The green hydrolysis technology of hemicellulose in corncob by the repeated use of hydrolysate☆
Lei Guo,Yangdong Hu*,Lianying Wu,Chen Liang,Weitao Zhang
College of Chemistry and Chemical Engineering,Ocean University of China,Qingdao 266100,China
1.Introduction
Biomass resources are the most abundant renewable resources on earth and the annual output of corncob has reached about 50 million tons in China[1].More importantly,the main rawmaterial for D-xylose production is hemicellulose.Hemicellulose takes up a large proportion in corncob as the structure of which is mainly composed of macromolecule xylan[2,3].Aside from producing furfural[4,5],xylitol[6,7],fumaric acid[8]and other products as an important rawmaterial,D-xylose is also widely used in baked food,top grade soy sauce and zero-calorie sweeteners[9].
Acid hydrolysis and enzymatic hydrolysis are main methods for D-xylose production in hemicellulose degradation.The process of using enzymatic hydrolysis to produce D-xylose is characterized by mild reaction and long-cycle[10,11].For instance,Hang et al.[10]took corncob as the rawmaterial,the corncob was pretreated by 1.25 mol·L−1sodium hydroxidefirstly,and then it was enzymolyzed by the enzyme of Rapidase Pomaliq at 50°C for 48 h under conditions of the solid/liquid ratio(by mass)of 1:21.2 and the p H of 5.0,respectively.The concentration of D-xylose in the hydrolysate turned to 10.4 g·L−1,while the ratio of D-xylose to water(by mass)was approx.1:96.The utilization of acid hydrolysis to produce D-xylose has fast and short-cycle characteristics[12,13].Sulfuric acid[14–16],hydrochloric acid[17],formic acid[18],phosphoric acid[19]and sulfurous acid[20]can be used as the acid medium in the course of acid hydrolysis of hemicellulose.In general,the concentration of acid is usually 3%–6%,the solid/liquid ratio(by mass)is 1:7–1:12;the concentration of D-xylose in hydrolysate is 17 g·L−1–32 g·L−1,the ratio of D-xylose to water(by mass)is 1:31–1:58,the ratio of D-xylose to acid(by mass)is 1:5–1:18 when the acid solutions are used to hydrolyze hemicellulose.For example,Ra fiqul et al.[16]used sulfuric acid solution to hydrolyze wood chips to produce D-xylose.The wood chips were hydrolyzed for 1 h at 125°C under conditions of the concentration of 4%and the solid/liquid ratio(by mass)of 1:8,respectively.After the hydrolysis,the D-xylose concentration in the hydrolysate was 17.9 g·L−1,the ratio of D-xylose to water(by mass)was 1:55.9 and the ratio of D-xylose to sulfuric acid(by mass)was 1:13.5,respectively.According to the ratios of D-xylose to water and D-xylose to acid,it is concluded that water and acid are in great demand in producing D-xylose.In the industrial production of D-xylose,the burden of the treatment of acid solution will be comparatively high if the acid is in high demand.The D-xylose is produced through the crystallization of the D-xylose mother liquor in which the sugar content has condensed to about 80 wt%.Therefore,the more the water contains,the higher consumption it produces during the concentration process.Consequently,it isof great significanceto develop anewtechnology in hydrolyzing hemicellulose with less acid consumption and high concentration of D-xylose.
In this paper,a newtechnology in producing hydrolysate with high concentration of D-xylose by the repeated use of corncob hydrolysate was studied at atmospheric pressure by taking corncob as the raw material and sulfuric acid as the catalyzer.Our research has evident effects on reducing the amount of acid and water used in D-xylose production.
2.Materials and Methods
2.1.Materials
Corncob was provided by a farm at Zhang Jialou town,Shandong,China.This material was ground and passed through a 120 mesh sieve to separate particles.Undersize product was dried to constant weight and used in this study.Detailed information of chemical reagents are shown in Table 1.
