Distinguished discriminatory separation of CO2 from its methane-containing gas mixture via PEBAX mixed matrix membrane
Pouria Abbasszadeh Gamali ,Abbass Kazemi *,Reza Zadmard ,Morteza Jalali Anjareghi 3,Azadeh RezakhaniReza Rahighi 4,Mohammad Madani
1 Nanotechnology Research Center,Research Institute of Petroleum Industry(RIPI),P.O.Box:14665-1998,Tehran,Iran
2 Chemical&Chemical Engineering Research Center of Iran,P.O.Box:14335-186,Tehran,Iran
3 College of Environment,Standard Square,P.O.Box:31746-118,Karaj,Iran
4 Department of Research and Development,Sharif Ultrahigh Nanotechnologists(SUN)Company,P.O.Box:13488-96394,Tehran,Iran
5 Nanotechnology Department,Agricultural Biotechnology Research Institute of Iran(ABRII),AREEO,3135933151 Karaj,Iran
1.Introduction
Acid gases such as carbon dioxide in natural gas cause serious damages to re finery equipment and pipelines due to their corrosive nature.Carbon dioxide reduces the heating value of natural gas as well.Removal of CO2from natural gas is of extreme importance in gas industry considering economic and HSE aspects[1].Recent advances in membrane technology make it as a convenient method of CO2separation comparing to the known conventional methods[2–6].MMMs consist of an organic polymer as continuous phase and an inorganic particle as dispersed phase.Although,these membranes offer promising separation properties,their brittleness and high production cost remain as very important challenges for their development and manufacturing[7].Different types of fillers have been tried to be dispersed into the polymer matrix including zeolites,carbon molecular sieves,C60,nanoparticles(e.g.TiO2,SiO2,and Zn O),carbon nanotubes,ionic liquids,and metal–organic frameworks.Incorporation of inorganic fillers within the polymer matrix can have a positive impact on gas selectivity of MMMs due to the increase in diffusion channels and selective gas permeation through their corresponding effective surface.Investigation of CO2/H2separation by MMMs(cellulose acetate–silicates)has been performed in UOP[8].As reported,incorporation of silicates into a polymer matrix can elaborate selectivity factor of CO2/H2in differential pressure of 50 psi(1 psi=6.895 k Pa)and 50/50 feed mixture of CO2/H2.
Poly(ether-block-amide)is a block copolymer which is known under the trade name of PEBAX.This copolymer includes linear chains of rigid polyamide(PA)segments as impermeable phase and a permeable phase of flexible polyether(PEO)segments.The polyether soft segment is responsible for gas permeation across the membrane.The polyamide hard segment gives an appropriate mechanical strength to the polymer membrane and also is known for its tendency to be crystallized.Further,temperature of glass transition for two phases of polyether and polyamide are below and above room temperature,respectively[9].Properties of PEBAX copolymers including chemical resistance to solvents,mechanical strength as well astheir considerable permeability property particularly for polar gases e.g.CO2and H2S have been extensively investigated by scientists.Kim and Lee employed a sol–gel method for preparation of PEBAX/silica hybrid membranes.The enhanced permeation properties were attributed to the interactions between CO2molecules and SiO2nanoparticles within the polymer matrix[10].Zoppi et al.reported hybrid films of poly(ethylene oxideb-amide-6)containing sol–gel silicon or titanium oxide as inorganic fillers.In the case of films containing silicon oxide,PEBAX/TEOS(50/50 wt%),the selectivity value increased,whereas the selectivity value of polymer film of PEBAX/TiOP(50/50 wt%)became lower than that of pure PEBAX membrane[11].Car et al.[12]haveintensely studied gas transport properties of PEBAX membranes.Their results showed PEBAX/PEG membranes loaded with different amounts of liquid additives could significantly increase CO2flux at the pressures between 0.5 and 2 MPa.Separation measurements of CO2/CH4(50/50 vol%)mixed gas indicated that the rate of CO2flux was enhanced.As authors claimed,the increase in CO2flux is related to swelling behavior of blend membranes.CO2/CH4selectivity for PEBAX/PEG blend membranes at 0.8 MPa is similar to the pristine polymer.Moreover,at pressures higher than 0.8 MPa,CO2/CH4selectivity declined,the behavior which can be more enhanced in PEBAX/PEG membranes with PEG contents of higher than 50 wt%.
