Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
Xin Bei Tan ,Man Kee Lam ,*,Yoshimitsu Uemura ,Jun Wei Lim ,Chung Yiin Wong ,Keat Teong Lee
1 Chemical Engineering Department,Universiti Teknologi PETRONAS,Seri Iskandar 32610,Perak,Malaysia
2 Centre for Biofuel and Biochemical Research,Universiti Teknologi PETRONAS,Seri Iskandar 32610,Perak,Malaysia
3 Fundamental and Applied Science Department,Universiti Teknologi PETRONAS,Seri Iskandar 32610,Perak,Malaysia
4 School of Chemical Engineering,Universiti Sains Malaysia,Engineering Campus,Seri Ampangan,Nibong Tebal 14300,Pulau Pinang,Malaysia
1.Introduction
The exhaustion of non-renewable energy resources on the Earth has initiated researchers to explore novel and alternative energy sources[1].Due to the concern of energy crisis and the negative environmental effects of burning fossil fuels,renewable energy with low level of carbon dioxide emission is highly explored in recent years.Sustainable and renewable energy sources such as hydroelectricity,solar energy,wind energy,wave power,geothermal energy and tidal power are able to generate clean electricity,improve energy efficiency,and power up small cities[2].Biomass and combustible renewable are the alternative energy sources with the greatest potential to meet all the energy needs[1].Examples of the renewable and combustible sources are biodiesel and bio-oil,which can be derived from seed oil and lignocellulosic material,respectively[3].
Of late,renewable biodiesel has gained certain attention globally,mainly due to their significant advantages to the community and environment.Biodiesel is biodegradable,non-toxic and able to reduce air pollution index by decreasing the emission of greenhouse gases to the atmosphere.Biodiesel can be categorized into three generations:(1) first generation biodiesel is derived from edible oil(e.g.rapeseed oil palm oil and soybean oil),(2)second generation biodiesel is mainly produced from non-edible oil(e.g.Jatropha oil,Pongamia pinnata oil and waste frying oil),and(3)third generation biodiesel is synthesized from microbial lipid.There are several drawbacks of the first generation biodiesel,such as competition with food sources,require large arable land,high demand of water and fertilizer,deforestation,and biodiversity loss[3].Therefore,the second generation biodiesel from non-edible oil sources is highly recommended by researchers,however,the feedstock is still limited for commercial exploitation[3,4].
Recently,microalgae have been highlighted as the most feasible feedstock for third generation production of biodiesel.Microalgae are a group of microorganisms which have a simple cellular structure[4,5].Microalgae are able to manufacture and store triacylglycerols(TAGs)within their cells,especially under stress environment.In these circumstances,microalgae will stop dividing and the TGAs will be stored within their cells as a survival way to withstand these adverse conditions[6].TAGs are usually present in the cell in the form of storage lipids and can be converted to biodiesel[7].Lipid storage in microalgae cell happens when the accessible nutrients are exhausted from the culture medium and become the growth limiting factor.Meanwhile,microalgae are able to assimilate the carbon source and process to TAG within the existing cells without cell proliferation[8].Advantages of producing biodiesel from microalgae include requirement of a small land area,it does not vie with food sources,environmentally friendly,and fast reproduction compared to other oil-bearing crops[4].
2.Microalgae
Of late,microalgae have become an important feedstock for biodiesel production due to their high lipid content within their cells and they can be reproduced rapidly[9,10].Microalgae are the oldest life-forms on the Earth and they are ancient plants which are called thallophytes[4,11].The characteristics of the thallophyte plant are deficiency of roots,stems and leaves,have chlorophyll to carry out the photosynthesis and the cells are surrounded with reproductive cells pigment[11].Microalgae are usually a single cell,which allows them to acclimate to universal environmental conditions and flourish thereafter[4,11].In addition,scientists have found more than 40,000 different types of microalgae containing 20%to 50%of lipid content from their total biomass[11],which posed a good characteristic for biodiesel production.
Microalgae can either be autotrophic or heterotrophic[3,4,11].The autotrophic microalgae require only inorganic components such as CO2,salts and sunlight for cell development;while the heterotrophic microalgae require extra source of organic components and nutrients as an energy source[4].Besides,approximately 10%to 20%of the microalgae biomass is comprised of nutrient components,e.g.,nitrogen and phosphorus[11].Photosynthesis is an essential process for autotrophic microalgae to gain exogenous organic nutrients and survive.The solar energy and carbon dioxide from environment are absorbed by chloroplasts and transformed into adenosine triphosphate(ATP)and oxygen.This energy is then utilized in respiration to provide energy and assist in cell development[4,12].The following equation shows the overall process of photosynthetic reaction:
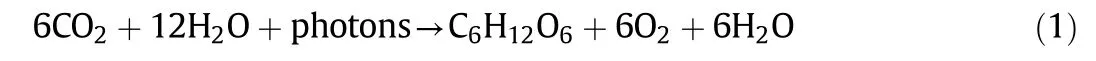
Microalgae are prokaryotic or eukaryotic photosynthetic microorganisms that can live under harsh condition due to their simple cellular structure[10].Table 1 shows the comparison between prokaryotic and eukaryotic cells[4,13].Prokaryotic cells do not consist of membranebound organelles and it is almost similar to bacteria or microalgae.Eukaryotic cells do have the membrane bound organelles which can carry out their function properly.The cells are able to concentrate and isolate enzymes and regulate chemical reactions efficiency.Besides,eukaryotic cells also can avoid harmful proteins and molecules in membrane-bound organelles and protect the rest of the cells from their harmful effects and allow them to grow and survive[3,4].

Table 1 Chemical composition of microalgae species[14]
Microalgae are essential producers in aquatic habitat and important organisms of the food web.Nowadays,microalgae are cultivated for many reasons,such as for sewage or waste treatment,controlling organic waste and bioconservation of solar energy[15].Besides,microalgae are one of the important feedstocks for the production of third generation biodiesel.The benefits of using microalgae as the biodiesel feedstock are as follows:no competition with food crops or agriculture land,low carbon emission and,high lipid and biomass productivity as compared with other terrestrial plants[16].
Microalgae require light,carbon dioxide,suitable temperature,p H and nutrient to carry out photosynthesis.Light supplies the energy whereas carbon dioxide provides the carbon source for biomass generation[17].Under lowlight intensity condition,microalgae will not reproduce as the photosynthesis rate may not reach the light saturation point.In contrast,if the light intensity is overloaded,it can reduce the microalgae growth rate due to photoinhibition effect[11,18].Besides,there is about 45%to 50%of carbon content in the microalgae biomass[11].Hence,additional carbon dioxide is needed to accelerate the growth of microalgae.Carbon sources for microalgae biomass production can be obtained from industrial exhaust gases that contain more than 15%CO2.This indicates that the microalgae are able to reduce the flue gases from industry and it also serves as an efficient way for carbon fixation[10].In the previous study,in order to produce 1 kg of microalgae biomass,1.8 kg of CO2was consumed.It also showed that microalgae not only contribute in carbon fixation but also can be a potential feedstock for biodiesel production[19].Meanwhile,CO2stream in microalgae cultivation plays a vital role as high concentration of CO2supply will be halted for carbon fixation and part of it will be released before being taken up by the microalgae[19].
In order to achieve ideal growth of microalgae,the cultivation temperature should be maintained at 20 °C to 30 °C.Normally,the microalgae biomass will increase when temperature rises.However,once the temperature attains optimal stage,the microalgae will stop growing resulting to biomass decrease[11].Besides,nutrients are heavily required to growmicroalgae.The nutrients can be supplied through wastewater,such as agricultural wastewater,industrial wastewater and livestock wastewater[19,20].In the process of microalgae cultivation,p H value in the cultivation medium is difficult to be fixed.Usually,the appropriate p H range for microalgae to grow is 6 to 8.Nevertheless,most of the microalgae species have their specific p H tolerance abilities[11].
Microalgae are able to produce different kinds of renewable biofuel[21],such as biomethane generation through anaerobic digestion process,[22]biohydrogen through photobiologically process under certain conditions and biodiesel through transesterification process.Microalgae cultivation for biofuel production is the current research focusasheavy dependenceon fossil fuelsincreasestheconcern of global energy security and pollution to the environment[23].
3.Nutrients to Grow Microalgae
3.1.Wastewater
Microalgae are the most essential primary producers in aquatic environments,including the food web based organisms.In fact,successful cultivation of microalgae is highly relying on the proper quantity and quality of nutrients[15].In industrial scale,large amounts of nutrients,such as nitrogen and phosphorus,are needed to cultivate microalgae for large scale biodiesel production.In order to achieve higher microalgae growth rate and bulk quantity production of biomass,inorganic fertilizers are usually used as the main nutrient source[24].However,inorganic nutrients are easily released to the water bodies and cause serious pollution,such as eutrophication[19].
Alternatively,wastewater is recommended as the nutrient source to cultivate microalgae because the wastewater contains high concentration of nitrogen and phosphorus.Researches have successfully demonstrated that nutrients are absorbed by microalgae for growing purpose.Besides,microalgae are also able to remove heavy and trace metals from wastewater[11,25].Using the wastewater to cultivate microalgae can improve the economics of microalgae biodiesel production,as well as improve the water quality.Normally,microalgae species which have high tolerance to contaminant,such as Chlorella and Dunaliella,are selected to grow in wastewater.These types of microalgae have more advantages,especially when cultivated in an open pond system that is usually exposed to unknown contaminates.However,care should be taken if the wastewater contains exceptionally high concentration of trace metals(e.g.copper)that can inhibit the growth of microalgae[26].Although the use of wastewater to cultivate microalgae in industrial scale for biodiesel production is still restricting,microalgae are still widely utilized for wastewater treatment[27].Wastewater treatment is essential to remove contaminants(e.g.,mineral nutrients)from wastewater[28].
Normally,wastewater treatment is divided into three classes which are physical,chemical and biological.Each of these wastewater treatment methods is integrated with different types of systems,such asprimary,secondary and tertiary wastewater systems in order to ensure that different stages of the contaminant are removed systematically[28].Fig.1 illustrates a typical wastewater treatment process.For physical treatment,mechanical force is used to remove the contamination,which serves as the most basic treatment method.Biological wastewater treatment is the most environmentally friendly and cheapest method.This process utilized microorganisms,such as microalgae and bacteria,to break down organic matters and remove mineral nutrients from wastewater[28].However,the main challenge of using wastewater as the nutrient sources to cultivate microalgae is the high contamination level in the wastewater and inconsistency of nutrient composition[9].
3.1.1.Municipal wastewater
Municipal wastewater is produced from different sections in a typical wastewater treatment plant[29].There are four kinds of wastewater streams,which are before primary sediment municipal wastewater,after primary sediment municipal wastewater,wastewater after activated sludge tank and concentrated municipal wastewater produced during activated sludge centrifuge,or known as “centrate”[30].Microalgae biomass productivity was greatly increased in the “centrate”stages of municipal wastewater because it contained high concentration of nitrogen and phosphorus compared with wastewater produced from other stages[30,31].Li et al.analyzed the feasibility of Chlorella sp.to growin “centrate”municipal wastewater and the findings showed that the microalgae could remove ammonia,nitrogen,phosphorus and chemical oxygen demand(COD)with efficiencies of 94%,89%,81%and 91%,respectively.Besides,Aslan and Kapdan[127]used Chlorella vulgaris to remove nitrogen and phosphorus in the municipal wastewater with removal efficiencies of 72%and 28%,respectively.All the above research works indicated that microalgae could be cultivated in municipal wastewater which is rich in nutrients and subsequently promote the growth of microalgae[30].
