Asymmetric reduction of chroman-4-one and its derivate by whole cell of Pseudomonas plecoglossicida
, ,
(School of Pharmacy,Zunyi Medical University,Zunyi Guizhou 563099,China)
Asymmetric reduction of prochiral ketones is a fundamentally accesses to formation of chiral and enriched alcohols[1-2].Traditional synthetic methods predominantly use toxic metals and expensive complex hydrides.To circumvent these drawbacks,many efforts were made in biocatalytic asymmetric reduction of ketones in the past decades[3-5].The vast majority of dehydrogenases[6-7]and reductases[8-9]could be used for ketones reduction.So far many biocatalysts were used for reduction of prochiral ketone to manufacture lots of pharmaceutical intermediates[10-12].

Fig 1 Synthesis applications of chiral aromatic heterocyclic alcohols
Optically active aromatic heterocyclic alcohols,such as (S)-chroman-4-ol,(S)-thiochroman-4-ol and their derivatives,are important intermediates for synthesis of fine chemicals and pharmaceuticals[13].For examples (Fig 1),(S)-chroman-4-ol is useful intermediate for synthesis of CP-199,3301and CP-199,3312that are cysLT1 receptor antagonists which are used for asthma disease[14].(S)-thiochroman-4-ol is also found in drug3which is employed in the treatment of high blood pressure[15].In addition,1,2,3,4-tetrahydroquinolin-4-ol derivatives serve as intermediates cAMP-specific phos-phodiesterase (PDE4) inhibitor4,and glucocorticoid agonists5[16].Although conventional chemical routes are used to obtain enantiopure chroman-4-ol and its core analogues[15,17],more efficient bioenzymatic methods are being developed.Lipase-mediated resolution route has been reported to generate the chiral chroman-4-ol and its analogues in the 1990s,which was carried out withCandidacylindracealipase giving moderate to goodeevalues[18].Soon afterwards Ramadas and Krupadanam reported the enantioselective acylation ofrac-chroman-4-ols using lipase fromPseudomonascepeciain n-hexane giving (R)-chroman-4-ol acetates and (S)-chroman-4-ols in higheevalue[19].In the paper of Olexandr,a better performance withee≥94% of products was obtained by resolution of racemic chroman-4-ol and its substituted analogues withBurkholderiacepacialipase[20].In addition,redox processes for preparation of chiral chroman-4-ol and its analogues were found inMortierellaisabellinaandHelminthosporiumspecies[21].

Fig 2 Asymmetric reduction of aromatic heterocyclic ketones by P.plecoglossicida ZMU-T06
In our previous study, a toluene-degrading strainP.plecoglossicidaZMU-T06 that isolated from soil samples exhibited the ability of stereoselective oxidation activity for methyl phenyl sulfide,indan and 1,2,3,4-tetrahydroquinoline[22].In this paper,we expanded the application ofP.plecoglossicidaZMU-T06 in asymmetric reduction of aromatic heterocyclic ketones chiral aromatic heterocyclic ketones (Fig 2).The results indicated ZMU-T06 was an efficient biocatalyst for preparing enantiopure aromatic heterocyclic alcohols,which gave (S)-chroman-4-ol,(S)-thiochroman-4-ol and (S)-1,2,3,4-tetrahydroquinolin-4-ol derivatives with 65%-96%eeand 87%-97% yields.
1 Materials and methods
1.1 Materials Compounds1a,2a,1band2bwere purchased from J&K.Compounds3a,4a,3band4bwere synthesized using 1,2,3,4-tetrahydroquinolin as the substrates[23].The other chemical reagents and solvents were purchased from commercial company and used without further purification.Column chromatography was performed with Merck silica gel 60 (200-300 mesh) as column material.TLC analysis was carried out with precoated Silica Gel 60 F254TLC-plate and the compounds were visualized with UV light.
1.2 Strains and cultivation Organism strainsP.plecoglossicidaZMU-T01,P.plecoglossicidaZMU-T04,PseudomonasmonteiliiM2013683,P.plecoglossicidaZMU-T06,P.monteiliiZMU-T10,P.monteiliiZMU-T11,P.monteiliiZMU-T16 andP.monteiliiZMU-T17 were isolated from Guizhou Province (China) and deposited in our laboratory.All the strains were grown in LB (Luria-Bertani,10 g/L tryptone,5 g/L yeast extract and 5 g/L NaCl) agar plate and cultured in 250 ml shaking flask containing 50 ml liquid LB medium.The cultures were shook at 300 rpm,30 (C for 24 h and the cells were harvested by centrifugation (12 000 rpm for 10 min,4 ℃).