In this work,we have conducted our research on the CO2/CH4separation by using MMMs.The goal was to study the impact of using silica nanoparticles on permeation of single and mixed gases through PEBAX/FS membranes.Highly CO2selective composite membranes were obtained when silica nanoparticles were homogeneously integrated within the polymer matrix.The permeation behavior of CO2and CH4through the pristine and mixed matrix membranes was demonstrated in that the permeability values of both penetrants are usually increased when the feed pressure is elevated from 1 to 3.5 MPa.However,the increase in the permeability of CO2is well above that of CH4so that a distinguished increase in both of ideal and binary gas selectivity of CO2/CH4can be approached.Furthermore,in the case of mixed matrix membrane containing 4.6 wt%FS,a unique selectivity increase was observed which was well above that of the pristine membrane that is the only detectable gas in the permeate side of the membrane was CO2as detected by gas chromatography(GC).As a result,the permeability properties of CO2/CH4gas pair were well beyond the Robeson upper bound[13,14].This approach will pave the way towards large scale gas sweetening process based on membrane technology.
2.Experimental
2.1.Materials
PEBAX® MH 1657 was supplied by Arkema(French).PEBAX® MH 1657 is a thermoplastic elastomer consisting of 60 wt%poly(ethylene glycol)as polyether phase and 40 wt% poly Poly[imino(1-oxohexamethylene)]as polyamide 6 phase(see Table 1).The hydrophobic FS under the trade name of AEROSIL R972 was provided by Degussa(Germany)(see Table 2).Silicananoparticles were synthesized based on hydrophilic silica.The nanoparticles were treated by dimethyldichlorosilane(DDS)as a hydrophobic surface modifier.
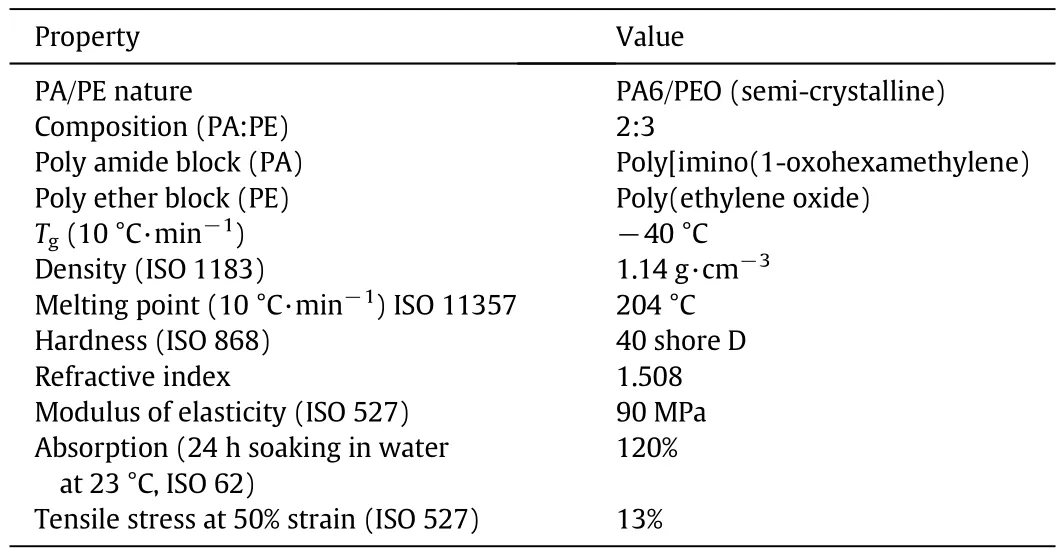
Table 1 Physical properties of PEBAX MH 1657
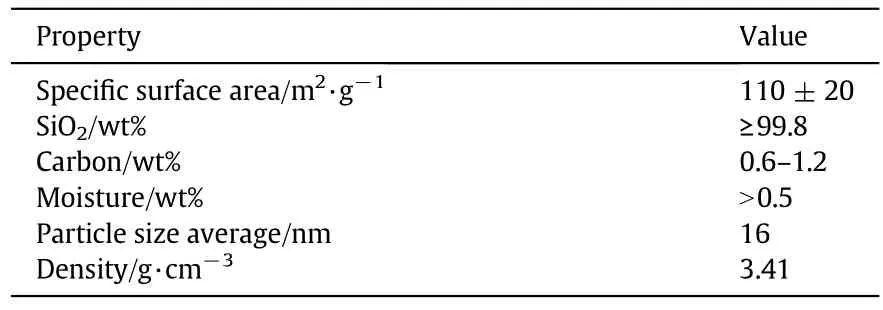
Table 2 Physical properties of FS
2.2.Stepwise membrane preparation
MMMs based on PEBAX 1657 copolymer were prepared by a solution-casting technique.The thermal treatment of PEBAX pellets and FS was performed at 80 °C and 120 °C for 6 h and 24 h,respectively.