However,the concern of using municipal wastewater to cultivate microalgae is the presence of toxic compounds in the wastewater.Recent researches showed that cyanobacteria and eukaryotic microalgae were able to bio-trans form naphthalene to 1-napthol,4-hydrox-4-tetralone,cis-naphthalene dihydrodiol and trans-naphthalene dihydrodiol to low level of toxicity[28,32,33].Pinto et al.reported that Ankistrodesmus braunii and Scenedesmus quadricauda were able to reducephenol concentration to more than 50%in olivemill wastewater under thedark environment.In addition,Lima et al.also indicated that consortium of C.vulgaris and Chlorella pyrenoidosa was able to remove 50 mg·L−1·d−1nitrophenol within 5 days[28,34,35].Fig.2 shows the simplified process of wastewater treatment using microalgae cultivation system with multiple benefits of water and nutrient recycling,biofuel and by-product generation,wastewater remediation and reduction of greenhouse gas emission[30].
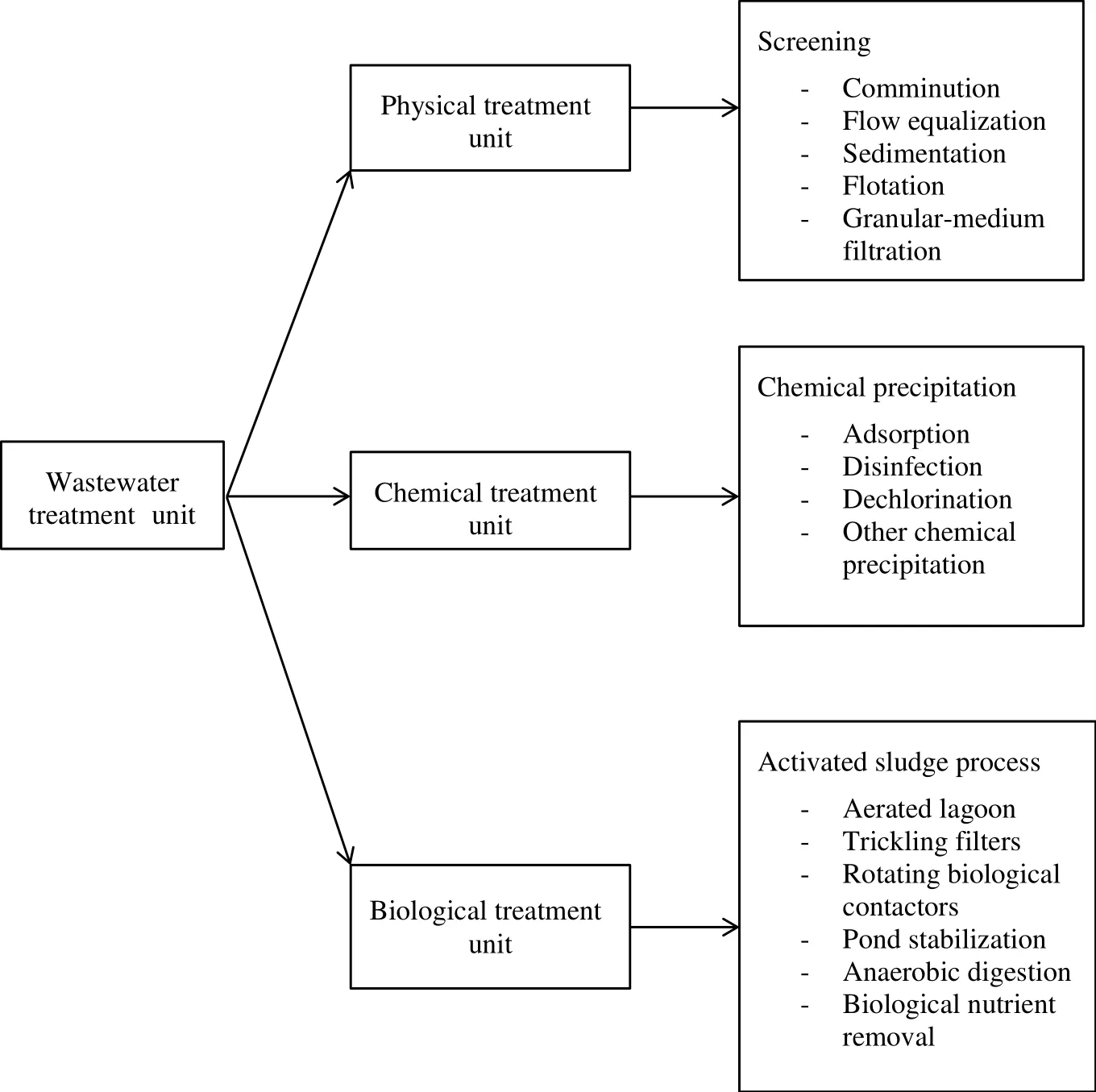
Fig.1.Wastewater treatment process[28].
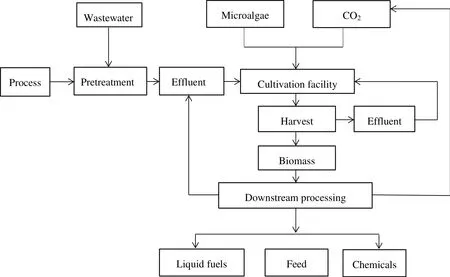
Fig.2.Advanced wastewater based microalgae cultivation system[30].
3.1.2.Industrial wastewater
Due to a variety of toxic components present in the industrial wastewater,it is foreseen to bring adverse effect on microalgae cultivation[30].Generally,the characteristic of the industrial wastewater relies on the nature of the product.Besides,the dissolved impurities can cause the wastewater to have unpleasant smell[28].Currently,researches are focusing on using microalgae to treat and eliminate the toxic components or heavy metals rather than to grow the microalgae for biodiesel production.Industrial wastewater is thought to be not suitable for microalgae cultivation because of the imbalance of nutrient composition and unknown compounds[30].Hence,utilization of industrial wastewater for microalgae cultivation in large scale is not favorable as compared with municipal or agriculture wastewater[36].Nevertheless,one of the recent studies showed that industrial wastewater produced from carpet mill could be used as nutrient sources to cultivate microalgae.The carpet mill wastewater had low concentration of toxin and adequate concentration of nitrogen and phosphorus to accelerate the growth of Botryococcus braunii,Chlorella saccharophila and Pleurochrysis carterae.All of the microalgae were able to survive and growusing the untreated carpet mill wastewater[27,30].The biomass productivity and lipid content produced by C.saccharophila were 23 mg·L−1·d−1and 18.10%respectively;whereas for Scenedesmus sp.the values were 126.54 mg·L−1·d−1and 12.80%respectively[30].Same observation was reported by Chinnasamy et al.,in which the native microalgae(fresh water microalgae such as B.braunii,Chlorella protothecoides,C.saccharophila,C.vulgaris and Spirulina maxima and marine microalgae such as Cricosphaera carterae,Dunaliella tertiolecta,Nannochloris oculata,Spirulina platensis,Tetraselmis suecica,Tetraselmis chui,Phaeodactylum tricornutum and P.carterae)were successfully grown in wastewater with 85%–90%of carpet industry effluents and 10%–15%of municipal sewage.The biomass productivity of microalgae was expected to be 9.2 to 17.8 t·ha−1·a−1with a lipid content of 6.82%[37].
Recently,Origin Oil Inc.discovered that some microalgae species were able to treat the wastewater from oil well(e.g.,water flooding and hydraulic cracking)[30].Based on the US Department Energy,huge amount of wastewater is generated daily from oil and gasonshore drilling.For example,every barrel of oil generated will generate 3 barrels of wastewater.In the United States,there are around 56 million wastewater produced daily during onshore oil and gas generation.If this amount wastewater or contaminated water is able to be utilized for microalgae cultivation,then around 0.7 million gallons of microalgae oil could be generated daily,assuming 1 g·L−1of microalgae biomass yield with 30%of lipid content[30].
In conclusion,the “ideal wastewater”that is able to supply optimum nutrient composition which is similar to commercial artificial media could be effectively used as alternative nutrient sources to cultivate microalgae.However,inconsistent concentration of nutrients in wastewater is usually the main factor affecting the microalgae biomass productivity and lipid yield.Hence,physical and chemical properties of wastewater must be analyzed prior to be used as nutrient sources to cultivate microalgae[30].
3.2.Compost and livestock waste
Composting is the process of organic matter decay or rotting by microorganisms under the controlled state.The organic matters include crop residues,animal waste and other suitable wastes.Compost contains an abundance of organic material which can improve the soil to withstand the stress and assist the crop to uptake more nutrients.Besides,the compost has active nutrient cycling capacity due to vigorous microbial activity[38,39].
There are two different composting processes,namely anaerobic composting and aerobic composting.In the anaerobic composting,oxygen is absent during the decomposition process and anaerobic microorganisms are producing intermediate components,such as methane,organic acids and others.All of these components are accumulated and unable to metabolize further.On the other hand,the aerobic composting process occurs in the presence of oxygen,in which an aerobic microorganism is able to decompose organic materials and generate carbon dioxide,ammonia and water.The intermediate compounds that are produced by aerobic microorganisms are able to be decomposed further and the resulting compost is unstable,causing phytotoxicity.The heat produced during aerobic decomposition will speed up the breakdown of protein,fats and carbohydrates[38].After the composting process,the compost is known as organic fertilizer[39,40].One of the studies reported by Kumaran et al.showed that 30%of the lipid can be extracted from C.vulgar is when the microalgae were cultivated by using waste-based compost under the following condition:50 ml of compost,p H of 9,and illuminated continuously for 15 days using fluorescent light[41].
Livestock waste is derived from animal manure and contains high level of nitrogen and phosphorus.Recent research works had shown that some microalgae species were more efficient at removing the mineral nutrients from livestock wastewater as compared with municipal wastewater[27,42].For example,B.braunii could grow efficiently in piggery wastewater,which contains 788 mg·L−1of NO3−ion,and are able to remove about 80%of the NO3−ion[27].In addition,Agwa and Abu,used poultry waste to cultivate Chlorella sp.The highest microalgae biomass produced was 2.5 mg·ml−1(dry matter)after culturing under sunlight,1.68 mg·ml−1under the aerated condition and 1.58 mg·ml−1under the unaerated conditions.The lipid yield from Chlorella sp.cultured under the sunlight was around 18.32%(w/w),11.19%(w/w)in the aerated condition and 7.17%(w/w)in the unaerated condition.The results showed that poultry waste could be utilized as an alternative nutrient source to grow Chlorella sp.[43].Fig.3 shows the process of using livestock waste for microalgae cultivation.