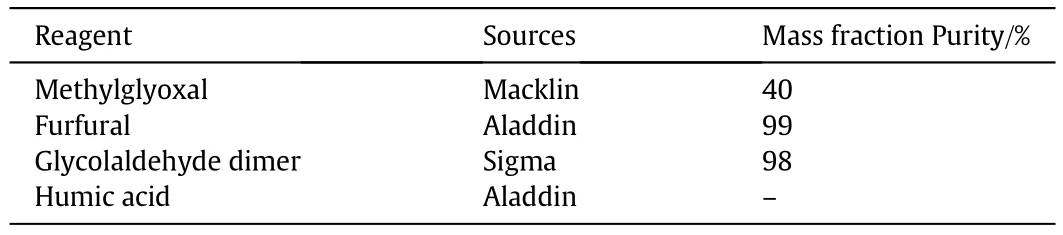
Table 1Detailed information of chemical reagents used in this study
2.2.Analytical methods
The content of D-xylose in solution was analyzed by HPLC(L-2000,Hitachi Limited,Japan)with a refractive index detector and a Aminex HPX-87H column(300 mm×7.8 mm,Bio-Rad,America).The mobile phase was an aqueous solution of sulfuric acid(0.005 mol·L−1),while the flow rate was 0.55 ml·min−1.The column temperature was 333.15 K,and the temperature of the refractive index detector was 308.15 K.
2.3.Experimental procedure
Corncob was pretreated by deionized water for 0.5 h at the condition that the solid/liquid ratio was 1:10 at 100°C.then adjusted the solution acidity to 0.1 wt%using sulfuric acid,and continue to pretreat for 1 h.The solution was stirred continuously by an electric stirring in the process of pretreatment.Corncob was separated by filtration under diminished pressure after pretreatment and was washed with deionized water,the amount of deionized water was twice that of dry corncob.The corncob was eventually dry to constant mass for hydrolysis.
Combined 30.0 g corncob with 2 wt%sulfuric acid aqueous solution(H+:0.408 mol·L−1)and the solid–liquid ratio was 1:10.The system reacted for 3 h at 103°C and was stirred simultaneously,the process was known as the first use of corncob hydrolysate.Then the hydrolysate was separated and the volume of it was measured,while the filter residue was washed for 3 times using 50–60 ml deionized water each time and the lotion was collected and measured for the volume of it.The right amount of lotion and sulfuric acid was added to adjust the volume of the hydrolysate and concentration of sulfuric acid equal to that before reaction.Continue to hydrolyze the corncob for 3 h using this hydrolysate with the solid/liquid ratio of 1:10 at 103°C.The process was known as the second repeated use of corncob hydrolysate.Based on this method,one can repeatedly use the hydrolysate of corncob to hydrolyze corncob using the repeated using.The concentration of D-xylose and L-arabinose in hydrolysate and lotion was analyzed by HPLC after each reaction.To minimize the experimental error,each experimental solution was measured at least three times,and expressed by the mean values.
3.Results and Discussion
3.1.The study of production technology of D-xylose using repeated-using corncob hydrolysate
3.1.1.The effects of times of repeated use of corncob hydrolysate on the concentration ofD-xylose andL-arabinose
The 2 wt%sulfuric acid aqueous solution(H+:0.408 mol·L−1)was used to hydrolyze corncob at 103°C.The concentrations of D-xylose and L-arabinose in the solution after repeated use of the hydrolysateare listed in Table 2.As shown in Table 2,the concentrations of D-xylose and L-arabinose increase with growing times of using corncob hydrolysate,which indicates the accumulation of D-xylose and L-arabinose in solution.