In order to prepare the FS containing membrane,an appropriate amount of FS was dispersed into a solvent mixture of ethanol/water(70/30 wt%)by continuous stirring(24 h at room temperature).Then,the suspension solution was sonicated for 1 h to obtain a uniform and homogenous suspension.Next,the suspension solution was warmed up to 80°C and PEBAX pellets were added under continuous stirring at re flux conditions at the desired temperature for 5 h.To eliminate gas bubbles trapped in the suspension,it was settled for 15 min at 80°C.To obtain a homogenous mixture,it was filtered and then poured into a Petri dish.The dried samples were obtained by placing them in a vacuum oven at 80°C for 24 h.The samples were detached by hexane solvent.
3.Membrane Characterization
To visualize fine morphology of top surface and cross-section of untreated PEBAX and PEBAX/FS membranes,a field emission scanning electron microscope(FESEM),MIRA TESCAN model at 15 kV voltage was applied.
The morphological structure of the PEBAX matrix containing silica nanoparticles was studied by Scanning Atomic Force Microscopy(AFM)as well.The qualitative surface scanning was done by using a universal scanning probe microscope(SPM)analyzer,Solver P-47H AFM(NT-MDT)model in the non-contact mode and at room temperature.
Characterization of functional groups of MMMs and pristine membrane was provided by FTIR analysis.The spectra of the samples were recorded in the range of 500–4000 cm−1wavenumber,by using an FTIR spectrometer,ISS-88 Bruker model.
The experimental density of the samples was determined by a standard buoyancy method.The experimental density for each sample was determined using the following equation[15,16]:

where ρfand ρ0(g·cm−3)present the densities of sample in dried form and auxiliary liquid,respectively.wtairis the mass of the dry sample at room temperature and the mass of the sample in the auxiliary liquid is shown as wtairand wtax,respectively.By using the volumefraction and known densities of PEBAX and silica nanoparticles,the theoretical density for PEBAX containing silica can be calculated according to the additive model which is expressed as the following equation:

where ρ1(2.1 g·cm−3)andρ2(1.02 g·cm−3)are theoretical densities of PEBAX and FS,respectively.ϕ1and ϕ2are the volume fractions of PEBAX and FS in the as-prepared MMMs.
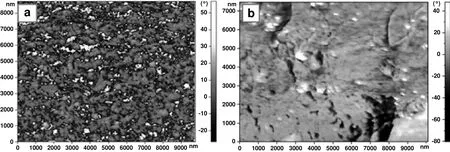
Fig.1.Surface scan of(a)pristine PEBAX,(b)PEBAX/4.6 wt%FS by AFM method.
As for the membranes with nonporous structure,the rate of gas transport through the polymer membrane is controlled by the so called unoccupied volume(free volume)which exists in between polymer chains.In the case of semi-crystalline polymers,gas molecules can be only diffused across amorphous regions while crystalline regions act as a barrier against gas diffusion.By using the results of determined densities,fractional free volume(FFV)can be estimated by Bondi's group contribution method as follows:

where V(cm3·g−1)presents the specific volume of the polymer chains and V0(cm3·g−1)stands for occupied volume by polymer chains at 0 K which is equal to 1.3Vvdwof the repeat units[16].
4.Gas Permeation Measurements
4.1.Gas permeation set-up
Gas permeation through membranes was measured by constant pressure,variable volume technique.The digital flow meter was used to measure gas flow rates and a GC analyzer was responsible for the gas flow analysis of permeate and retentate.The measurements were conducted at various feed pressures at room temperature.The analysis of mixed gas was performed with a Varian Gas Chromatograph which was equipped with a TCD detector using the integral gas permeation also called time-lag method.The mass flow control(MFC)unit was used to read the feed gas flow to the inlet chamber and also a pressure transducer and a temperature transducer were applied to control the feed flow into the reservoir[17].The permeation cell was sealed by a pair of O-rings(Viton®).The feed gas after passing through the membrane was sent to the GC analyzer.The effective surface area of membrane cell was 56.71 cm2.
4.2.Gas permeability determination
Single gas permeability through polymer membrane can be calculated in a steady-state by the following equation:


Fig.2.FESEM images of top surface of a)pristine and b)PEBAX/4.6 wt%FS membranes.