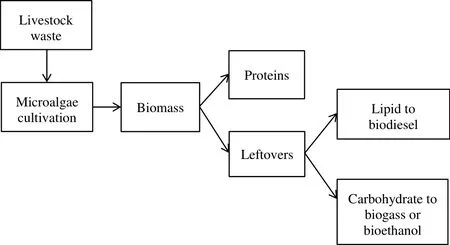
Fig.3.Process of using livestock waste compost for microalgae cultivation[15].
4.Microalgae Cultivation System
4.1.Raceway pond
Generally,there are two types of cultivation systems to growmicroalgae,which are open raceway pond and closed photobioreactor.The open raceway pond is more cost effective than a closed photobioreactor and it is more suitable to be used to remove nutrients from domestic wastewater[28].The open pond system for microalgae cultivation has been well established since the 1950s and it is classified into natural water pond and artificial pond[4].Generally,the raceway pond is constructed with a paddle wheel to ensure that the pond integrity is always maintained[21].Although the open raceway pond system has a lower capital cost for microalgae cultivation,the system is easily contaminated by other microorganisms[44].In addition,microalgae biomass productivity in this system is always low because the raceway ponds are poorly mixed and it is unable to sustain an optically dark zone[28].There are three main designs of the raceway pond:(1)endless loop to circulate microalgae continuously using paddle wheels;(2)rotating arm which is used for agitation purpose;and(3)inclined system which combines pumping and gravity flow[10,16].
The depth of the raceway pond is usually designed in a range of 0.2 m to 0.5 m to ensure high growth rate of microalgae[4,16].A paddle wheel will generate mixing in the raceway pond and the motion is monitored around the baffles located at the flow channel as shown in Fig.4.The raceway pond is made with concrete and sometimes lined with white plastic.During the day time,the cultivation medium is fed permanently to the raceway pond before circulation is started.The paddle wheel is operated continuously throughout the cultivation cycle to avoid sedimentation of microalgae and the broth is harvested behind the paddle wheel upon the completion of the circulation loop[4,23,45].One of the disadvantages of this system is the excessive evaporation of water to the atmosphere.Due to the consistent water loss,utilization of CO2by microalgaein this system is less efficient as compared with the photobioreactor.In addition,microalgae biomass yield is also affected by other microorganisms(e.g.,rotifer)that may contaminate or consume the microalgae[23,45].
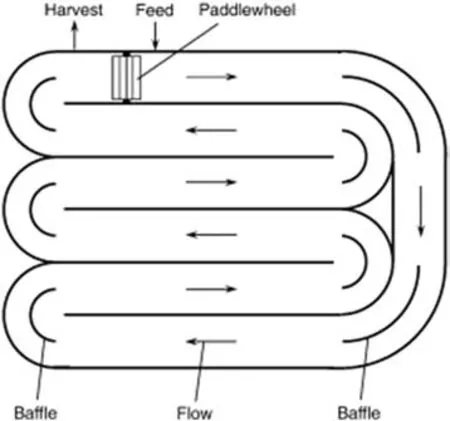
Fig.4.Raceway pond[23].
Hence,in the current design of the raceway pond,the pond is covered with greenhouse to prevent debris,pollution,water loss and rainfall.Besides,in order to allow the highest sunlight intensity to reach the raceway pond,water depth in the pond should not exceed 30 cm.The cascading system is better than a single channel raceway pond due to extension of retention time[16,28].Raceway-shape culture ponds are applied in Israel,US and China with an average microalgae biomass yield of 0.5 g·L−1[46].Besides,this system is also applied in Czech Republic to cultivate Chlorella with a maximum cell density of 10 g·L−1and areal productivity of 25 g·m−2[46–48].
4.2.Photobioreactor
A photobioreactor is a reactor which utilizes light as energy source to perform a photobiological reaction [10]. In microalgae cultivation, a photobioreactor is usually used to produce bulk quantity of microalgae biomass under controlled condition [23]. A photobioreactor allows mono-culture of microalgae for a long time with low risk of contamination as compared with open raceway ponds which are easily contaminated by microflora [4,28]. However, it should be noted that a photobioreactor is not an ideal option for commercial scale phytoremediation of wastewater because huge volumes of wastewater are involved daily. However,the wastewater could be concentrated and diverted to microalgae cultivation in a photobioreactor for further treatment. For example,organic pollutant and nutrients existing in piggery wastewater were successfully remediated by microalgae at a lower cost in a photobioreactor powered with solar energy [28].
From an economic perspective,a closed photobioreactor is much costly as compared with open raceway ponds.Nevertheless,the closed photobioreactor requires less agriculture land for microalgae cultivation.For example,microalgae species with high lipid content and cultivated under optimized environment in a photobioreactor was estimated to produce about 19000 to 57000 of microalgae oil per acre per year[14].This productivity of microalgae oil is more than 200 times as compared with oil-bearing crops[14].
4.2.1.Tubular photobioreactor
In the last 50 years,there are several designs of closed photobioreactors,such as tubular photobioreactor, flat-plate photobioreactor or bubble column photobioreactor[21].These photobioreactors are suitable to be used to cultivate environment-sensitive microalgae strains as closed construction is relatively easy to control the contamination.For a tubular photobioreactor,transparent straight glass or plastic tubes are arrayed horizontally,vertically,inclined or helix[4].The transparent tubes are known as a solar collector,which is used to receive sunlight as shown in Fig.6.The diameter of the solar collector should be less than 0.1 m to enable sufficient light penetration to the culture medium and hence,to promote high biomass productivity.The microalgae culture medium in the reservoir is circulated to the solar collector and returned back to the reservoir as shown in Fig.5[23].In addition,the microalgae cultivated in a tubular photobioreactor are recycled with a mechanical or airlift pump;thus,permitting carbon dioxide and oxygen to be exchanged in the cultivation medium while maintaining the mixing process[4,23].
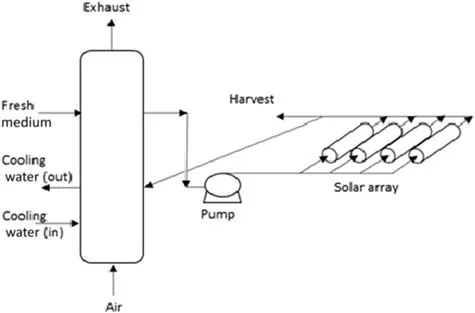
Fig.5.Tubular photobioreactor[23].
Oxygen is produced during the photosynthesis process.In the condition of high irradiance,oxygen is produced at a rate of 10 g·m−3·min−1.However,it should be noted that high dissolved oxygen level in water can inhibit the photosynthesis process.Besides,photooxidation may occur when the concentration of dissolved oxygen and sunlight intensity are high,which subsequently results in bleaching of microalgae cells.Therefore,it is suggested that the level of dissolved oxygen should not bemorethan 400%of air saturation.Hence,in the tubular photobioreactor design,the microalgae culture is constantly flowed back to the degassing column to remove the accumulated oxygen(Fig.6).Cooling water is pumped via a heat exchanger in the degassing column to control the water temperature[23].

Fig.6.Column photobioreactor[52].
Microalgae growth is always stagnated at light saturation due to the effect of photoinhibition[21].In order to accelerate the growth of microalgae,a photobioreactor is designed to disperse the light over high surface area,so that suitablelight intensity is exposed to microalgae cells.This can be attained by using a tubular photobioreactor in a fencelike structure.The fences are aligned in the direction of the north/south in order to avoid straight light striking on the surface of the photobioreactor[21].This allows the surface area of the photobioreactor to scale up to 10 times higher than the corresponding footprint area.Similar design is also applicable to a bubble column and flat-plate photobioreactor to fit at asuitableangle to receiveoptimum sunlight[21].
4.2.2.Flat plate photobioreactor
Another type of photobioreactor is a flat plate photobioreactor that is usually placed horizontally or vertically on the ground[48].The advantage of a flat plate photobioreactor is high surface area for illumination which would result to high cell density.The photobioreactor is manufactured with transparent materials to maximize the light exposure to microalgae.The thickness of the flat-plate is only a few millimeters to allow optimum radiance penetration to the microalgae cultivation.If 1.2 to 12.3 cm of light path is used in a flat-plate photobioreactor,around 20 g·L−1of cell concentration and 0.25–3.64 g·L−1·d−1of biomass yield could be attained under outdoor semi-continuous cultivation[48].Thus,a flat plate photobioreactor is highly recommended for mass cultivation of microalgae.In fact,a flat-plate photobioreactor tends to have lower accumulation of dissolved oxygen and higher photosynthetic efficiency as compared to a tubular photobioreactor[4].However,the flat-plate photobioreactor is difficult to sterilize uniformly by heat because of the large surface area to volume ratio[48].
4.2.3.Column photobioreactor
Acolumn photobioreactor provides efficient mixing,better gas–liquid mass transfer rate and excellent control of cultivation environment.The advantages of a column photobioreactor are low cost,compact and easy to operate.A column photobioreactor tended to produce higher microalgae biomass yield as compared to a horizontal photobioreactor due to the effect of hydrodynamics[49].The column reactor is in vertical direction,which allows gases to flow from the bottom and move up to the top of the column(Fig.6).The gas bubbles rise fast along the column and dispersed when they have arrived to the surface of the photobioreactor.Hence,the liquid will circulate throughout the column;upright close to the cylinder axis and downward close to the walls[50,51].This design could enhance gas–liquid exchange because of its inherent design.The rate of mass transfer is able to operate by controlling the residence time of the gas bubbles[50,51].
4.2.4.Hybrid system
The open raceway pond is a low cost and efficient way to cultivate microalgae,but the system is easily contaminated by other microorganisms;whereas the closed-photobioreactor is excellent in maintaining axenic culture,however the cost is exceptionally expensive.Hence,a combination of both systems or hybrid system is recommended to reduce the overall cost and to improve the microalgae biomass productivity[5,21].In this system,the closed-photobioreactor is used prior to the raceway pond to control or reducecontamination from other microorganisms and to encourage continuous cell production[4].Then,the dense microalgae cells are diverted to the open raceway pond for further cell reproduction and to attain bulk quantity of biomass.It should be noted that high inoculum concentration is required in the open raceway pond to ensure the cultivation is always dominated by microalgae population instead of other microorganisms.This hybrid system is currently used by Aquasearch,in which Haematococcus pluvialis is cultivated to produce astaxanthin[21].One part of the Aquasearch infrastructure is constructed with the closed-photobioreactor and another part is open ponds.H.pluvialis is reproduced in a photobioreactor under nutrient sufficient condition before the cultivation is diverted to the open pond system under nutrient deficient condition to promote astaxanthin generation[21,53].Besides cost saving and high biomass productivity,the hybrid system also has a lesser carbon footprint in the production line.It was proved that through utilization of a hybrid system in microalgae cultivation for biodiesel production,42%and 38%in global warming potential(GWP)and fossil energy requirements(FER),respectively,were saved by producing 1 ton of microalgae-derived biodiesel under the aegis of hypothetical downstream process compared to fossil-derived diesel[5].Table 2 shows the comparison between the raceway pond,photobioreactor and hybrid system.