1.3 Analytical methods Theeevalue was determined by ShimadzuTM Prominence HPLC analysis on a DaicelTMOJ-H or OD-H chiral column (250×4.6 mm,5 μm) at 25 °C with UV detection at 254 nm.The parameters for all the chemicals were detailed in Table 1.1H and13C NMR spectrum were recorded on Angilent (400/100 MHz) spectrometer using DMSO and CDCl3as solvent and TMS as an internal standard.Optical rotations were measured on a Perkin-Elmer 241 Polarimeter.LC-MS spectrum were obtained from microTOF-Q II 10203 MS.
Tab1HPLCparametersforanalysisofchemicals

EntryHPLCparametersRetentiontime(min)1ChiralpakOD-H,hexane/i-PrOH=95/5,flowrate1.0mL/min(S)-1b:12.1;(R)-1b:10.42ChiralpakOD-H,hexane/i-PrOH=90/10,flowrate0.5mL/min,(S)-2b:16.4;(R)-2b:19.63ChiralpakOD-H,hexane/i-PrOH=90/10,flowrate0.5mL/min(S)-3b:31.3;(R)-3b:29.14ChiralpakOJ-H,hexane/i-PrOH=90/10,flowrate0.8mL/min(S)-4b:41.4;(R)-4b:33.7
1.4 Screening reductase-producing strains for bioreduction of1aThe harvested cells were resuspended in 5 ml of 100 mM KH2PO4-K2HPO4buffer solution (PBS,pH 7.0) to a density of 10 g dcw/L.To it 2 mM substrate of1awas added,and the mixture was shaken at 300 rpm and 30 °C for 24 h.All reactions were ended by adding 5 ml ethyl acetate to extract the chemicals.The organic phases were separated and analyzed by HPLC.
1.5 Characterization of the reaction temperature and pH Bio-reductions of1awere carried out in various temperatures ranging from 25 to 45 ℃.All the reactions were performed at 100 mM PBS (pH 7.0).On the other hand,the different pH values ranging from 7.0 to 9.0 were chose to test the influences on the bio-reduction process.The later reactions were carried out at 30 ℃.Data analysis was using the same method that described above.
1.6 Influence of cosolvents on the bio-reduction reaction Glycerol,ethylene glycol,methanol,ethanol,dimethyl sulfoxide and n-hexane were used as cosolvents in the bio-reduction reaction.The reaction were proceeded with 4 ml of 100 mM PBS (pH 8.0),1 ml of cosolvent,50 g dcw/L cells and 5 mM1a.All the mixtures were stirred at 30℃ and 300 rpm for 24 h.The yields andeevalues of prodoucts were analyzed by HPLC.
1.7 Substrate specificity ofP.plecoglossicidaZMU-T06 Four aromatic heterocyclic ketones1a,2a,3aand4awere used as substrates to investigate the substrate specificity ofP.plecoglossicidaZMU-T06.The reactions were performed in 5 ml of 100 mM PBS (pH 7.0) with the 40 g dcw/L cells of and 2 mM ketones.The mixtures were reacted at 300 rpm and 30 °C for 24 h.The recation mixtures were extracted with 5 ml ethyl acetate,dried over anhydrous Na2SO4and concentrated by evaporation at reduced pressure.The proudts were submited for chiral HPLC,LC-MS and NMR analysis.

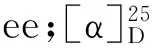
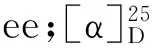
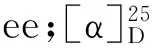
2 Results and discussion
Ketoreductases are a reliable source of enantiopure alcohols matching and often exceeding the ability of chemical catalyst to perform the same reactions[24-25].Chiral aromatic heterocyclic alcohols are important intermediates for preparing many pharmaceuticals.In oder to discovery a reduction process for synthesis of chiral aromatic heterocyclic alcohols,ketone compound1awas used as a standard substrate to screen microbial strains.Eight microbial strains that deposited in our laboratory were selected to catalyze the asymmetric reduction of1a(Tab 2).All the strains showedS-selectivity to1a,which generated (S)-1bwith theeefrom 78% to 94%.The highest 82% conversion and 94%eewere obtained with the strainP.plecoglossicidaZMU-T06.Though conversion of1abyP.monteiliiZMU-T10 gave (S)-1bwith 94%ee,the yield was only 73%.