wherein J[GPU,1 GPU=10−6cm3(STP),cm·cm−2·s−1·cmHg−1]is the gas flux according to Fick's first law,pf(cm Hg)and pp(cm Hg)represent feed and permeate pressures,respectively.T denotes the absolute temperature(K),A is the effective surface area of membranes for gas transport(cm2),l is the membrane thickness(cm),d V/d t represents the permeation volumetric flow rate(cm3(STP)·s−1)and P refers to the gas permeability[Barrer,1 Barrer=10−10cm3(STP)·cm·cm−2·s−1·cmHg−1].The selectivity factor(α∗)of ideal gas can be expressed by the following equation:

where Piand Pjare the permeability coefficients of pure gases i and j.Mixed gas permeance of gas i is calculated as follows:
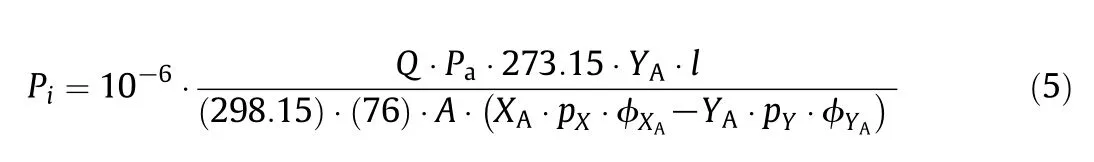
Q is the permeate flow rate in ml·s−1.XAand YAare the upstream and permeate mole fractions,respectively.pXand pYare the upstream and downstream total pressures(cm Hg),respectively.ϕXAand ϕYArepresent the fugacity coefficients of upstream and downstream,respectively.A and l are the effective area and thickness for permeation in cm2and μm respectively.Pais the atmospheric pressure(cm Hg).
The separation selectivity can be expressed as:

5.Results and Discussion
5.1.Exploring morphology of membranes by AFM characterization
PEBAX as a semi-crystalline block copolymer consists of two phases:amorphous and crystalline phases that impart its unique properties.Fig.1a represents an AFM image of PEBAX in which components are irregularly oriented.The phase image(Fig.1a)indicated microphase separation of block copolymers,which resulted from an incompatibility of crystallinerigid and amorphous flexible blocks.As for phasic analysis,bright regions oriented randomly correspond to the crystalline domains,introducing a meso-structure consisting of stiff PA phase and glassy phase of PE as dark areas.The crystalline phases are surrounded by PE phase a soft continuous phase.The self-organized crystals indicate co-continuous morphology[18].PEBAX complex nature can be strongly related to the type of the steric conformations of polymer chains,caused by specific interactions of functional groups[16,19,20].
The microphase separation in PEBAX copolymer between crystalline and amorphous phases can be clearly observed for pristine PEBAX whereas in the case of PEBAX/FS,the microphase separation cannot be distinguished(see Fig.1b).Fig.1 indicatesthat co-continuousstructures of PEBAX/FS are disorganized comparing to those of the pristine PEBAX.This results in decrease of crystallinity.AFM observation also revealed the integration of FS nanoparticles within the polymer matrix(see Fig.1b).
5.2.SEM characterization
Fig.2 shows the top-surface morphology of neat and FS containing membranes.SEM image of the pristine membrane(see Fig.2a)looks different from what was previously reported in the literature[21].The top-surface photomicrographs of pristine membrane clearly showseparate phases of PA proposed as crystalline rigid phase and PEO as amorphous phase.In fact,the systematic orientation can be attributed to the feature of copolymer structure,steric effect and also process of film preparation.
Preparation of MMMs by loading silica nanoparticles within the PEBAX matrix results in significantly modified surface morphology.The crystalline phase undergoes more changes caused by FS existence within the PEBAX matrix,leading to a decrease in crystallinity value which is the result of disorganization of the PA phase.
As can be seen from Fig.3,the cross-section scanning of neat membrane showed mesomorphic morphology[16,22].
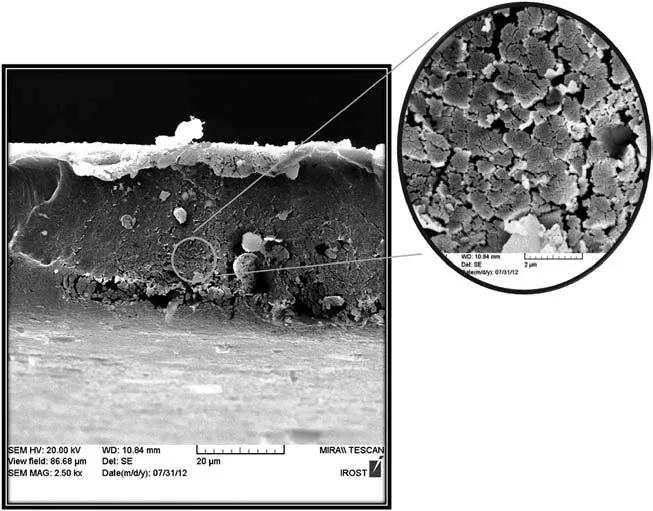
Fig.3.The cross-section image of PEBAX sample.