5.Heterotrophic and Mixotrophic Cultivation
5.1.Heterotrophic cultivation
Heterotrophic cultivation used organic carbon(e.g.,glucose,acetate,crop flours,wastewater and others)as substrate to reproduce microalgae.The growth of the microalgae is independent of solar or light energy,which permits scale-up possibility due to the small surface to volume ratio of reactor[4,12,58,59].Heterotrophic growth is an aerobic process where assimilation of organic substrates produces energy via oxidative phosphorylation accompanied by oxygen consumption as the final electron acceptor[59].There are also other processes of metabolism used by microorganisms for aerobic glycolysis(breakdown of glucose),such as the Embden–Meyerh of pathway and the Pentose Phosphate path way[59].Under the condition of a dark heterotrophic system,glucose is mainly metabolized through the Pentose Phosphate pathway[59].This process has high cell production and promotes easy harvest due to higher cell density.However,care should be taken as heterotrophic cultivation might utilize more energy than autotrophic cultivation due to the requirement of organic carbon source.Miao and Wu reported that the lipid content of C.protothecoides was 4 times higher when cultivated under heterotrophic environment[4,60].Nevertheless,the main disadvantages of heterotrophic cultivation are:(1)limited types of microalgae strains that can grow heterotrophically,(2)expensive due to the addition of organic substrate(e.g.,glucose,nitrogen,phosphorus and trace elements),(3)easily contaminated by other microorganism and,(4)unable to generate light-induced metabolite[61].It was reported that Chlorella sp.cultivated under heterotrophic condition in a conventional stirred tank fermentor by using glucose as the organic substrate could attain 45 g·L−1of cell concentration and 20 g·L−1·d−1of biomass productivity[48,62].
5.2.Mixotrophic cultivation
Mixotrophic cultivation is a process wherein microbes could reproduce their cells under autotrophic and heterotrophic conditions.This indicates that light energy and organic carbon are not the limiting factors for the cell to reproduce as the microbes could utilize both energy sources to sustain their growth[4,12,58].For example,Chlorella was successfully cultivated under the mixotrophic condition.The ratios of carbon between the microalgae biomass and glucose are 577.4 kJ·C mol−1and 478.2 kJ·C mol−1,respectively[48].These energy ratios are not suf ficient to support the process of conversion of organic substrate to all carbon.Therefore,under heterotrophic condition,extra carbon is converted into carbon dioxide and the microalgae could further fix the carbon dioxide into glucose via photosynthesis.It was reported that the biomass productivity of Chlorella was around 127 g·m−2·d−1during daylight and 68.7 g·m−2·d−1at night under heterotrophic condition[48].In addition,a recent study also compared Spirulina sp.growth under photoautotrophic,heterotrophic and mixotrophic conditions[4].The study showed that mixotrophic culture reduced the effect of photoinhibition and improved the cell reproduction rate in comparison with other cultivation conditions.Successful mixotrophic microalgae cultivation allows the unification of both photosynthetic and heterotrophic compounds during diurnal cycle.In fact,this could further reduce biomass loss during dark respiration and organic matter use during cell reproduction under autotrophic condition[4].Although mixotrophic method attained higher biomass and lipid yields than phototrophic cultivation,the cost of the organic carbon substrate is appraised to be around 80%of the total cultivation medium cost.Thus,low cost organic sources needed to be intensively explored to reduce the overall processing cost under mixotrophic culturecondition[63,64].
6.Cultivation Methods
6.1.Batch cultivation
Currently,there are three main methods to cultivate microalgae,which are batch,fed-batch(semi-continuous)and continuous[65].Batch cultivation refers to the microalgae cultivated in a closed container or environment.In this process,all there quired materials(e.g.,microalgaeseed,water,nutrients)are introduced at the start of the process and additional nutrients are not added during the cultivation.Hence,the concentration of nutrients in the cultivation will be reduced along with the cultivation time[66].Microalgae cultivated in batch cultivation mode are currently technology-ready.There are a number of microalgae species cultivated via batch system,such as Chlorella zo fingiensis,C.vulgaris,Scenedesmus sp.,and Nannochloropsis salina[11].Factors that directly affect the microalgae growth,such as temperature,p H and dissolved oxygen,are constantly fixed in the batch cultivation[66].The main disadvantages of the batch cultivation are inconsistent irradiance due to cell self-shading effect and continuous nutrient consumption by microalgae[11,67].Fig.7 shows the batch cultivation photobioreactor.

Table 2 Comparison of raceway pond,closed photobioreactor and hybrid system[4,54–57]

Fig.7.Microalgae batch cultivation reactor[66].
6.2.Continuous cultivation
For continuous cultivation,nutrients are added constantly to the system,while the effluent which may consist of product or waste is constantly discharged[68,69].The continuous cultivation system starts with batch cultivation,in which the microalgae growth pattern follows the ordinary growth cycle.Then,new mediums or nutrients are introduced during the exponential growth phase,which allow the microalgae to reproduce continuously at an indeterminate rate[70].As a result,the volume of microalgae biomass will be greatly increased[70].The volumetric flow rates for in fluent and effluent streams are maintained once the stationary growth phase is achieved.However,the continuous cultivation system is not preferred in industrial scale as the system is easier to be contaminated by bacteriophage[68].In addition,the biomass yield produced is low at steady state operation[68].The advantages of this cultivation method are the nutrient concentration and p H can be easily manipulated[70].Fig.8 shows the continuous cultivation in a photobioreactor.
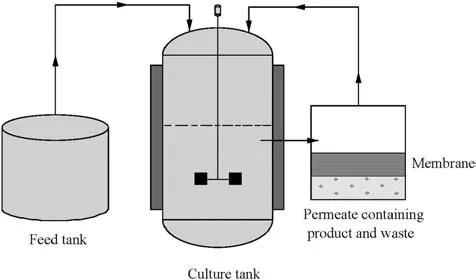
Fig.8.Microalgae continuous cultivation reactor[66].
6.3.Semi-continuous cultivation
Semi-continuous cultivation is a more practicable process than batch cultivation in which part of the cultivation medium is regularly discharged and the remaining culture is utilized as the seed to continue the cultivation.In addition,high inoculum ratio must be maintained at the moment of introducing a new cultivation cycle.The amount of fresh culture added into the cultivation is known as “renewal rate”and the biomass concentration is known as “blend concentration”[71].Semi-continuous cultivation process can be operated for multiple cycles,depending on the microalgae reproducibility.This will help increase the overall biomass productivity due to the elimination of lag phase,resulting in high biomass yield[68,72].Another advantage of using semi-continuous cultivation process is it will maintain the quantity of inoculum in the cultivation and ensure microalgae are always remained at high specific growth rate[71,73].Fig.9 shows the photobioreactor of a semi-continuous cultivation system.

Fig.9.Microalgae semi-continuous cultivation reactor[66].
Reichert et al.cultivated S.platens is in a two liter Erlenmeyer flask at 30°C(90 days,2500 lx of illumination)with 12 h of photoperiod via semi-continuous cultivation.From the study,0.5 g·L−1of microalgae biomass concentration and 50%v/v of renewal rate were attained with a specific growth rate of 0.111 per day and biomass productivity of 42.3 mg·L−1·d−1.This result was much better than batch cultivation,in which the biomass productivity was only 21.2 mg·L−1·d−1[71].Besides,Ashokkumar et al.carried out batch cultivation of B.braunii for 6 days then followed by semi-continuous cultivation.40%of the microalgae culture was removed every three days and similar water volume was added into the photobioreactor at the same time for a total cultivation time of 24 days.Consequently,33.8 g·L−1·d−1of microalgae biomass yield and 8.2–13 g·L−1·d−1of lipid productivity were achieved from each of the harvesting cycle.Similar study was also carried out by Tang et al.in which the biomass productivities of Chlorella minutissima and D.tertiolecta were 137 mg·L−1·d−1and 91 mg·L−1·d−1,respectively,for a total cultivation time of more than 3 months[11,74,75].Table 3 shows the comparison of advantages and disadvantages of batch,semi-continuous and continuous cultivation methods of microalgae[72].
7.Harvesting Process
Microalgae biomass usually contains high water content and hence,downstream processing is required to eliminate the water content.The main concern in the microalgae harvesting process is the cost and energy requirement to process bulk quantity of wet microalgae in an economical way.Therefore,microalgae strain selection is essential,since some of the microalgae are easy to be harvested than others.For instance,Spirulina have a long spiral pattern which allows them to attach together and be easily harvested through sedimentation.Although filtration is always used in laboratory to harvest microalgae,it will be an issue if it is used in large scale due to membrane clogging[21].In order to reduce processing of large amount of water,the harvesting method may include one or more stages of physical,chemical or biological way to attain the desired solid–liquid dissociation degree[10].
The existing technologies used to harvest microalgae biomass comprise centrifugation, flocculation, filtration,gravity sedimentation,flotation and electrocoagulation[4,76,77].Harvesting of microalgae biomass is usually divided into two stages,which are bulk harvesting and thickening.The aim of the bulk harvesting is to separate microalgae biomass from bulk suspension to achieve 2%to 7%of solid matter through flocculation, flotation or gravity sedimentation.On the other hand,the aim of the thickening is to enrich the slurry by using filtration and centrifugation.This process required higher energy input than bulk harvesting[4,76].The selection of harvesting method mainly relies on the characteristics of microalgae,such as density and size[4,76].
7.1.Chemical coagulation and flocculation
In chemical coagulation,chemical is used to aggregate the microalgae cells to cause flocculation to occur[76].Microalgae cells are negatively charged and will repel each other leading to cell suspension.However,by introducing metal salts,such as ferric chloride,the charges surrounding the microalgae cells are neutralized and result in cell aggregation[27,78].The coagulants are usually divided into two main types,which are inorganic and organic coagulants.Inorganic coagulants such as ferric or aluminum coagulant are always used to harvest Scenedesmus and Chlorella.Organic coagulants,such as chitosan,are bio-polymers that can enhance the microalgae floc size(more than 100 μm);thus,improving the settling efficiency.Up to now,the best coagulants to recover microalgae cells from water are cationic coagulant.Anionic and nonionic polyelectrolytes have failed to flocculate microalgae due to the repulsion present among charges or inadequate distance to bridge the particles.Nevertheless,Bilanovic et al.reported that flocculation by cationic polymer was prevented by high salinity of marine condition.High concentration of microalgae biomass in the cultivation is also able to assist flocculation due to regular cell-to-cell interaction[4,76,79,80].
7.2.Electrocoagulation
Electrocoagulation process comprises coagulant production by electrolytic oxidation of the sacrificial electrode,destabilization of particulate suspension and aggregation of destabilized particulate to become flocs[81].Azarian et al.reported that electrocoagulation was successfully applied to harvest microalgae from wastewater.However,this process needs to sacrifice electrode in comparison with chemical flocculation.Electrolytic flocculation is conducted when microalgae cells are moving to anode,neutralizing the microalgae surface charge and aggregating them.Poelman et al.indicated that 80%to 95%of the microalgae were successfully recovered when electrolytic flocculation was used[4,76,81,82].