For improving the catalysis efficient of bio-reduction of 1a with strain ZMU-T06,the optimal temperature and pH were investigated and the results were displayed in Fig 3.The conversion increased significantly with the increase of temperature.The maximum conversion was reached 87% at 30 ℃ and rapidly decreased to only 2.5% at 40 ℃ (Fig 3a).Strain ZMU-T06 showed the optimal temperature at 30 ℃ that was as the same as most other carbonyl reductase[26-28].However,the highesteewas found at 25 ℃ that just gave 21% conversion.The conversion was also dramatically influenced by pH value (Fig 3b).Alkaline condition was propitious to obtaining higheeof (S)-1b,while only 12% conversion was found at pH 9.0.Even though 92%eewas generated,pH 8.0 was the optimal condition for production of (S)-1b with the highest conversion.
Tab2Asymmetricreductionof1abydifferentmicrobialstrains
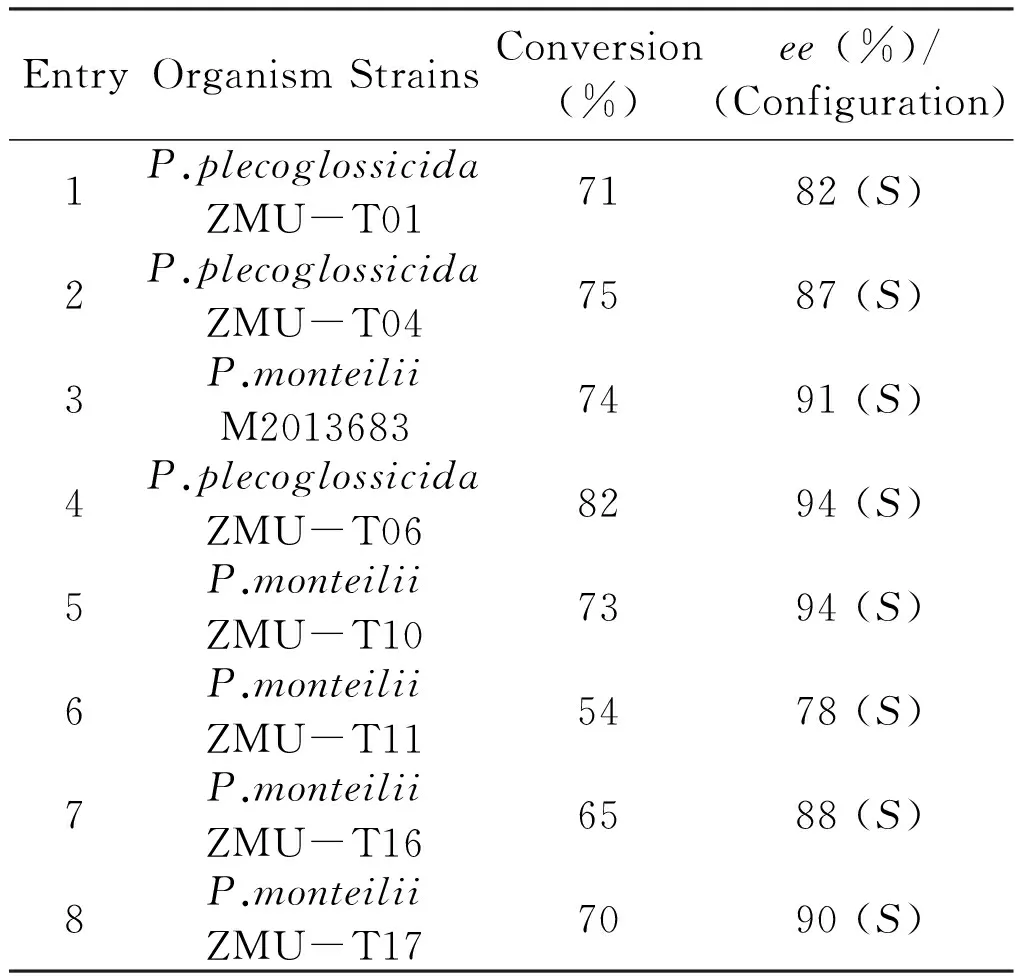
EntryOrganismStrainsConversion(%)ee(%)/(Configuration)1P.plecoglossicidaZMU-T017182(S)2P.plecoglossicidaZMU-T047587(S)3P.monteiliiM20136837491(S)4P.plecoglossicidaZMU-T068294(S)5P.monteiliiZMU-T107394(S)6P.monteiliiZMU-T115478(S)7P.monteiliiZMU-T166588(S)8P.monteiliiZMU-T177090(S)

Fig 3 Effects of temperature (a) and pH (b) on the asymmetric synthesis of (S)-1b using P.plecoglossicida ZMU-T06
The influence of cosolvents to the bio-reduction of1awith strain ZMU-T06 was also studied (Fig 4).Methanol,ethanol and n-hexane were highly reduced the conversion and theeeof (S)-1b.Unconspicuous changes were found by using ethylene glycol or DMSO as the cosolvent.The conversion and enantioselectivity were improved with adding of glycerol,while the advancements were slight.Based on the above results,none excellent cosolvent was found from these organic solvents.It could be inferred that the carbonyl reductase in strain ZMU-T06 was not tolerated to hydrophilic solvents.