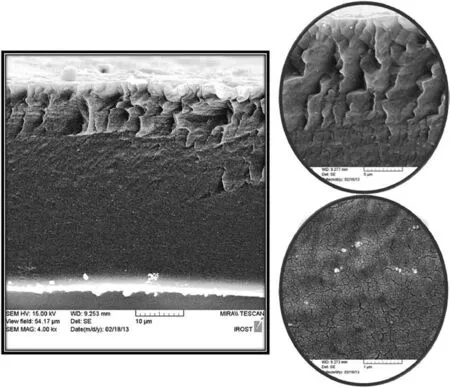
Fig.4.The cross-section image of PEBAX/4.6 wt%FS membrane.
Ascan beseen from Fig.4 at 5μm magnification,change in morphology is distinguishable for the FS containing film as compared with untreated membrane.It showed a zigzag-like morphology with circumstances of the organization along the cross-section and lateral stresses at the interface which may be related to process of solvent evaporation.Fig.4 particularly at 1 μm magnification displays silica nanoparticles dispersed within the polymer matrix.
5.3.FFV determination
The densities of the mixed matrix membranes are decreased by introducing various amounts of FS within the PEBAX matrix.Using FS within the PEBAX matrix leads to the decrease in the density value from 1.076 g·cm−3to 1.02 g·cm−3in the case that pristine membrane and membrane containing 4.6 wt%FS were employed,respectively.A change in density of PEBAX film can have an impact on free volume of the film.As for pristine membrane,FFV is calculated to be 0.374 while PEBAX/4.6 wt%FS membrane exhibits finely tuning free volume architecture(see Fig.5).The increase in FFV value corresponds to the decrease in the film density when FS content within the polymer matrix is increased.In fact,CO2transport can be enhanced when desirable free volumes are constructed[16].
5.4.Feed pressure dependence of perm selectivity
Generally,PEBAX membranes possess a nonpolar/polarizablebased gas separation property that makes this type of membranes applicable to separation of polarizable gases such as CO2from nonpolar gases like CH4.In this section,the effect of an increase in upstream pressure on permeability properties of samples is investigated.In order to obtain an ideal permeation property of a PEBAX membrane,the singlegas measurements of CO2and CH4gasesthrough the polymer films were performed at ambient temperature and at upstream pressures of 1.0,1.5,2.0,2.5,3.0,and 3.5 MPa.The permeation of the binary gas mixture of CO2/CH4(4.8 mol%CO2in CH4)was also investigated and analyzed by GC.Ascan beseen from Figs.6 and 7,the CO2permeability as a function of fugacity strongly increases whereas the least changes for CH4permeability as a function of fugacity are recorded.
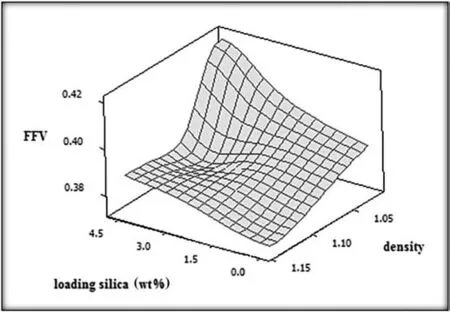
Fig.5.FFV of the films as a function of FS loading and density values.
In the case of CO2,the results clearly show that by increasing upstream pressure from 1.0 to 3.5 MPa,permeability values elaborated from 80 to 162 Barrer(see Figs.6 and 7).Permeability values of CH4are elevated from 1.7 to 2.5 Barrer by increasing upstream pressure from 1 to 3.5 MPa.
Figs.8 and 9 indicate the permeation behavior of CO2and CH4through the BEBAX/4.6 wt%FS membrane.Gas permeabilities of both CO2and CH4are increased when the feed pressure is elevated from 1 to 3.5 MPa.The result is strongly correlated to the effect of FFV,which is calculated to be 0.381 for BEBAX/4.6 wt%FS membrane as compared with FFV of pristine PEBAX with the value of 0.374.