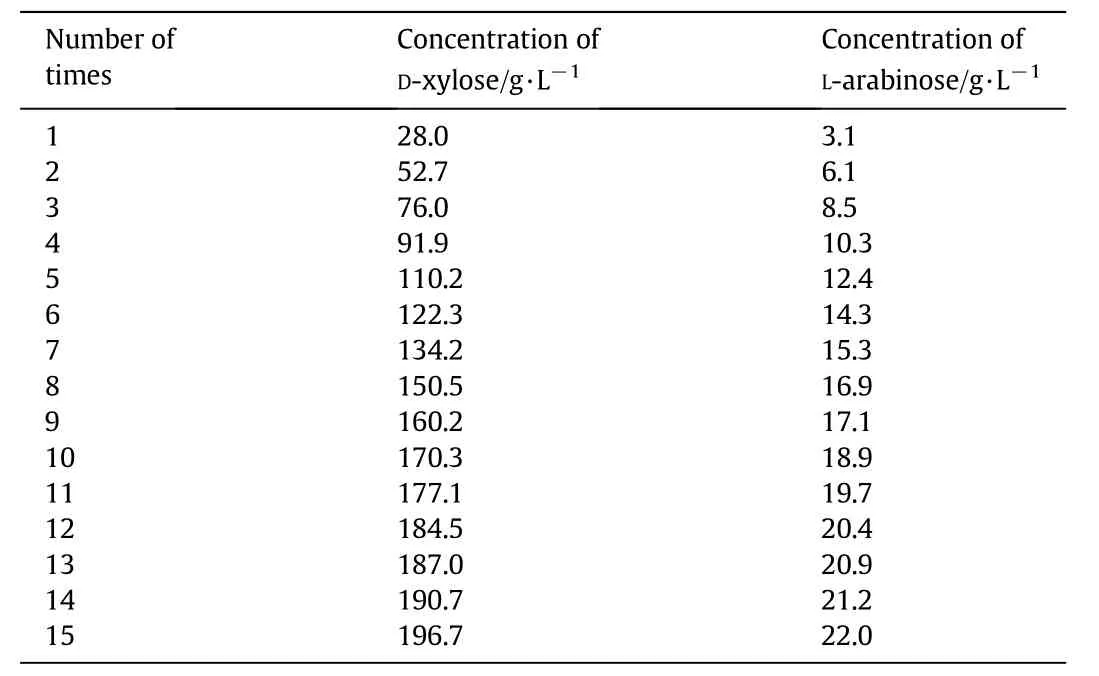
Table 2 The concentrations of D-xylose and L-arabinose in the solution after repeated use of the hydrolysate
3.1.2.The effects of times of repeated use of corncob hydrolysate on the D-xylose yield
Table2 showsthat the D-xylosecontent in hydrolysate isthehighest,which accounts for almost 90%of the total pentose.Hence,we focus on discussing the production technology of D-xylose using repeated use of corncob hydrolysate.
The D-xylose is8.4 g in the solution after the first time use of corncob hydrolysate.This study takes the mass of D-xylose as its theoretical increase with the repeated use of hydrolysate each time,while the method for calculating the D-xylose yield after repeatedly using hydrolysis is shown in Eq.(1):

where η is the yield of D-xylose,%.ΔmNis the increase of D-xylose when the hydrolysate was repeated used for the N th time,g.N is the number of times of repeated use of hydrolysate.m1is theoretical increase in the repeatedly-using hydrolysate,m1=8.4 g.
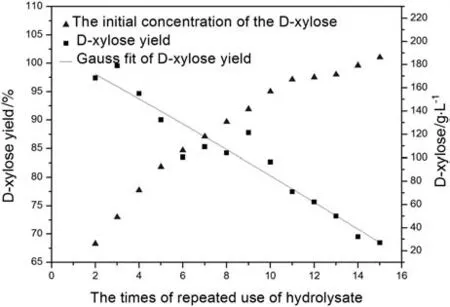
Fig.1.The variation of D-xyloseyield and the initial concentrations of the D-xylose in solution when hydrolysate was repeatedly used over the times of repeated use of hydrolysate.
The variation of D-xylose yield and the initial concentrations of the D-xylose in solution when hydrolysate was repeatedly used over the times of repeated use of hydrolysate are shown in Fig.1.It can be seen from Fig.1 that the yield of D-xylose decreases with the increase of initial concentrations of the D-xylose in solution when hydrolysate was repeatedly used.When the hydrolysate is repeatedly used for two or three times,the increases of D-xylose are 8.2 g and 8.4 g,respectively,while the yield of D-xylose is 97.4%and 99.5%,respectively.When it comesto the fifteenth time,the concentration of D-xylosein hydrolysate is 196.7 g·L−1,the D-xylose increases by 5.8 g and the yield of D-xylose is 68.4%.
3.1.3.The effects of times of repeated use of corncob hydrolysate on byproduct acetic acid
Acetic acid is the byproduct in the process of producing D-xylose by the acidolysis of corncob.The variation of concentrations of acetic acid in solution over the times of repeated use of hydrolysate is shown in Fig.2.It can be seen that the concentration of acetic acid increases with growing times of repeated use of corncob hydrolysis.The variation of increase of acetic acid and the initial concentrations of D-xylose and acetic acid in solution when hydrolysate was repeatedly used over the times of repeated use of hydrolysate are shown in Figs.3 and 4,respectively.From Figs.3 and 4,the increase of acetic acid fluctuate isbetween 0.8 g and 1.5 g,and it has no relations with the initial concentrations of D-xylose and acetic acid.It demonstrates that the main source of acetic acid in the solution is not derived from the degradation of D-xylose.There are branched chains of xylosyl and acetyl in graminaceous hemicellulose,while the main source of acetic acid in hydrolysate is derived from the hydrolysis of graminaceous hemicellulose.