Fig 4 Effects of cosolvents on the asymmetric synthesis of (S)-1b using P.plecoglossicida ZMU-T06
To check whether strain ZMU-T06 could accept other substrates,a representative set of aromatic heterocyclic ketones,including2a,3aand4awere subjected to the optimal reaction conditions (Tab 3).The proudts were identifed by chiral HPLC,LC-MS and NMR analysis (NMR spectrum were listed in S1-S4).Interestingly,strain ZMU-T06 was not restricted to 1a,and it worked well against other aromatic heterocyclic ketones.Of the four substrates tested,all led to theS-selectivity and 65%-94%eeof chiral alcohols.Comparion of3aand4a,it could be concluded that the big substituent group in N1 position of ketone substrate would reduce the enantio-selectivity of strain ZMU-T06.
Tab3SubstratespecificityofP.plecoglossicidaZMU-T06
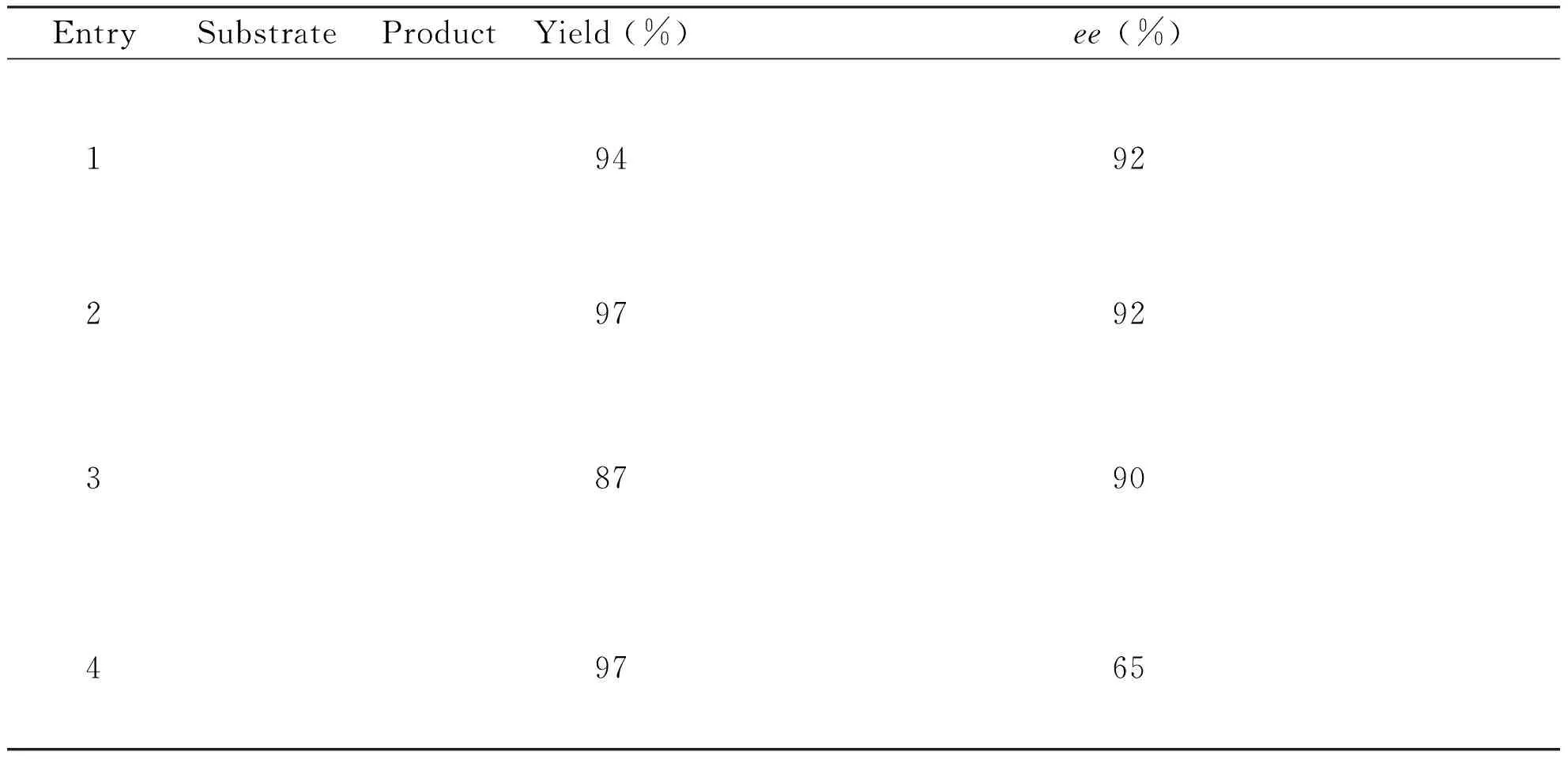
EntrySubstrateProductYield(%)ee(%)19492297923879049765
3 Conclusion
In conclusion,we identified a microbial strainP.plecoglossicidaZMU-T06 for asymmetric reduction of aromatic heterocyclic ketones.High yields and moderateeeof (S)-chroman-4-ol,(S)-thiochroman-4-ol and (S)-1,2,3,4-tetrahydroquinolin-4-ol derivatives were obtained by suspending whole-cells biotransformation of ZMU-T06 as biocatalyst.It might be a promising biotransformation process for preparation of chiral aromatic heterocyclic alcohols.Further studies for cloning,heterologous expression and characterization of carbonyl reductases inP.plecoglossicidaZMU-T06 are in progress.
[References]
[1] Touchard F,Gamez P,Fache F,et al.Chiral thiourea as ligand for the asymmetric reduction of prochiral ketones[J].Tetrahedron Lett,1997,28(30):2275-2278.