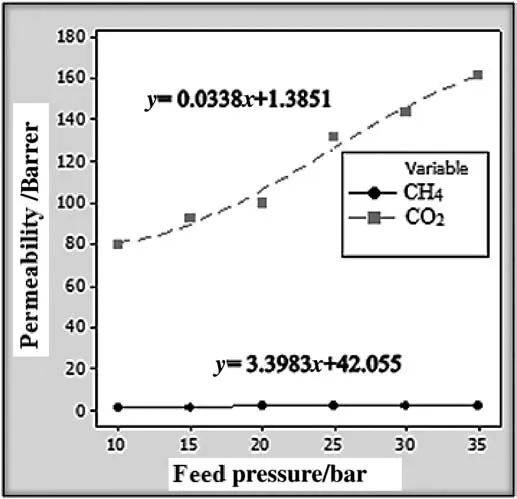
Fig.6.Representation of the feed pressure dependency of CO2 and CH4 permeabilities where pristine membrane is utilized.(1 bar=105 Pa)
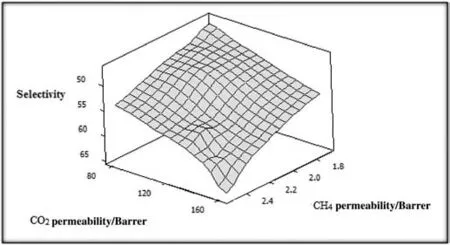
Fig.7.Feed pressure dependency of CO2 and CH4 permeabilities,and the resultant ideal selectivity of CO2/CH4 where pristine membrane is utilized.
The silica nanoparticles can trigger conformational changes in the matrix framework,thereby modifying the pore size and shape.The mutual interactions between FS and PEO/PA units can lead to a permeability improvement[23,24].As a consequence,CO2/CH4selectivity was found to be 64.54 for pristine membrane whereas the selectivity reached to 74.5 by loading 4.6 wt%of FS within the polymer matrix.The results showthat CO2/CH4selectivity increases slowly as feed pressure is increased.The ideal selectivity as a function of penetrant permeability is plotted in Fig.9.
5.5.Permeation behavior of binary gas mixture
In the case of the single gas permeation mentioned previously,permeability values are elaborated when PEBAX/4.6 wt%FS is utilized and the enhanced ideal selectivity of the treated membrane is a result of its permeability modification.In binary gas separation,the similar gas permeation behavior is also observed.
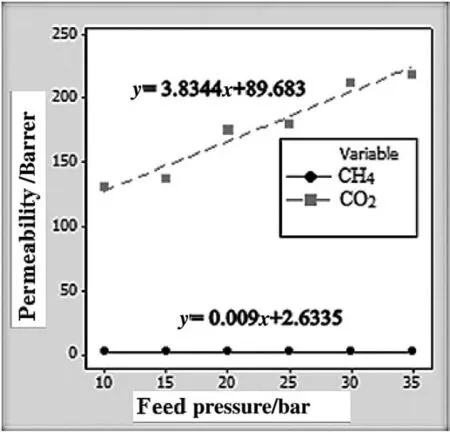
Fig.8.Feed pressure dependency of CO2 and CH4 permeabilities where PEBAX/4.6 wt%FS membrane is utilized.(1 bar=105 Pa)
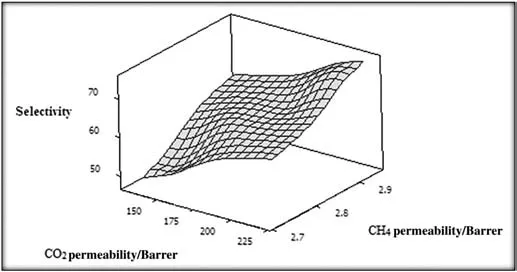
Fig.9.Feed pressure dependency of CO2 and CH4 permeabilities,and the resultant ideal selectivity of CO2/CH4 where PEBAX/4.6 wt%FS membrane is utilized.
Fig.10 shows chromatogram of CO2/CH4(4.8/95.2 mol%)mixture used as feed gas pair in this study.
Figs.11 and 12 represent permeate-side CO2/CH4composition for pristine and PEBAX/4.6 wt%FS films,respectively.For all of the experiments feed pressure of 3 MPa was applied.According to Eq.(6),the value of 15.5 is calculated for separation selectivity,αij,of pristine membrane when the binary gas mixture of CO2/CH4was used.
By utilizing FS within the polymer matrix,predominant changes in the permselectivity of the filled membrane can be observed.Surprisingly,the membrane containing 4.6 wt%FS exhibited astonishing selectivity result for separation of binary CO2/CH4gas mixture.As can be seen from Fig.12,CH4gas was not detected by GC in the permeate side.Therefore,the only detectable gas in the permeate side was CO2which can penetrate through the treated membrane.
A very lownonpolar gas,CH4,transport through the membrane containing 4.6 wt%of FS was observed.The linear molecules of CO2shown an enhanced permeation through the membrane containing 4.6 wt%of nanosilicaparticlesin comparison to the permeation of larger CH4molecules that do not have dipole and quadrupole moment[25,26].
