan,C.Srinivasakannan,X.Wang,F.Wang,X.Y.Liu,Synthesis of activated carbon fibers from cotton by microwave induced H3PO4activation,J.Taiwan Inst.Chem.Eng.70(2017)374–381.
[4]T.Robinson,G.McMullan,R.Marchant,P.Nigam,Remediation of dyes in textile effluent:a critical review on current treatment technologies with a proposed alternative,Bioresour.Technol.77(3)(2001)247–255.
[5]M.Yusuf,M.A.Khan,M.Otero,E.C.Abdullah,M.Hosomi,A.Terada,S.Riya,Synthesis of CTAB intercalated graphene and its application for the adsorption of AR265 and AO7 dyes from water,J.Colloid Interface Sci.493(2017)51–61.
[6]M.Oz,D.E.Lorke,M.Hasan,G.A.Petroianu,Cellular and molecular actions of Methylene Blue in the nervous system,Med.Res.Rev.31(1)(2011)93–117.
[7]R.Pradhananga,L.Adhikari,R.Shrestha,M.Adhikari,R.Rajbhandari,K.Ariga,L.Shrestha,Wool carpet dye adsorption on nanoporous carbon materials derived from agro-product,J.Carbon Res.3(2)(2017)12.
[8]A.A.Spagnoli,D.A.Giannakoudakis,S.Bashkova,Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride:the role of surface and structural parameters,J.Mol.Liq.229(2017)465–471.
[9]G.Orlandi,J.Cavasotto,F.R.Machado Jr.,G.L.Colpani,J.D.Magro,F.Dalcanton,J.M.Mello,M.A.Fiori,An adsorbent with a high adsorption capacity obtained from the cellulose sludge of industrial residues,Chemosphere 169(2017)171–180.
[10]E.V.Rosa,L.Mater,M.M.Souza-Sierra,L.R.Rorig,L.M.Vieira,C.M.Radetski,Textile sludge application to non-productive soil:physico-chemical and phytotoxicity aspects,Ecotoxicol.Environ.Saf.68(1)(2007)91–97.
[11]E.Kacan,C.Kutahyali,Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges,J.Anal.Appl.Pyrolysis 97(2012)149–157.
[12]K.S.A.Sohaimi,N.Ngadi,H.Mat,I.M.Inuwa,S.Wong,Synthesis,characterization and application of textile sludge biochars for oil removal,J.Environ.Chem.Eng.5(2)(2017)1415–1422.
[13]E.Kacan,Optimum BET surface areas for activated carbon produced from textile sewage sludges and its application as dye removal,J.Environ.Manag.166(2016)116–123.
[14]F.Rozada,M.Otero,A.Moran,A.I.Garcia,Activated carbons from sewage sludge and discarded tyres:production and optimization,J.Hazard.Mater.124(1–3)(2005)181–191.
[15]B.Stuart,Infrared spectroscopy,Kirk-Othmer Encyclopedia of Chemical Technology,John Wiley&Sons Inc.,2000
[16]S.Wong,N.Ngadi,T.A.T.Abdullah,I.M.Inuwa,Catalytic cracking of LDPE dissolved in benzene using nickel-impregnated zeolites,Ind.Eng.Chem.Res.55(9)(2016)2543–2555.
[17]Y.S.Ho,G.McKay,A two-stage batch sorption optimized design for dye removal to minimize contact time,Process Saf.Environ.76(B4)(1998)313–318.
[18]M.Fatiha,B.Belkacem,Adsorption of methylene blue from aqueous solutions using natural clay,J.Mater.Environ.Sci.7(1)(2016)285–292.
[19]M.A.Ahmad,R.Alrozi,Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon:equilibrium,kinetic and thermodynamic studies,Chem.Eng.J.171(2)(2011)510–516.
[20]J.Febrianto,A.N.Kosasih,J.Sunarso,Y.H.Ju,N.Indraswati,S.Ismadji,Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent:a summary of recent studies,J.Hazard.Mater.162(2–3)(2009)616–645.
[21]K.Y.Foo,B.H.Hameed,Coconut husk derived activated carbon via microwave induced activation:effects of activation agents,preparation parameters and adsorption performance,Chem.Eng.J.184(2012)57–65.
[22]M.A.Martin-Lara,I.L.R.Rico,I.D.A.Vicente,G.B.Garcia,M.C.de Hoces,Modi fication of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions,Desalination 256(1–3)(2010)58–63.
[23]I.A.Aguayo-Villarreal,A.Bonilla-Petriciolet,R.Muniz-Valencia,Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions,J.Mol.Liq.230(2017)686–695.
[24]S.Álvarez-Torrellas,M.Martin-Martinez,H.T.Gomes,G.Ovejero,J.García,Enhancement of p-nitrophenol adsorption capacity through N2-thermal-based treatment of activated carbons,Appl.Surf.Sci.414(2017)424–434.
[25]K.M.Smith,G.D.Fowler,S.Pullket,N.J.Graham,Sewage sludge-based adsorbents:a review of their production,properties and use in water treatment applications,Water Res.43(10)(2009)2569–2594.