[2] Nie Y,Xiao R,Xu Y,et al.Novel anti-Prelog stereospecific carbonyl reductases fromCandidaparapsilosisfor asymmetric reduction of prochiral ketones[J].Organic & Biomolecular Chemistry,2011,9(11):4070-4078.
[3] Nakamura K,Yamanaka R,Matsuda T,et al.Recent developments in asymmetric reduction of ketones with biocatalysts[J].Tetrahedron-Asymmetry,2003,34(52):2659-2681.
[4] Dudzik A,Snoch W,Borowiecki P,et al.Asymmetric reduction of ketones and β-keto esters by (S)-1-phenylethanol dehydrogenase from denitrifying bacteriumAromatoleumaromaticum[J].Applied Microbiology and Biotechnology,2015,99(12):5055-5069.
[5] Sun Z,Lonsdale R,Ilie A,et al.Catalytic asymmetric reduction of difficult-to-reduce ketones:Triple-code saturation mutagenesis of an alcohol dehydrogenase[J].ACS Catalysis,2016,6(3):1598-1605.
[6] Zhang D,Zhang R,Zhang J,et al.Engineering a hydroxysteroid dehydrogenase to improve its soluble expression for the asymmetric reduction of cortisone to 11 β-hydrocortisone[J].Applied Microbiology and Biotechnology,2014,98 (21):8879-8886.
[7] Chen X,Liu Z Q,Huang J F,et al.Asymmetric synthesis of optically active methyl-2-benzamido-methyl-3-hydroxy-butyrate by robust short-chain alcohol dehydrogenases fromBurkholderiagladioli[J].Chemical Communications,2015,51(61):12328-12331.
[8] He Y C,Zhang D P,Lu Y,et al.Biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with an NADH-dependent reductase (ClCR) discovered by genome data mining using a modified colorimetric screening strategy[J].Bioengineered Bugs,2015,6(3):170-174.