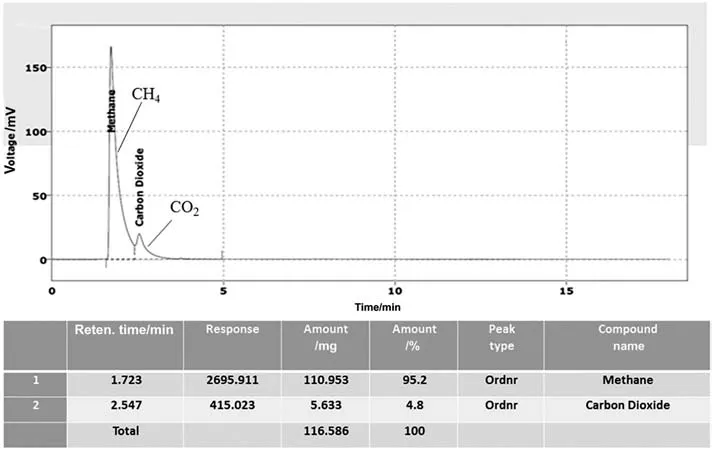
Fig.10.Chromatogram of CO2/CH4 gas pair obtained by GC.
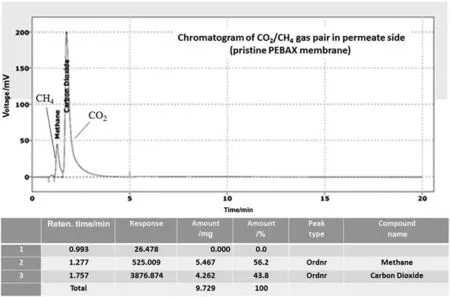
Fig.11.Chromatogram of CO2/CH4 gas pair in permeate side of pristine PEBAX membrane.
6.Conclusions
PEBAX/4.6 wt%FS membrane exhibited a distinguished CO2/CH4selectivity for both single and binary gas systems.The results showed that the modified membrane indicates much higher permeance of CO2comparing to that of the pristine membrane when the feed pressure is elevated from 1 to 3.5 MPa.The silica nanoparticles give a permanent exclusivity to the PEBAX morphotropic matrix so that the membrane is prohibited from plasticization.Furthermore,adding 4.6 wt%FS within the polymer matrix causes structural changes of the membrane,leading to distinguished gas permeation properties and highly CO2selective characteristics.Fig.12 shows when PEBAX/4.6 wt%FS membrane is used for the permeation test of the binary gas mixture,CO2gas is the only detectable permeate gas,in other words,no CH4gas can be detected in the permeate side.
Acknowledgements
The authors gratefully acknowledge the financial support of Research Institute of Petroleum Industry.
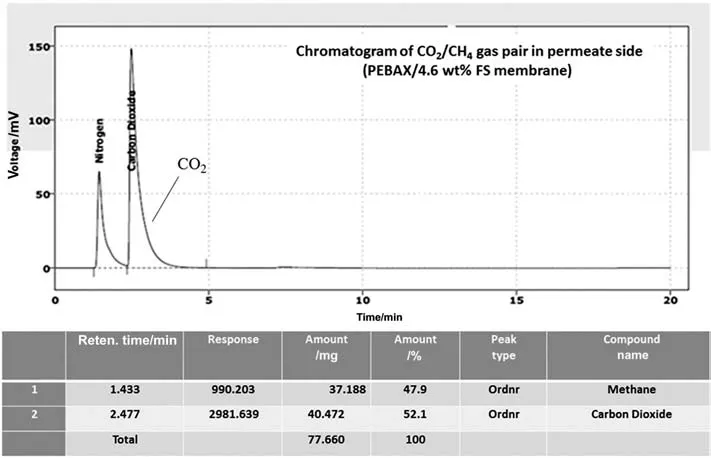
Fig.12.Chromatogram of CO2/CH4 gas pair in permeate side of PEBAX/4.6 wt%FS membrane.
[1]A.L.Lee,H.L.Feldkrichner,S.A.Stern,A.Y.Houde,J.P.Gamez,H.S.Meyer,Field tests of membrane modules for the separation of carbon dioxide from low-quality natural gas,Gas Sep.Purif.9(1995)35–43.
[2]Y.Dai,X.Ruan,Z.Yan,G.He,Imidazole functionalized graphene oxide/PEBAX mixed matrix membranes for efficient CO2capture,Sep.Purif.Technol.166(2016)171–180.
[3]R.S.Murali,A.F.Ismaeil,M.A.Rahman,S.Sridhar,Mixed matrix membranes of Pebax-1657 loaded with 4A zeolite for gaseous separations,Sep.Purif.Technol.129(2014)1–8.
[4]Y.Shen,H.Wang,X.Zhang,Y.Zhang,MoS2nanosheets functionalized composite mixed matrix membrane for enhanced CO2capture via surface drop-coating method,ACS Appl.Mater.Interfaces 35(2016)1–8.