[26]S.H.Md Arshad,N.Ngadi,A.A.Aziz,N.S.Amin,M.Jusoh,S.Wong,Preparation of activated carbon from empty fruit bunch for hydrogen storage,J.Energy Storage 8(2016)257–261.
[27]N.V.Farinella,G.D.Matos,M.A.Arruda,Grape bagasse as a potential biosorbent of metals in effluent treatments,Bioresour.Technol.98(10)(2007)1940–1946.
[28]B.Nabgan,W.Nabgan,T.A.Tuan Abdullah,M.Tahir,Y.Gambo,M.Ibrahim,W.Syie Luing,Parametric study on the steam reforming of phenol-PET solution to hydrogen production over Ni promoted on Al2O3-La2O3catalyst,Energy Convers.Manag.142(2017)127–142.
[29]I.M.Inuwa,R.Arjmandi,A.N.Ibrahim,M.K.M.Haa fiz,S.L.Wong,K.Majeed,A.Hassan,Enhanced mechanical and thermal properties of hybrid graphene nanoplatelets/multiwall carbon nanotubes reinforced polyethylene terephthalate nanocomposites,Fiber Polym.17(10)(2016)1657–1666.
[30]B.A.Marekha,K.Sonoda,T.Uchida,T.Tokuda,A.Idrissi,T.Takamuku,ATR-IR spectroscopic observation on intermolecular interactions in mixtures of imidazoliumbased ionic liquids CnmimTFSA(n=2–12)with DMSO,J.Mol.Liq.232(2017)431–439.
[31]C.-L.Huang,Y.-J.Wang,Y.-C.Fan,C.-L.Hung,Y.-C.Liu,The effect of geometric factor of carbon nano fillers on the electrical conductivity and electromagnetic interference shielding properties of poly(trimethylene terephthalate)composites:a comparative study,J.Mater.Sci.52(5)(2016)2560–2580.
[32]E.Altıntıg,H.Altundag,M.Tuzen,A.Sarı,Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent,Chem.Eng.Res.Des.122(2017)151–163.
[33]G.Wendimu,F.Zewge,E.Mulugeta,Aluminium-iron-amended activated bamboo charcoal(AIAABC)for fluoride removal from aqueous solutions,J.Water Process Eng.16(2017)123–131.
[34]W.H.Li,Q.Y.Yue,B.Y.Gao,Z.H.Ma,Y.J.Li,H.X.Zhao,Preparation and utilization of sludge-based activated carbon for the adsorption of dyes from aqueous solutions,Chem.Eng.J.171(1)(2011)320–327.
[35]M.Greluk,Z.Hubicki,Kinetics,isotherm and thermodynamic studies of Reactive Black 5 removal by acid acrylic resins,Chem.Eng.J.162(3)(2010)919–926.
[36]A.E.Pirbazari,E.Saberikhah,M.Badrouh,M.S.Emami,Alkali treated Foumanat tea waste as an efficient adsorbent for methylene blue adsorption from aqueous solution,Water Resour.Ind.6(2014)64–80.
[37]F.Rozada,L.F.Calvo,A.I.Garcia,J.Martin-Villacorta,M.Otero,Dye adsorption by sewage sludge-based activated carbons in batch and fixed-bed systems,Bioresour.Technol.87(3)(2003)221–230.
[38]M.Y.Chang,R.S.Juang,Adsorption of tannic acid,humic acid,and dyes from water using the composite of chitosan and activated clay,J.Colloid Interface Sci.278(1)(2004)18–25.
[39]Z.Aly,A.Graulet,N.Scales,T.Hanley,Removal ofaluminium from aqueous solutions using PAN-based adsorbents:characterisation,kinetics,equilibrium and thermodynamic studies,Environ.Sci.Pollut.Res.Int.21(5)(2014)3972–3986.
[40]S.Dawood,T.K.Sen,Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent:equilibrium,thermodynamic,kinetics,mechanism and process design,Water Res.46(6)(2012)1933–1946.
[41]N.K.Lazaridis,D.D.Asouhidou,Kinetics of sorptive removal of chromium(VI)from aqueous solutions by calcined Mg-Al-CO(3)hydrotalcite,Water Res.37(12)(2003)2875–2882.
[42]E.Inam,U.J.Etim,E.G.Akpabio,S.A.Umoren,Process optimization for the application of carbon from plantain peels in dye abstraction,J.Taibah Univ.Sci.11(1)(2017)173–185.
[43]M.A.Islam,S.Sabar,A.Benhouria,W.A.Khanday,M.Asif,B.H.Hameed,Nanoporous activated carbon prepared from karanj(Pongamia pinnata)fruit hulls for methylene blue adsorption,J.Taiwan Inst.Chem.Eng.74(2017)96–104.
[44]H.Xiao,H.Peng,S.Deng,X.Yang,Y.Zhang,Y.Li,Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3activation—application in reactive black 5 adsorption from aqueous solution,Bioresour.Technol.111(2012)127–133.
[45]S.Rio,L.Le Coq,C.Faur,D.Lecomte,P.Le Cloirec,Preparation of adsorbents from sewage sludge by steam activation for industrial emission treatment,Process Saf.Environ.84(B4)(2006)258–264.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- 17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
- Transesteri fication of sun flower oil in microchannels with circular obstructions
- The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
- Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination☆