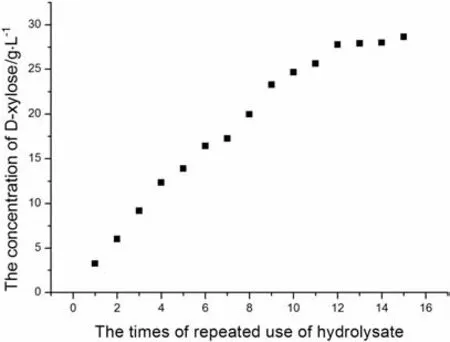
Fig.2.Thevariation of concentrationsof acetic acid in solution versus thetimesof repeated use of hydrolysate.
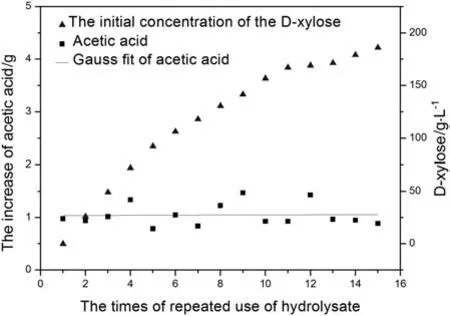
Fig.3.The variation of increase of acetic acid and the initial concentrations of D-xylose in solution when hydrolysate is repeatedly used versus the times of repeated use of hydrolysate.
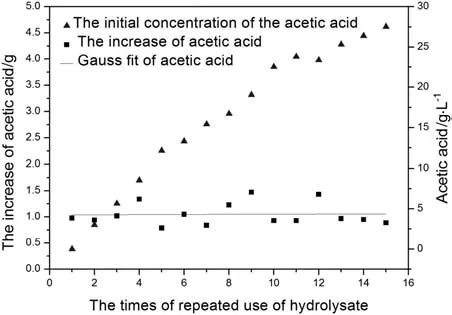
Fig.4.The variation of increase of acetic acid and the initial concentrations of the acetic acid in solution when hydrolysate is repeatedly used versus the times of repeated use of hydrolysate.
3.2.The comparison of acidolysis of D-xylose solutions both with and without corncob
According to the study on repeatedly-using corncob hydrolysate,when the hydroly sate is repeatedly used,the yield of D-xylose decreases with the increase of the initial D-xylose concentration.We study the variation of D-xylose loss rates in acid solution over different initial concentrations.D-xylose solutions with different concentrations have two sources,one is the repeatedly-using corncob hydrolysate,the other is the prepared solution of pure D-xylose.The acidity of the prepared solutions is the same as the repeatedly-using corncob hydrolysate(H+:0.408 mol·L−1,H2SO4).The concentrations of D-xylose in prepared solutions without corncob are 100,150 and 200 g·L−1,respectively.The concentrations of D-xylose in prepared solutions with corncob(the solid to liquid ratio is 1:10,the same as the repeated use of corncob hydrolysate)are 96.0,142.0 and 193.0 g·L−1,respectively.D-xylose solutions with different initial concentrations both with and without corncob were heated at 103°C for 3 h,then the D-xylose loss rates were investigated.The method of calculating D-xylose loss rate is shown in Eq.(2):

where λ is the loss rate of D-xylose,%;m′is the initial mass of D-xyloseat the N th time of repeatedly using of the hydrolysate;m1is the theoretical increase in the repeatedly-using hydrolysate,m1=8.4 g,if the corncob is not added,then m1=0;m is the mass of D-xylose in the solution after repeatedly using of the hydrolysate,g.
The loss rates of D-xylose in solutions with different initial concentrations with and without corncob at 103°Cfor 3 h are shown in Fig.5.It can be seen from Fig.5 that the D-xylose loss rate in repeatedly-using hydrolysate with corncob is the lowest;the D-xylose loss rate in D-xylose preparation solution with corncob,is higher after being heated;while the D-xylose loss rate in D-xylose preparation solution without corncob is the highest.