7.3.Flotation
Flotation is one of the microalgae harvesting methods that utilized micro-air bubble without adding any chemical.In some cases,microalgae cells can float on the water surface naturally when the lipid content increased[4,83].Flotation is able to bind microalgae cells in less than 500 μm of diameter by collision and adhesion between the bubble and cells.Flotation can be classified into dissolved air flotation and dispersed air flotation,depending on the bubble size.Dissolved air flotation is the process where small bubbles are produced,with a mean size of 40 μm and ranging between 10 and 100 μm.Fine air bubbles are generated in the dissolved air flotation in which the process is depending on the solubility of air in water.There are three paths to achieve dissolved air flotation:(1)saturation at atmospheric pressure and flotation under vacuum condition,(2)saturation in static head with flowupward causing bubble formation(micro- flotation)and(3)saturation with pressure which is higher than atmospheric.Sometimes,dissolved air flotation utilized a flocculant and pressurized air bubbles(heat or entrained air)to accelerate the microalgae cells to aggregate and float to the water surface.Although dissolved air flotation is an efficient method,it is an energy intensive operation due to high pressure requirement[84].Onthe other hand, dispersed air flotation is the process where continuous air bubbles are generated through porous material. This process requires less energy input as compared with micro bubble production method.However,small bubbles are difficult to be generated [85].
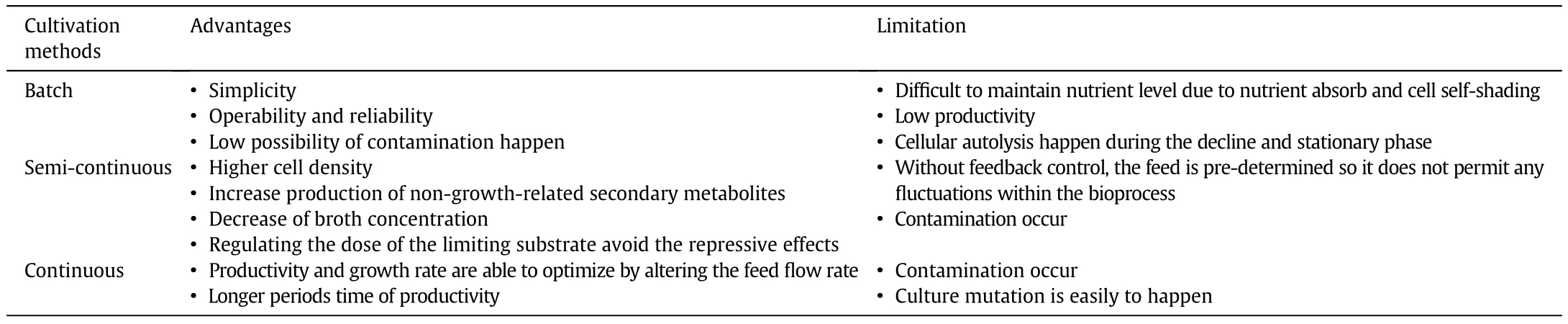
Table 3 Comparison of advantages and limitation of batch,semi-continuous and continuous cultivation methods[72]
7.4.Gravity sedimentation
Gravity sedimentation is a common practice to separate microalgae from water.Density and radius of microalgae cell are the main factors that affect the sedimentation rate of the microalgae.A lamella separator and sedimentation tank could be used to improve the microalgae harvesting efficiency[4].Usually,microalgae with high density are easy to be removed by gravity settling as compared with low density cells[4,76,86].
7.5.Filtration
A conventional filtration process is used to harvest bigger sizes of microalgae(usually more than 70 mm)such as Coelastrum and Spirulina[10,27].Micro filtration and ultra filtration are used to harvest smaller size of microalgae;however,these filtration methods require regular replacement of costly membrane[10,27].Tangential flow filtration is a high rate method to harvest fresh type microalgae and the result showed that Stephanodiscus hantzschii,S.Astraea,Cyclotella sp.,and Rhodomonas minuta could be recovered with efficiencies of 70%to 89%,respectively[87,88].Rossignol et al.reported that polymer membranes were effective in recovering marine microalgae species(e.g.Haslea ostrearia and Skeletonema costatum),but the performance relied on the hydrodynamic conditions,microalgae properties and the microalgae cell concentration[88,89].
7.6.Centrifugation
Centrifugation process is used to separate a mixture by utilizing centrifugal force.Nowadays,centrifugation method is widely utilized in harvesting the microalgae biomass.Under optimum condition,the maximum microalgae recovery rate could achieve up to 95%.The limitation of this method is high operation and maintenance costs,which are not suitable to be used for large scale[86,90].However,centrifugation is rather easy to clean and prevents bacteria contamination of raw product[10].
8.Drying
Drying of wet microalgae biomass is one of the important steps prior to biodiesel production.If high water content is present in the microalgae biomass,it can affect the biodiesel yield due to the reducing efficiency in biodiesel processing steps.One of the conventional and low cost methods to dry microalgae biomass is by utilizing natural sunlight.However,this method demands high drying surface,risk of matter loss and inconsistent sunlight throughout the year.Therefore,additional heat produced from fossil fuels is applied to dry the wet microalgae biomass constantly to ensure optimum dry microalgae production for every cultivation cycle.Usually,natural gas is used as fuel to dry the microalgae biomass.However,care should be taken as it may cause negative energy balance in producing microalgae biodiesel due to high energy input requirement[9].
Besides drying using sunlight,spray drying is usually used prior to extraction of high value-added product(e.g.food with high protein content).However,this method is costly and may destroy microalgae pigments.Although freeze drying is costly as well for large scale process,it is effective to disrupt the microalgae cells for high lipid extraction efficiency.This is because intracellular components like lipid are difficult to be extracted from wet microalgae biomass with chemical solvent in the condition without cell breaking down[4,91,92].
9.Lipid Extraction
After harvesting and drying of microalgae biomass,the next step is lipid extraction from the biomass for biodiesel production.The extraction step should be effective,does not destroy the lipid content and is easy to scale-up[93].The typical methods of oil extraction from microalgae biomass comprise cell drying,cell disruption through chemical,mechanical or biological ways,and lipid extraction by chemical solvent[94].Solvent extraction and supercritical fluid extraction are the common techniques for lipid extraction from microalgae biomass[95].The Folch method has been widely used in microalgae lipid extraction as it is a simple process of extracting total lipids from animal tissues.However,this process requires plenty amount of solvents and it also depends on the presence of mineral salts in the crude extract.In the absence of mineral salts,most of the acidic lipids are washed out during the washing step.Thus,the modified Bligh and Dyer method is always recommended for microalgae lipid extraction,however,it strongly depends on microalgae species[28].Besides,the solvent used should be low cost,non-volatile,non-toxic and non-polar[28].Prior to lipid extraction,the microalgae cell wall and cell membrane should be disrupted before extraction of intracellular lipid from microalgae biomass[96,97].This is because the microalgae cell wall is a strong modulator to protect the cell from degradation by any extraction operation.The presence of a rigid and complicated cell wall structure in microalgae will impact the oil extraction efficiency in the case of wet biomass as it is hard to liberate the extractable oil from the unyielding cell wall[99].Cell rupture can be completed via sonication,homogenization or freezing treatment[4,28].Then,the microalgae lipid can be further extracted by chemical cool press technology,enzymatic extraction or supercritical fluid extraction[96,97].
9.1.Chemical cool press
Chemical cool press is one of the simplest methods to extract lipid from microalgae biomass.A mechanical machine is used to press the microalgae biomass together with a chemical solvent,such as benzene,ether and hexane,in order to extract the microalgae lipid.The purpose of adding these chemical solvents is to break the microalgae cell wall,so that the lipid is easily liberated within the microalgae cells.Additional heat energy is not required in this process.Through this process,95%of oil content from total oil could be attained[97].The selection of solvent depends on the microalgae species,cost,volatility and toxicity[1,28,96].
9.2.Enzymatic extraction
Enzymatic extraction utilized enzyme to break the microalgae cell wall in order to extract the entrapped lipid[97].The enzymatic extraction method could be applied on wet microalgae biomass without drying process.Liang et al.reported that enzyme extraction assisted with sonication could enhance the microalgae lipid extraction efficiency(49.8%of lipid was attained)[98].Zuorro et al.reported that there were around 90%of lipids recovered from Nannochloropsis cells by using cellulase and mannanase under the following conditions:temperature 53°C,p H 4.4,pre-treatment time 210 min,cellulose dosage 13.8 mg·g−1and mannanase dosage 1.5 mg·g−1[99].In another study,it was found that by mixing cellulase and two hemicellulases,37.2 g of lipid per 100 g of dry microalgae(Nannochloropsis sp.)biomass was able to be recovered[100].
9.3.Supercritical extraction
Supercritical extraction is a simple extraction method that is able to extract 70 to 75%of microalgae oils[14].It utilized high pressure and temperature to rupture the microalgae cell membrane[16,96].In the process of CO2supercritical extraction,the CO2is lique fied and heated up until it reaches a supercritical state.The liquefied CO2acts as the main solvent in the oil extraction process[14].There are several advantages of using supercritical fluid extraction to obtain microalgae lipids,such as high selectivity,shorter extraction time and use of non-toxic solvents.Santana et al.reported the performance of supercritical CO2extraction of lipid from B.braunii for biodiesel production.The optimum condition for the supercritical extraction was at 22 to 25 MPa at 50°C.The result showed that lipid extraction efficiency decreased with increasing temperature and increased with increasing pressure.High recovery of polyunsaturated fatty acids and essential fatty acids was observed from this study[101].
10.Biodiesel and Bio-oil Production Process
10.1.Transesterification
Biodiesel which is derived from triglycerides through transesterification reaction has been focused during the past decade due to the characteristics of being renewable, biodegradable and nontoxic. The process usually involved short-chain alcohol (e.g. methanol) and alkaline (base) catalyst to achieve high conversion of triglycerides to fatty acid methyl esters(FAME) at a short reaction time.
The vegetable and microalgaeoil is not suitable to be directly used in diesel engine due to its relatively high viscosity than gasoline and diesel[102].Microalgae oil is extremely high in viscosity,hence conversion to lower molecular weight components in the structure of FAME(biodiesel)is required through transesterification reaction[28].However,the process is reversible and thus,excess of alcohol is required to shift equilibrium to product side and to enhance the rate of reaction.There are three reversible stages involved in the transesterification reaction,in which triglycerides are converted to diglycerides,diglycerides are converted to monoglycerides and monoglycerides are converted to ester[103].
A catalyst can be acid,base or enzyme.The most common catalyst used in transesterification process is a base catalyst,such as NaOH and KOH.The catalyst can be carried out at low temperature and pressure with 98%of biodiesel conversion[83].However,the base catalyst will be inhibited if the oil contains high concentration of free fatty acids(2%to 5%),in which saponification reaction will occur instead of transesterification reaction[104].Application of a base catalyst in transesterification of microalgae was successfully demonstrated by Ahmad et al.[31].The study revealed that 95%of biodiesel yield can be attained from C.vulgaris by using sodium methoxide as a catalyst at a temperature of 160°C and reaction time of 51 min[105].