[5]M.Rahmani,A.Kazemi,F.Talebnia,P.Abbasszadeh Gamali,Fabrication and characterization of brominated matrimid®5218 membranes for CO2/CH4separation:Application of response surface methodology(RSM),e-Polymers 16(6)(2016)481–492.
[6]M.Rahmani,A.Kazemi,F.Talebnia,Matrimid mixed matrix membranes for enhanced CO2/CH4separation,J.Polym.Eng.36(5)(2016)499–511.
[7]R.W.Baker,Future directions of membrane gas separation technology,Ind.Eng.Chem.Res.41(2002)1393–1404.
[8]S.Kulprathipanja,R.W.Neuzil,and N.N.Li,Separation of fluids by means of mixedmatrix membranes.U.S.Patent 4(1988),740,219.
[9]L.Liu,J.H.Kim,S.Y.Ha,Y.M.Lee,Gas permeation of poly(amide-6-b-ethylene oxide)copolymer,J.Membr.Sci.190(2001)179–193.
[10]J.H.Kim,Y.M.Lee,Gas permeation properties of poly(amide-6-b-ethylene oxide)-silica hybrid membranes,J.Membr.Sci.193(2001)209–225.
[11]R.A.Zoppi,S.das Neves,S.P.Nunes,Hybrid films of poly(ethylene oxide-b-amide-6)containing sol–gel silicon or titanium oxide as inorganic fillers:Effect of morphology and mechanical properties on gas permeability,Polymer 41(2000)5461–5470.
[12]A.Car,C.Stropnik,W.Yave,K.Peinemann,PEBAX®/polyethylene glycol blend thin film composite membranes for CO2separation:Performance with mixed gases,Sep.Purif.Technol.62(2008)110–117.
[13]L.M.Robeson,Correlation of separation factor versus permeability for polymeric membranes,J.Membr.Sci.62(1991)165–185.
[14]L.M.Robeson,The upper bound revisited,J.Membr.Sci.320(2008)390–400.
[15]B.D.Freeman,H.Lin,in:H.Czichos,T.Saito,L.Smith(Eds.),Springer Handbook of Materials Measurement Methods,Springer,Berlin,German 2006,pp.371–387.
[16]Y.Yumpolskii,B.D.Freeman,Membrane Gas Separation,John Wiley&Sons,2010.
[17]W.Yave,A.Szymczyk,N.Yave,Z.Roslaniec,Design,synthesis,characterization and optimization of PTT-b-PEO copolymers:A newmembrane material for CO2separation,J.Membr.Sci.362(2010)407–416.
[18]A.J.Ryan,Polymer science:designer polymer blends,Nat.Mater.1(2002)8–10.
[19]N.Sergei,N.Magonov,D.H.Reneker,Characterization of polymer surfaces with atomic forced microscopic,Annu.Rev.Mater.Sci.27(1997)175–222.
[20]W.K.Lee,C.S.Hab,Miscibility and surface crystal morphology of blends containing poly(vinylidene fluoride)by atomic force microscopy,Polymer 39(26)(1998)7131–7134.
[21]L.Mengdie,Z.Xiangping,Z.Shaojuan,B.Lu,G.Hongshuai,D.Jing,Y.Qingyuan,Z.Suojiang,Pebax-based composite membranes with high gas transport properties enhanced by ionic liquids for CO2separation,RSC Adv.7(2017)6422–6431.
[22]W.Yave,A.Car,K.V.Peinemann,Nanostructured membrane material designed for carbon dioxide separation,J.Membr.Sci.350(2010)124–129.
[23]W.J.Koros,Gas separation membranes:needs for combined materials science and processing approaches,Macromol.Symp.188(2002)13–22.
[24]J.R.Fried,N.Hu,The molecular basis of CO2interaction with polymers containing fluorinated groups:Computational chemistry of model compounds and molecular simulation of poly[bis(2,2,2-trifluoroethoxy)p hosphazene],Polymer 44(2003)4363–4372.
[25]F.Li,Y.Li,T.S.Chung,S.Kawi,Facilitated transport by hybrid POSS®–Matrimid®–Zn2+nanocomposite membranes for the separation of natural gas,J.Membr.Sci.356(2010)14–21.
[26]I.H.Musselman,K.J.Balkus,J.P.Ferraris,Mixed-matrix membranes for CO2and H2gas separations using metal-organic frameworks and mesoporous hybrid silicas,Final Scientific/Technical Report,University of Texas at Dallas,USA,2008.
 Chinese Journal of Chemical Engineering2018年1期
Chinese Journal of Chemical Engineering2018年1期
- Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