According to Fig.5,the D-xylose loss rate in D-xylose preparation solution with corncob is lower than that without corncob.It demonstrates that,by adding corncob to solution,the loss rate of D-xylose can be significantly reduced.This can be explained that the crudefiber,which owns negative electricity,is apt to combine with H+in solution to reduce the concentration of H+.To verify the effect of corncob on the concentration of H+,30 g of corncob was added into the solution with the concentration of H+of 0.408 mol·L−1and the concentration of D-xylose of 100.0 g·L−1.When the solution was heated up to 103 °C,the concentration of H+decreasesto 0.371 mol·L−1.Therefore,corncob can reduce the concentration of H+in solution.The effect of the concentration H+on the D-xylose loss rate was studied.The loss rates of D-xylose in solutions with different mass fractions of sulfuric acid at 103°C for 3 h are shown in Fig.6.It can be seen from Fig.6 that,under the experimental heating of D-xylose solutions with different concentrations of H+at 103°C for 3 h,the higher the concentration of H+,the higher the loss rates of D-xylose it reached.To conclude,the concentration of H+decreases by adding the corncob into the D-xylosesolution,which brings about a decrease of the loss rate of D-xylose.Hence the loss rate of D-xylose in D-xylose solutions with corncob is lower than that without corncob.

Fig.5.The loss rates of D-xylose in solutions with and without corncob in it at 103°C for 3 h:prepared solution without corncob;prepared solution with corncob;repeatedly using hydrolysate with corncob.
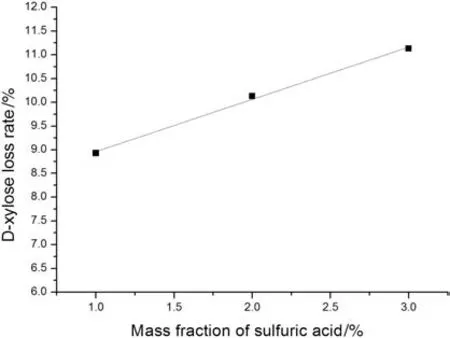
Fig.6.The loss rates of D-xylose in solutions with different mass fraction of sulfuric acid at 103°C for 3 h.
Fig.5 shows that the D-xylose loss rate in repeated-using corncob hydrolysate is lower than that of D-xylose preparation solution with corncob.Therefore,it can be deduced that the appearance of a certain substance inhibits the degradation of D-xylose in the repeated-using process of corncob hydrolysate.To verify this,the relations between D-xylose loss rate and reaction time under acidic conditions were studied.The solution with D-xylose(150 g·L−1)and sulfuric acid(2 wt%)was heated at 103°C,the variation of both the concentration and the loss rate of D-xylose over the reaction time is shown in Fig.7.It can be seen from Fig.7 that the concentration of the D-xylose gradually decreases as the solution starts to be heated.However,as the reaction time goes on,the D-xylose loss rate tends to be stable.This reflects that a certain kind of substance which inhibits the degradation of the D-xylose is generated during the process.Also,it demonstrates that a certain substance which helps inhibit the degradation of D-xylose arises in the repeated-using process of corncob hydrolysate.

Fig.7.The solution with D-xylose(150 g·L−1)and sulfuric acid(2 wt%)was heated at 103°C,the variation of concentrations of D-xylose and D-xylose loss rate of the solution versus the reaction time.
3.3.The analysis of D-xylose degradation
A certain inhibitor of D-xylosede gradation has been made in the process of repeated using corncob hydrolysate(see Section 3.2).The D-xylose loss rate tends to be stable with the increase of the heating time under acidic condition.In our analysis,the main reasons for that change can be divided into two aspects:(1)the dehydration process is accompanied in the degradation of D-xylose.The degradation products of D-xylose may increase water activity of the solution,the dehydration of D-xylose is thereby inhibiting.(2)The degradation product of D-xylosemay be combined with D-xylose molecules,which makes pro to nation of hydroxyl and intramolecular is omerization of D-xylose more difficult.