An acid catalyst is not favorably used in transesterification reaction due to its corrosive property and long reaction time[83].An acid catalyst will only be used if the oil contains high level of free fatty acid in transesterification process.In order to increase the reaction rate of transesterification by using an acid catalyst,high temperature and pressure are required to attain high biodiesel yield.However,the process would not be economically feasible when applied in large scale[28,106].El-Shimi et al.[128]showed that 84.7%of biodiesel was successfully produced from non-edible S.platensis through acid catalysts via in-situ transesterification at the following conditions:80 ml methanol volumes,8 h reaction time and 65°C reaction temperature with continuous stirring at 650 r·min−1.
There are some limitations in the process of chemical catalyzed transesterification,such as requirement of high energy input,difficult to remove the catalyst from product,alkaline water produced from washing step(purification)required further treatment,saponification may occur and difficult to recover the glycerol[28].In order to mitigate the problem of high free fatty acid level,the microalgae lipid is firstly pre-treated with an acid catalyst before a base catalyst is used in the process of transesterification.However,the main limitation of this two-step reaction is that an extra base catalyst is required to neutralize an acid catalyst which increases the production cost of biodiesel[9].Currently,transesterification process is further modified and reaction rate is enhanced with a sequencing baf fled reactor,microwave irradiation and cavitation or ultrasonic effect[10].
Since last ten years,enzymes have been used in the process of transesterification to produce biodiesel.The advantages of using enzyme in the reaction are easy recovery of product without producing a by product,insensitivity to free fatty acid and reusability enzymes can be reused[107].Other advantages include moderate reaction conditions,less energy requirement and low ratio of alcohol and oil[108,109].However,it is not economically feasible at the moment for large scale utilization due to high enzyme cost and the reaction is usually not complete[28,110].Extracellular and intracellular lipases are the main biocatalyst[83].Enzymatic catalyzed transesterification is usually conducted in the form of immobilized lipase,whole cell catalyst and liquid lipase mediated[28].Immobilized extracellular lipase is more suitable in transesterification compared with free lipase due to its high stability state and potential for reutilization.However,the activity of the immobilized lipase is always reduced due to the contamination by glycerol(by-product of transesterification).Purification of the contaminated immobilized lipase isacomplicated processand thus,hindersthe continuity of large scale biodiesel production[28,111].On the other hand,whole cell biocatalysis used intracellular lipases and this method is able to reduce lipase cost by neglecting the requirement of isolation,purification and immobilization[112].Besides,liquid lipase mediated catalysis is used because it is easy to prepare and cheaper than immobilized lipase.Liquid lipase mediated catalysis is usually carried out in water consisting an operation which helps regain the enzyme activity[28].Xiong et al.reported that C.protothecoides produced 98%of biodiesel by using 30%Candida sp.lipase[83,113].Similar study was also reported by Li et al.that transesterification of C.protothecoides achieved 98.15%of oil conversion at the following reaction condition:oil catalyzed by immobilized lipase from Candida sp.75%lipase(12000 U·g−1,based on lipid quantity),3:1 molar ratio of methanol to oil(batch-fed for three times),and 12 h reaction time[83,114].
10.2.Pyrolysis
Pyrolysis of microalgae biomass to generate liquid fuel has been developed since the year 1986[28].Report has shown that catalytic pyrolysis is able to produce gasoline with high content of aromatic hydrocarbon and octane number.Pyrolysis is a decomposition of biomass process in the absence of oxygen at high temperature.Pyrolysis of biomass can generate charcoal,organic liquid,acetic acid,acetone,methanol and gases.Slow pyrolysis generates high charcoal ingredient while fast pyrolysis can generate 60 to 75 wt%of liquid bio-oil,15 to 25%solid charcoal and gases[28,115].
In contrast with slow pyrolysis,fast pyrolysis is relatively a new technology that generates bio-oil in the condition of absent air at atmospheric pressure,low temperature but high heating rate,and short gas residence time to break the biomass into short chain molecules[102].Pyrolysis is first utilized for generation of bio-oil from lignocellulosic biomass.However,this process is more appropriate for microalgae biomass due to its lower operating temperature with high quality of bio-oil[102].Furthermore,pyrolysis of lignocellulosic biomass is more expensive than microalgae.As compared to lignocellulosic biomass,microalgae consisted of high cellular lipid,dissolvable polysaccharides and protein that are easier to be pyrolyzed to bio-oils.Specifically,microalgae biomass that has high lipid content could generate higher bio-oil yield with improved heating value[28,115].One of the recent studies showed successful fast pyrolysis of microalgae in the fluidize bed reactor.The experiments were conducted at a temperature of 500 °C with a heating rate of 60 °C·s−1,a sweep gas(N2) flow rate of 0.4 m3·h−1and a vapor residence time of 2–3 s.The result showed that 18%and 24%of bio-oil yield was produced from C.protothecoides and Microcystis aeruginosa,respectively.The result was considered good compared with pyrolysis of wood[116].

Table 4 Comparison of different methods to produce microalgae biodiesel and bio-oil[125,126]
10.3.Thermo-chemical liquefaction
Thermal decomposition of microalgae biomass is able to produce different ranges of energy fuel depending on the operating temperature.Gasification generates syngas by partial oxidation of microalgae biomass at high temperature.Microalgae biomass reacts with oxygen and water to become steam to generate syngas,which consists of carbon dioxide,hydrogen gas,nitrogen and methane.The syngas can be burned directly as fuel.
Thermo-chemical liquefaction of microalgae biomass is used to convert wet biomass to bio-oil at low operating temperature and high pressure in the presence of a catalyst[28,117].One of the thermochemical processes is hydrothermal liquefaction,in which the wet microalgae biomass is converted to smaller molecular weight components by using high water activity under sub-critical condition.This method allows direct conversion of wet microalgae biomass to bio-oil without the need of drying the microalgae.This could subsequently reduce the overall processing cost and energy input.Besides,this process can be applied to lipid-extracted microalgae biomass(residue),in which the remaining carbohydrates and protein can be further converted to bio-oil[118–120].It was reported that the microalgae(C.vulgaris)and cyanobacteria(Spirulina)with different biochemical contents were liquefied under hydrothermal conditions at 350°C around 200 bar in water,1 mol·L−1Na2CO3and 1 mol·L−1formic acid.The bio-crude yields were 5 to 25 wt%which were higher than the lipid content of the microalgae[118,121].In another study by Dote et al.and Minowa et al.,dried biomass from B.braunii and D.tertiolecta,were used as the feedstock in hydrothermal liquefaction process in abatch reactor.Theres ult showed that 37 wt%and 57 wt%–64 wt%of bio-oil could be derived from B.braunii and D.tertiolecta,respectively[122–124].Recently,this approach is modified by using wet microalgae biomass.For example,Yu et al.[129]reported that wet C.pyrenoidosa,was converted through hydrothermal liquefaction into bio-crude oil.Carbon recovery,nitrogen and energy in the bio-crude oil fraction increased with the increase of reaction temperature and retention time.The highest energy recovery of bio-crude oil was 65.4%at 280°C with a 120 min retention time.Table 4 summarized the advantages and limitations of different technologies to convert microalgae biomass to biodiesel and bio-oil.
As revealed,there are several technical methods for microalgaederived biodiesel and bio-oil such as direct use and blending,microemulsions,thermal cracking(pyrolysis),and transesterification.All of these methods have their own advantages and drawbacks which are shown in Table 4.Among these methods,transesterification route is performing better than other methods and it produces more desired end product(biodiesel)[125].
11.Conclusions
Microalgae biodiesel isthecurrent research niche that addresses the concerns of energy and environmental sustainability.Developing a large-scale microalgae cultivation system is urgently required to explore the economic feasibility of this new feedstock for alternative energy and valuable by-product production.Microalgae cultivation requires huge amount of water and chemical nutrients,high energy input,and high initial cost,and is prone to contamination.In the future,a hybrid semi-continuous cultivation system(combination of airlift tubular photobioreactors and raceway ponds)seems to be the best among the other cultivation methods as this system is able to lower the production cost,prevent culture contamination,and produce high biomass yield.It is foreseen that the advancement of current biodiesel conversion technology will help accelerate the microalgae biodiesel production,especially in terms of energy and cost saving.
Acknowledgments
Technical support from Green Technology Mission Oriented Research and Centre for Biofuel and Biochemical Research(CBBR)of UTP is highly appreciated.
[1]A.Piasecka,I.Krzemińska,J.Tys,Physical methods of microalgal biomass pretreatment,Int.Agrophys.28(3)(2014)341–348.
[2]S.L.Homsy,Processing Algal Biomass to Renewable Fuel:Oil Extraction and Hydrothermal Liquefaction,University of Dayton,2012.
[3]A.Pandey,Biofuels:Alternative Feedstocks and Conversion Processes,Academic Press,Amsterdam,2011.
[4]L.Brennan,P.Owende,Biofuels from microalgae—A reviewof technologies for production,processing,and extractions of biofuels and co-products,Renew.Sust.Energ.Rev.14(2)(2010)557–577.
[5]V.O.Adesanya,E.Cadena,S.A.Scott,A.G.Smith,Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system,Bioresour.Technol.163(2014)343–355.
[6]R.R.Narala,S.Garg,K.K.Sharma,S.R.Thomas-Hall,M.Deme,Y.Li,P.M.Schenk,Comparison of microalgae cultivation in photobioreactor,open raceway pond,and a two-stage hybrid system,Front.Energy Res.4(2016)29.
[7]L.Barsanti,P.Gualtieri,Algae:Anatomy,Biochemistry,and Biotechnology,CRC Press,United States,2014.
[8]C.C.Fu,T.C.Hung,J.Y.Chen,C.H.Su,W.T.Wu,Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction,Bioresour.Technol.101(22)(2010)8750–8754.
[9]M.K.Lam,K.T.Lee,Microalgae biofuels:A critical reviewof issues,problems and the way forward,Biotechnol.Adv.30(3)(2012)673–690.
[10]T.M.Mata,A.A.Martins,N.S.Caetano,Microalgae for biodiesel production and other applications:a review,Renew.Sust.Energ.Rev.14(1)(2010)217–232.
[11]L.Zhu,Microalgal culture strategies for biofuel prod uction:A review,Biofuels Bioprod.Biore fin.9(6)(2015)801–814.
[12]G.A.Lutzu,Analysis of the Growth of Microalgae in Batch and Semi-batch Photobioreactors,Universita'degli Studi di Cagliari,2012.
[13]R.D.Storey,Textbook errors&misconceptions in biology:Cell structure,Am.Biol.Teach.(1990)213–218.
[14]A.Demirbas,M.F.Demirbas,Importance of algae oil as a source of biodiesel,Energy Convers.Manag.52(1)(2011)163–170.
[15]F.M.F.de Oliveira,M.C.B.Crispim,Compost extract as a nutrient source for algal cultures,J.Aquac.Res.Dev.4(5)(2013)100–195.