[9] He Y C,Tao Z C,Zhang X,et al.Highly efficient synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate and its derivatives by a robust NADH-dependent reductase fromE.coliCCZU-K14[J].Bioresource Technology,2014,161(6):461-464.
[10]Pollard D J,Woodley J M.Biocatalysis for pharmaceutical intermediates:the future is now[J].Trends in Biotechnology,2007,25(2):66-73.
[11]Patel R N.Synthesis of chiral pharmaceutical intermediates by biocatalysis[J].Coordination Chemistry Reviews,2008,252(11):659-701.
[12]Woodley J M.New opportunities for biocatalysis:making pharmaceutical processes greener[J].Trends Biotechnol,2008,26(6):321-327.
[13]Voight E A,Daanen J F,Hannick S M,et al.Efficient and general asymmetric syntheses of (R)-chroman-4-amine salts[J].Tetrahedron Lett,2010,51(45):5904-5907.
[14]Chambers R J,Marfat A,Antognoli G W,et al.Discovery of CP-199,330 and CP-199,331:Two potent and orally efficacious cysteinyl LT1 receptor antagonists devoid of liver toxicity[J].Bioorganic & Medicinal Chemistry Letters,1999,30(52):2773-2778.
[15]Stepanenko V,Correa W,Bermúdez L,et al.Chiral spiroaminoborate ester as a highly enantioselective and efficient catalyst for the borane reduction of furyl,thiophene,chroman,and thiochroman-containing ketones[J].Tetrahedron-Asymmetry,2009,20(23):2659-2665.
[16]Regan J,Lee T W,Zindell R M,et al.Quinol-4-ones as steroid A-ring mimetics in nonsteroidal dissociated glucocorticoid agonists[J].Journal of Medicinal Chemistry,2006,49(26):7887-7896.
[17]Mao J,Guo J.Chiral amino amides for the ruthenium(II)‐catalyzed asymmetric transfer hydrogenation reaction of ketones in water[J].Chirality,2010,22(1):173-181.
[19]Ramadas S,David K G L.Enantioselective acylation of chroman-4-ols catalysed by lipase fromPseudomonascepecia(Amano PS)[J].Tetrahedron-Asymmetry,1997,8(18):3059-3066.
[20]Kucher O V,Kolodyazhnaya A O,Smolii O B,et al.Enzymatic resolution of chroman-4-ol and its core analogues withBurkholderiacepacialipase[J].Tetrahedron-Asymmetry,2014,45(39):563-567.
[21]Holland H L,Manoharan T S,Schweizer F,et al.Preparation of homochiral chroman-4-ols and thiochroman-4-ols by microbial biotransformation[J].Tetrahedron-Asymmetry,1991,2(5):335-338.
[22]Zheng D,Yang M,Zhuo J,et al.Regio- and stereoselective benzylic hydroxylation to synthesize chiral tetrahydroquinolin-4-ol and tetrahydro-1H-benzo[b]azepin-5-ol withPseudomonasplecoglossicidas[J].Journal of Molecular Catalysis B:Enzymatic,2014,110(22):87-91.
[23]Chen Y,Zheng D,Liu X,et al.Diffusion-weighted magnetic resonance imaging for early response assessment of chemoradiotherapy in patients with nasopharyngeal carcinoma[J].Chinese Journal Synther Chemical,2014,32(6):630-637.
[24]Ni Y,Xu J H.Biocatalytic ketone reduction:A green and efficient access to enantiopure alcohols[J].Biotechnology Advances,2012,30(6):1279-1288.
[25]Hoyos P,Sinisterra J V,Molinari F,et al.Biocatalytic strategies for the asymmetric synthesis of α-hydroxy ketones[J].Accounts Chemical Research,2010,43(8):288-299.
[26]Chen X,Liu Z Q,Lin C P,et al.Chemoenzymatic synthesis of (S)-duloxetine using carbonyl reductase fromRhodosporidiumtoruloides[J].Bioorganic Chemistry,2016,65(4):82-89.
[27]Xu Q,Tao W Y,Huang H,et al.Highly efficient synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by a novel carbonyl reductase fromYarrowialipolyticaand using mannitol or sorbitol as cosubstrate[J].Biochemical Engineering Journal,2016,106(15):61-67.
[28]Chen R,Liu X,Wang J,et al.Cloning,expression,and characterization of an anti-Prelog stereospecific carbonyl reductase fromGluconobacteroxydansDSM2343[J].Enzyme Microbial Technology,2015,70(20):18-27.