3.4.The additives effects to the D-xylose degradation
Conclusion can be made from Section 3.2 that the degradation of D-xylose will generate a substance which inhibits its degradation under acidic conditions.As the ingredients of D-xylose degradation products are complex,the effects of parts of degradation products on the D-xylose degradation were studied.The products include methylglyoxal,furfural,glycolaldehyde dimer,humic acid,etc.[21,22].Put different additives into D-xylose solutions with 134.0 g·L−1D-xylose and 2 wt%sulfuric acid(H+:0.408 mol·L−1)respectively,and heated up for 1.5 h at 103°C.Final concentrations and loss rates of D-xylose are listed in Table 3.The results indicate that there are no changes when methyl-glyoxal,furfural,glycolaldehyde dimer or humic acid was added into the solution,as compared to no added additive.Thus,it has little Influence on D-xylose degradation.Accordingly,the aforementioned substances are not D-xylose degradation inhibitors,i.e.,playing no significant role in inhibiting D-xylose degradation.
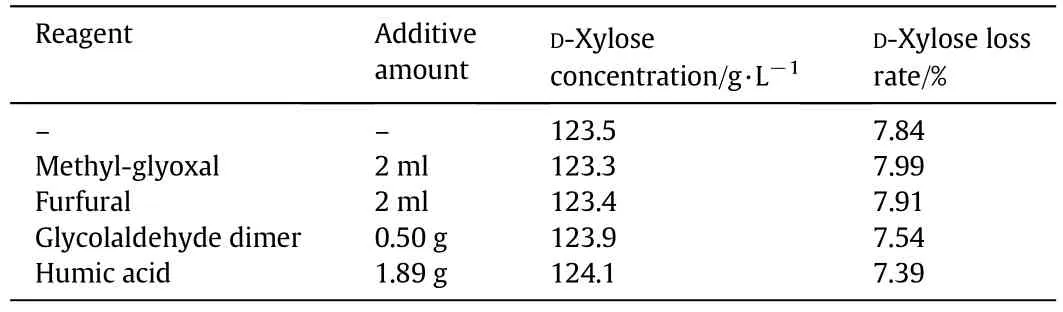
Table 3 The effect of additive on the concentration and loss rate of D-xylose in solution heated for 1.5 h at 103°C
4.Conclusions
•The newtechnology of producing D-xylose by repeatedly using corncob hydrolysate was established.The hydrolysate was separated after the hydrolyzed corncob with 2 wt%sulfuric acid aqueous solution.The acidity and volume of the hydrolysate were adjusted to be equal to the initial sulfuric acid aqueous solution,then it was used to hydrolyze corncob.The hydrolysate was repeatedly used for several times by this method.After the first hydrolysis,the concentration of D-xylose was 28.0 g·L−1,and the concentrations in the third,seventh,twelfth and fifteenth experiment were 76.0,134.2,184.5,and 196.7 g·L−1,respectively.The concentrations of acetic acid,i.e.,the by-product of the experiment,were 9.2,17.3,27.8,and 28.7 g·L−1,respectively.From the experiments,the conclusion can be made that repeated use of corncob hydrolysate contributes to an increase of D-xylose content in hydrolysate significantly.
•The loss rates of D-xylose in different solutions with and without corncob at 103°C for 3 h were compared.The results show that the loss rate of D-xylose in repeated-using hydrolysate with corncob is the lowest;while it goes higher after being heated;the loss rate of D-xylose in D-xylose preparation solution without corncob is the highest.Under acidic conditions,the concentration of the D-xylose decreases gradually as the solution starts to be heated;whereas the loss rate of D-xylose tends to be stable as the reaction time goes on.Considering these,the certain inhibitor of D-xylose degradation was generated in the repeated-using process of corncob hydrolysate.
•The effects of methyl-glyoxal,furfural,glycolaldehyde dimer and humic acid on D-xylose degradation under acidic conditions were investigated.The experiments prove that none of them play significant role on D-xylose degradation.
[1]H.Wang,X.Zhang,D.Wang,C.Cui,C.Gao,L.Wang,Y.Wang,Y.Bi,Estimation and utilization of corn cob resources in china,Chin.J.Agric.Res.Reg.Plann.37(2016)1–8.
[2]H.S.Jeong,S.K.Jang,H.Y.Kim,H.Yeo,J.W.Choi,I.G.Choi,Effect of freeze storage on hemicellulose degradation and enzymatic hydrolysis by dilute-acid pretreatment of Mongolian oak,Fuel 165(2015).