[16]G.Dragone,B.D.Fernandes,A.A.Vicente,J.A.Teixeira,Third Generation Biofuels From Microalgae,Current Research,Technology and Education Topics in Applied Microbiology and Microbial Biotechnology,2,2010 1355–1366.
[17]M.Arnold,Sustainable Algal Biomass Products by Cultivation in Waste Water Flows,VTT Technology,147,2013,pp.1–84.
[18]T.Ren,Primary Factors Affecting Growth of Microalgae Optimal Light Exposure Duration and Frequency,Master Thesis,Iowa State University,2014.
[19]N.Abdel-Raouf,A.Al-Homaidan,I.Ibraheem,Microalgae and wastewater treatment,Saudi J.Biol.Sci.19(3)(2012)257–275.
[20]O.K.Dalrymple,T.Halfhide,I.Udom,B.Gilles,J.Wolan,Q.Zhang,S.Ergas,Wastewater use in algae production for generation of renewable resources:a review and preliminary results,Aquat.Biosyst.9(1)(2013)1–11.
[21]P.M.Schenk,S.R.Thomas-Hall,E.Stephens,U.C.Marx,J.H.Mussgnug,C.Posten,O.Kruse,B.Hankamer,Second generation biofuels:high-efficiency microalgae for biodiesel production,Bioenergy Res.1(1)(2008)20–43.
[22]M.Hannon,J.Gimpel,M.Tran,B.Rasala,S.May field,Biofuelsfrom algae:Challenges and potential,Biofuels 1(5)(2010)763–784.
[23]Y.Chisti,Biodiesel from microalgae,Biotechnol.Adv.25(3)(2007)294–306.
[24]M.K.Lam,K.T.Lee,Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production,Appl.Energy 94(2012)303–308.
[25]K.Larsdotter,Microalgae for Phosphorus Removal From Wastewater in a Nordic Climate,Ph.D Thesis,KTH,2006.
[26]K.K.Sharma,H.Schuhmann,P.M.Schenk,High lipid induction in microalgae for biodiesel production,Energies 5(5)(2012)1532–1553.
[27]J.K.Pittman,A.P.Dean,O.Osundeko,The potential of sustainable algal biofuel production using wastewater resources,Bioresour.Technol.102(1)(2011)17–25.
[28]I.Rawat,R.R.Kumar,T.Mutanda,F.Bux,Dual role of microalgae:Phycoremediation of domestic wastewater and biomassproduction for sustainable biofuelsproduction,Appl.Energy 88(10)(2011)3411–3424.
[29]Y.Li,Y.F.Chen,P.Chen,M.Min,W.Zhou,B.Martinez,J.Zhu,R.Ruan,Characterization of a microalga Chlorella sp.well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production,Bioresour.Technol.102(8)(2011)5138–5144.
[30]W.Zhou,P.Chen,M.Min,X.Ma,J.Wang,R.Grif fith,F.Hussain,P.Peng,Q.Xie,Y.Li,Environment-enhancing algal biofuel production using wastewaters,Renew.Sust.Energ.Rev.36(2014)256–269.
[31]F.Ahmad,A.U.Khan,A.Yasar,The potential of Chlorella vulgaris for wastewater treatment and biodiesel production,Pak.J.Bot.45(S1)(2013)461–465.
[32]C.E.Cerniglia,C.Van Baalen,D.T.Gibson,Metabolism of naphthalene by the cyanobacterium Oscillatoria sp.,strain JCM,Microbiology 116(2)(1980)485–494.
[33]C.E.Cerniglia,D.T.Gibson,C.Van Baalen,Algal oxidation of aromatic hydrocarbons:Formation of 1-naphthol from naphthalene by Agmenellum quadruplicatum,strain PR-6,Biochem.Biophys.Res.Commun.88(1)(1979)50–58.
[34]G.Pinto,A.Pollio,L.Previtera,M.Stanzione,F.Temussi,Removal of lowmolecular weight phenols from olive oil mill wastewater using microalgae,Biotechnol.Lett.25(19)(2003)1657–1659.
[35]S.A.Lima,P.M.Castro,R.Morais,Biodegradation of p-nitrophenol by microalgae,J.Appl.Phycol.15(2–3)(2003)137–142.
[36]C.Zheng,A.Li,L.Zhao,X.Zhou,Z.Fu,Treatment Technologies for Organic Wastewater,INTECH Open Access Publisher,Croatia,2013.
[37]S.Chinnasamy,A.Bhatnagar,R.W.Hunt,K.Das,Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications,Bioresour.Technol.101(9)(2010)3097–3105.
[38]R.Misra,R.Roy,H.Hiraoka,On-farm Composting Methods,UN-FAO,Rome,Italy,2016.
[39]L.Zhu,E.Hiltunen,Z.Li,Continuous production of high-value products,biodiesel and biogas from microalgae cultivated with livestock waste compost:A feasible study,J.Environ.Sci.4(1)(2015)1–4.
[40]E.Bomans,K.Fransen,A.Gobin,J.Mertens,P.Michiels,H.Vandendriessche,N.Vogels,Addressing phosphorus related problems in farm practice,Soil Sci.15(1)(2006)23–44.
[41]K.Kumaran,M.K.Lam,X.B.Tan,Y.Uemura,J.W.Lim,C.G.Khoo,K.T.Lee,Cultivation of Chlorella vulgaris using plant-based and animal waste-based compost:A comparison study,Procedia Eng.148(2016)679–686.
[42]J.M.Martín-Marroquín,D.Hidalgo,Livestock waste:fears and opportunities,Environment,Energy and Climate Change,Springer 2014,pp.341–373.
[43]O.Agwa,G.Abu,Utilization of poultry waste for the cultivation of Chlorella sp.for biomass and lipid production,Int.J.Curr.Microbiol.Appl.Sci.3(8)(2014)1036–1047.
[44]M.Balat,Potential alternatives to edible oils for biodiesel production—A reviewof current work,Energy Convers.Manag.52(2)(2011)1479–1492.
[45]P.Collet,A.Hélias,L.Lardon,M.Ras,R.A.Goy,J.P.Steyer,Life-cycle assessment of microalgae culture coupled to biogas production,Bioresour.Technol.102(1)(2011)207–214.
[46]A.Richmond,E.Lichtenberg,B.Stahl,A.Vonshak,Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways,J.Appl.Phycol.2(3)(1990)195–206.
[47]I.Šetlík,V.Šust,I.Málek,Dual purpose open circulation units for large scale culture of algae in temperate zones.I.Basic design considerations and scheme of a pilot plant,Arch.Hydrobiol.Algol.Stud.(1970)111–164 Supplement Volumes.
[48]Y.K.Lee,Microalgal massculture systems and methods:their limitation and potential,J.Appl.Phycol.13(4)(2001)307–315.
[49]A.S.Miron,A.C.Gomez,F.G.Camacho,E.M.Grima,Y.Chisti,Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae,J.Biotechnol.70(1)(1999)249–270.
[50]J.Merchuk,F.Garcia-Camacho,E.Molina-Grima,Photobioreactor design and fluid dynamics,Chem.Biochem.Eng.Q.21(4)(2007)345–355.
[51]N.Chavada,Optimization of Vertical Photobioreactors,University of Dayton,2012.
[52]A.Sánchez Mirón,F.Garcia Camacho,A.Contreras Gomez,E.M.Grima,Y.Chisti,Bubble-column and airlift photobioreactors for algal culture,AICh E J.46(9)(2000)1872–1887.
[53]Y.Chisti,Biodiesel from microalgae beats bioethanol,Trends Biotechnol.26(3)(2008)126–131.
[54]A.Burns,Photobioreactor Design for Improved Energy Ef ficiency of Microalgae Production,Master Thesis,California Polytechnic State University,2014.
[55]J.W.Richardson,M.D.Johnson,X.Zhang,P.Zemke,W.Chen,Q.Hu,A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability,Algal Res.4(2014)96–104.
[56]T.Cai,S.Y.Park,Y.Li,Nutrient recovery from wastewater streams by microalgae:Status and prospects,Renew.Sust.Energ.Rev.19(2013)360–369.
[57]A.Demirbas,Use of algae as biofuel sources,Energy Convers.Manag.51(12)(2010)2738–2749.
[58]X.Zhang,J.Rong,H.Chen,C.He,Q.Wang,Current status and outlook in the application of microalgae in biodiesel production and environmental protection,Front.Energy Res.2(32)(2014)1–15.
[59]O.Perez-Garcia,Y.Bashan,Microalgal heterotrophic and mixotrophic culturing for bio-re fining:from metabolic routes to techno-economics,Algal Biore fineries,Springer 2015,pp.61–131.
[60]X.Miao,Q.Wu,Biodiesel production from heterotrophic microalgal oil,Bioresour.Technol.97(6)(2006)841–846.
[61]O.Perez-Garcia,F.M.Escalante,L.E.de-Bashan,Y.Bashan,Heterotrophic cultures of microalgae:metabolism and potential products,Water Res.45(1)(2011)11–36.
[62]Y.K.Lee,Commercial production of microalgae in the Asia-Pacific rim,J.Appl.Phycol.9(5)(1997)403–411.
[63]A.P.Abreu,B.Fernandes,A.A.Vicente,J.Teixeira,G.Dragone,Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source,Bioresour.Technol.118(2012)61–66.
[64]A.Bhatnagar,S.Chinnasamy,M.Singh,K.Das,Renewable biomass production by mixotrophic algae in the presence of various carbon sources and waste waters,Appl.Energy 88(10)(2011)3425–3431.
[65]O.M.Amaya,M.T.C.Barragán,F.J.A.Tapia,Microbial biomass in batch and continuous system,Biomass Now—Sustainable Growth and Use,2013.
[66]H.C.Lim,H.S.Shin,Fed-batch Cultures:Principles and Applications of Semi-batch Bioreactors,Cambridge University Press,United States,2013.
[67]R.A.Andersen,Algal Culturing Techniques,Academic Press,2005.
[68]T.Egli,Microbial growth and physiology:a call for better craftsmanship,Front.Microbiol.6(2015)287.
[69]P.Dorka,Modelling Batch and Fed-batch Mammalian Cell Cultures for Optimizing MAb Productivity,UWSpace,2007.
[70]P.A.Hoskisson,G.Hobbs,Continuous culture—Making a comeback?Microbiology 151(10)(2005)3153–3159.
[71]C.D.C.Reichert,C.O.Reinehr,J.A.V.Costa,Semicontinuous cultivation of the cyanobacterium Spirulina platensis in a closed photobioreactor,Braz.J.Chem.Eng.23(1)(2006)23–28.
[72]B.McNeil,L.M.Harvey,Practical Fermentation Technology,Wiley Online Library,2008.
[73]J.Fábregas,M.Patiño,E.D.Morales,A.Dominguez,A.Otero,Distinctive control of metabolic pathways by Chlorella autotrophica in semicontinuous culture,Can.J.Microbiol.42(11)(1996)1087–1090.
[74]V.Ashokkumar,E.Agila,P.Sivakumar,Z.Salam,R.Rengasamy,F.N.Ani,Optimization and characterization of biodiesel production from microalgae Botryococcus grown at semi-continuous system,Energy Convers.Manag.88(2014)936–946.