[3]H.Li,Q.Dai,J.Ren,L.Jian,F.Peng,R.Sun,G.Liu,Effect of structural characteristics of corncob hemicelluloses fractionated by graded ethanol precipitation on furfural production,Carbohydr.Polym.136(2016)203–209.
[4]C.Sánchez,L.Serrano,M.A.Andres,J.Labidi,Furfural production from corn cobs autohydrolysis liquors by microwave technology,Ind.Crop.Prod.42(2013)513–519.
[5]Y.Xing,B.Yan,Z.Yuan,K.Sun,Mesoporous tantalum phosphates:Preparation,acidity and catalytic performance for xylose dehydration to produce furfural,RSC Adv.6(2016).
[6]J.M.Dominguez,N.Cao,C.S.Gong,G.T.Tsao,Dilute acid hemicellulose hydrolysates from corn cobs for xylitol production by yeast,Bioresour.Technol.61(1997)85–90.
[7]L.Ribeiro,J.J.Delgado,J.Orfao,M.F.Pereira,A one-pot method for the enhanced production of xylitol directly from hemicellulose(corncob xylan),RSC Adv.(2016).
[8]H.Liu,W.Wang,L.Deng,F.Wang,T.Tan,High production of fumaric acid from xylose by newly selected strain Rhizopus arrhizus RH 7-13-9,Bioresour.Technol.186(2015)348–350.
[9]E.Lim,J.Y.Lim,J.H.Shin,P.R.Seok,S.Jung,S.H.Yoo,Y.Kim,D-Xylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet-induced obese mice,Nutr.Res.35(2015)626–636.
[10]Y.D.Hang,E.E.Woodams,Enzymatic production of reducing sugars from corn cobs,LWT Food Sci.Technol.34(2001)140–142.
[11]Y.D.Hang,E.E.Woodams,Enzymatic production of soluble sugars from corn husks,Lebensm.Wiss.Technol.32(1999)208–210.
[12]W.C.Ji,Z.M.Shen,Y.J.Wen,Hydrolysis of wheat straw by dilute sulfuric acid in a continuous mode,Chem.Eng.J.260(2015)20–27.
[13]O.Akpinar,O.Levent,S.Bostanci,U.Bakir,L.Yilmaz,The optimization of dilute acid hydrolysisof cotton stalk in xylose production,Appl.Biochem.Biotechnol.163(2011)313–325.
[14]M.Cuevas,Influence of solid loading on D-xylose production through dilute sulphuric acid hydrolysis of olive stones,Grasas Aceites 66(2015).
[15]B.P.Lavarack,G.J.Grif fin,D.Rodman,The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose,arabinose,glucose and other products,Biomass Bioenergy 23(2002)367–380.
[16]I.Ra fiqul,A.M.Sakinah,Design of process parameters for the production of xylose from wood sawdust,Chem.Eng.Res.Des.90(2012)1307–1312.
[17]A.Herrera,S.J.Téllezluis,J.A.Ramırez,M.Vázquez,Production of xylose from sorghum strawusing hydrochloric acid,J.Cereal Sci.37(2003)267–274.
[18]T.Zhu,P.Li,X.Wang,W.Yang,H.Chang,S.Ma,Optimization of formicacid hydrolysis of corn cob in xylose production,Korean J.Chem.Eng.31(2014)1624–1631.
[19]H.T.Tan,G.A.Dykes,T.Y.Wu,S.Leefong,Enhanced xylose recovery from oil palm empty fruit bunch by efficient acid hydrolysis,Appl.Biochem.Biotechnol.170(2013)1602–1613.
[20]J.Yan,Y.Hou,S.Ren,M.Niu,W.Wu,Two-step treatment of corn cob in H2O–SO2system,Ind.Eng.Chem.Res.53(2014)13256–13263.
[21]J.Huang,C.He,L.Wu,H.Tong,Thermal degradation reaction mechanism of xylose:a DFT study,Chem.Phys.Lett.658(2016)114–124.
[22]M.J.Antaljr,M.G.N.Richards,Mechanism of formation of 2-furaldehyde from D-xylose,Carbohydr.Res.217(1991)71–85.
 Chinese Journal of Chemical Engineering2018年1期
Chinese Journal of Chemical Engineering2018年1期
- Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