[75]H.Tang,M.Chen,K.Simon Ng,S.O.Salley,Continuous microalgae cultivation in a photobioreactor,Biotechnol.Bioeng.109(10)(2012)2468–2474.
[76]C.Y.Chen,K.L.Yeh,R.Aisyah,D.J.Lee,J.S.Chang,Cultivation,photobioreactor design and harvesting of microalgae for biodiesel production:A critical review,Bioresour.Technol.102(1)(2011)71–81.
[77]S.O.Gultom,B.Hu,Reviewof microalgae harvesting via co-pelletization with if lamentous fungus,Energies 6(11)(2013)5921–5939.
[78]E.Molina,J.Fernández,F.Acién,Y.Chisti,Tubular photobioreactor design for algal cultures,J.Biotechnol.92(2)(2001)113–131.
[79]D.Vandamme,Flocculation Based Harvesting Processes for Microalgae Biomass Production,UGent,2013.
[80]D.Bilanovic,A.Andargatchew,T.Kroeger,G.Shelef,Freshwater and marine microalgae sequestering of CO2at different C and N concentrations—Response surface methodology analysis,Energy Convers.Manag.50(2)(2009)262–267.
[81]G.Azarian,A.Mesdaghinia,F.Vaezi,R.Nabizadeh,D.Nematollahi,Algae removal by electro-coagulation process,application for treatment of the effluent from an industrial wastewater treatment plant,Iran.J.Public Health 36(4)(2007)57–64.
[82]E.Poelman,N.De Pauw,B.Jeurissen,Potential of electrolytic flocculation for recovery of micro-algae,Resour.Conserv.Recycl.19(1)(1997)1–10.
[83]H.Taher,S.Al-Zuhair,A.H.Al-Marzouqi,Y.Haik,M.M.Farid,A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology,Enzym.Res.2011(2011)1–25.
[84]J.H.Shah,A.Deokar,K.Patel,K.Panchal,A.V.Mehta,A comprehensive overviewon various method of harvesting microalgae according to Indian perspective,International Conference on Multidisciplinary Research&Practice,International Journal of Research and Scientific Innovation(IJRSI)India,2014,pp.313–317.
[85]J.Hanotu,H.Bandulasena,W.B.Zimmerman,Micro flotation performance for algal separation,Biotechnol.Bioeng.109(7)(2012)1663–1673.
[86]G.Shelef,A.Sukenik,M.Green,Microalgae Harvesting and Processing:A Literature Review,Technion Research and Development Foundation Ltd.,Haifa(Israel),1984.
[87]B.Petrusevski,G.Bolier,A.Van Breemen,G.Alaerts,Tangential flow filtration:A method to concentrate freshwater algae,Water Res.29(5)(1995)1419–1424.
[88]M.Al Hattab,A.Ghaly,A.Hammoud,Microalgae harvesting methods for industrial production of biodiesel:Critical reviewand comparative analysis,J.Fundam.Renew.Energy Appl.5(2015)1–26.
[89]N.Rossignol,L.Vandanjon,P.Jaouen,F.Quemeneur,Membrane technology for the continuous separation microalgae/culture medium:compared performances of cross- flow micro filtration and ultra filtration,Aquac.Eng.20(3)(1999)191–208.
[90]L.Gouveia,Microalgae as a Feedstock for Biofuels,Springer,2011.
[91]L.Lin,Microstructure of spray-dried and freeze-dried microalgal powders,Food Struct.4(2)(1985)17.
[92]H.Desmorieux,N.Decaen,Convective drying of Spirulina in thin layer,J.Food Eng.66(4)(2005)497–503.
[93]S.J.Kumar,G.V.Kumar,A.Dash,P.Scholz,R.Banerjee,Sustainable green solvents and techniques for lipid extraction from microalgae:a review,Algal Res.21(2017)138–147.
[94]Y.Pan,M.A.Alam,Z.Wang,D.Huang,K.Hu,H.Chen,Z.Yuan,One-step production of biodiesel from wet and unbroken microalgae biomass using deep eutectic solvent,Bioresour.Technol.238(2017)157–163.
[95]R.Halim,M.K.Danquah,P.A.Webley,Extraction of oil from microalgae for biodiesel production:a review,Biotechnol.Adv.30(3)(2012)709–732.
[96]D.Özçimen,M.Ö.Gülyurt,B.İnan,Algal biore finery for biodiesel production,Biodiesel— Feedstocks,Production and Applications,InTech 2012,pp.25–57.
[97]Y.Wang,Microalgae as the Third Generation Biofuel:Production,Usage,Challenges and Prospects,Master Thesis,Uppsala University,2013.
[98]K.Liang,Q.Zhang,W.Cong,Enzyme-assisted aqueous extraction of lipid from microalgae,J.Agric.Food Chem.60(47)(2012)11771–11776.
[99]A.Zuorro,G.Maffei,R.Lavecchia,Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae,J.Taiwan Inst.Chem.Eng.67(2016)106–114.
[100]A.Zuorro,S.Miglietta,G.Familiari,R.Lavecchia,Enhanced lipid recovery from Nannochloropsis microalgae by treatment with optimized cell wall degrading enzyme mixtures,Bioresour.Technol.212(2016)35–41.
[101]A.Santana,S.Jesus,M.Larrayoz,Supercritical carbon dioxide extraction of algal lipids for the biodiesel production,Procedia Eng.42(2012)1755–1761.
[102]G.Huang,F.Chen,D.Wei,X.Zhang,G.Chen,Biodiesel production by microalgal biotechnology,Appl.Energy 87(1)(2010)38–46.
[103]G.Anastopoulos,Y.Zannikou,S.Stournas,S.Kalligeros,Transesterification of vegetable oils with ethanol and characterization of the key fuel prop erties of ethyl esters,Energies 2(2)(2009)362–376.
[104]R.Banani,S.Youssef,M.Bezzarga,M.Abderrabba,Waste frying oil with high levels of free fatty acids as one of the prominent sources of biodiesel production,J.Mater.Environ.Sci.6(4)(2015)1178–1185.
[105]F.Ahmad,A.U.Khan,A.Yasar,Transesterification of oil extracted from different species of algae for biodiesel production,Afr.J.Environ.Sci.Technol.7(6)(2013)358–364.
[106]J.Sebastian,C.Muraleedharan,A.Santhiagu,A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production,Cogent Eng.3(1)(2016)1178370.
[107]M.K.Lam,K.T.Lee,A.R.Mohamed,Homogeneous,heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil(waste cooking oil)to biodiesel:a review,Biotechnol.Adv.28(4)(2010)500–518.
[108]A.Sivasamy,K.Y.Cheah,P.Fornasiero,F.Kemausuor,S.Zinoviev,S.Miertus,Catalytic applicationsin theproduction of biodiesel from vegetableoils,ChemSusChem 2(4)(2009)278–300.
[109]A.A.Refaat,Different techniques for the prod uction of biodiesel from waste vegetable oil,IJEST 7(1)(2010)183–213.
[110]J.Yan,X.Zheng,L.Du,S.Li,Integrated lipase production and in situ biodiesel synthesis in a recombinant Pichia pastoris yeast:an efficient dual biocatalytic system composed of cell free enzymes and whole cell catalysts,Biotechnol.Biofuels 7(1)(2014)1.
[111]S.Sharma,S.S.Kanwar,Organic solvent tolerant lipases and applications,Sci.World J.2014(2014)1–15.
[112]G.Jin,T.J.Bierma,Whole-cell Biocatalysts for Producing Biodiesel From Waste Greases,Illinois Sustainable Technology Center,Champaign,IL,2010.
[113]W.Xiong,X.Li,J.Xiang,Q.Wu,High-density fermentation of microalga Chlorella protothecoides in Bioreactor for microbio-diesel production,Appl.Microbiol.Biotechnol.78(1)(2008)29–36.
[114]X.Li,H.Xu,Q.Wu,Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors,Biotechnol.Bioeng.98(4)(2007)764–771.
[115]M.I.Jahirul,M.G.Rasul,A.A.Chowdhury,N.Ashwath,Biofuels production through biomass pyrolysis— A technological review,Energies 5(12)(2012)4952–5001.
[116]X.Miao,Q.Wu,C.Yang,Fast pyrolysis of microalgae to produce renewable fuels,J.Anal.Appl.Pyrolysis 71(2)(2004)855–863.
[117]C.Tian,B.Li,Z.Liu,Y.Zhang,H.Lu,Hydrothermal liquefaction for algal biore finery:a critical review,Renew.Sust.Energ.Rev.38(2014)933–950.
[118]P.Biller,A.Ross,Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content,Bioresour.Technol.102(1)(2011)215–225.
[119]T.M.Brown,P.Duan,P.E.Savage,Hydrothermal liquefaction and gasification of Nannochloropsis sp,Energy Fuel 24(6)(2010)3639–3646.
[120]S.Sawayama,T.Minowa,S.-Y.Yokoyama,Possibility of renewable energy production and CO2mitigation by thermochemical liquefaction of microalgae,Biomass Bioenergy 17(1)(1999)33–39.
[121]Y.Yang,C.Feng,Y.Inamori,T.Maekawa,Analysis of energy conversion characteristics in liquefaction of algae,Resour.Conserv.Recycl.43(1)(2004)21–33.
[122]Y.Dote,S.Sawayama,S.Inoue,T.Minowa,S.-y.Yokoyama,Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction,Fuel 73(12)(1994)1855–1857.
[123]D.C.Elliott,T.R.Hart,A.J.Schmidt,G.G.Neuenschwander,L.J.Rotness,M.V.Olarte,A.H.Zacher,K.O.Albrecht,R.T.Hallen,J.E.Holladay,Process development for hydrothermal liquefaction of algae feedstocks in a continuous- flowreactor,Algal Res.2(4)(2013)445–454.
[124]T.Minowa,S.-y.Yokoyama,M.Kishimoto,T.Okakura,Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction,Fuel 74(12)(1995)1735–1738.
[125]F.Ma,M.A.Hanna,Biodiesel production:A review,Bioresour.Technol.70(1)(1999)1–15.
[126]B.J.Gallagher,The economics of producing biodiesel from algae,Renew.Energy 36(1)(2011)158–162.
[127]S.Aslan,I.K.Kapdan,Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae,Ecol.Eng 28(1)(2006)64–70.
[128]H.I.El-Shimi,N.K.Attia,S.T.El-Shetawy,G.I.El-Diwani,Biodiesel production from spirulina-platensis microalgae by in-situ transesterification p rocess,J.Sustain.Bioenergy Syst 3(3)(2013)224–233.
[129]G.Yu,Y.Zhang,L.Schideman,T.L.Funk,Z.Wang,Hydrothermal liquefaction of lowlipid content microalgae into bio-crude oil,Trans.ASABE 54(1)(2011)239–246.
 Chinese Journal of Chemical Engineering2018年1期
Chinese Journal of Chemical Engineering2018年1期
- Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
- Study on extraction kinetics of α-cyclopentylmandelic acid enantiomers with hydroxyethyl-β-cyclodextrin as chiral selector☆
