石墨相氮化碳的制备、功能化及应用
徐溥群 吴惠霞 杨仕平
摘要:
由于石墨相氮化碳(g-C3N4)优越的光电性能以及“无金属”的特性,吸引了化学、物理学、生物医学、材料学等各个领域对其的深入研究和探索,成为当前研究的热点之一.介绍了g-C3N4的基本性质和制备方法,探讨了g-C3N4的元素掺杂以及稀土元素的修饰,简述了g-C3N4在光催化降解领域和生物医学领域的应用.
关键词:
石墨相氮化碳; 制备; 元素掺杂; 应用
中图分类号: O 613.7文献标志码: A文章编号: 1000-5137(2018)01-0113-10
Graphitic carbon nitride:synthesis,functionalization and applications
Xu Puqun, Wu Huixia*, Yang Shiping
(College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
Abstract:
Due to the superior optical and electrical performance and the unique property of ″no metal″,graphite phase carbon nitride (g-C3N4) has attracted researchers of chemistry,physics,biomedicine,materials science and other fields to carry out in-depth studies.It has become one of the hotspots of the current research.In this paper,we introduced the basic properties and preparation methods of g-C3N4.Then,we discussed the elemental doping of g-C3N4 and the modification of rare earth elements.Finally,we briefly described the applications of g-C3N4 in the fields of photocatalytic degradation and biomedicine.
Key words:
graphitic carbon nitride; preparation; elemental doping; application
收稿日期: 2017-09-10
基金项目: 教育部环境功能材料创新团队(IRT_16R49)
作者简介: 徐溥群(1993-),男,硕士研究生,主要从事纳米生物材料方面的研究.E-mail:xupuqun@126.com
*通信作者: 吴惠霞(1972-),女,博士,教授,主要从事纳米生物材料方面的研究.E-mail:wuhuixia@shnu.edu.cn
引用格式: 徐溥群,吴惠霞,杨仕平.石墨相氮化碳的制备、功能化及应用 [J].上海师范大学学报(自然科学版),2018,47(1):113-122.
Citation format: Xu P Q,Wu H X,Yang S P.Graphitic carbon nitride:synthesis,functionalization and applications[J].Journal of Shanghai Normal University(Natural Sciences),2018,47(1):113-122.
0引言
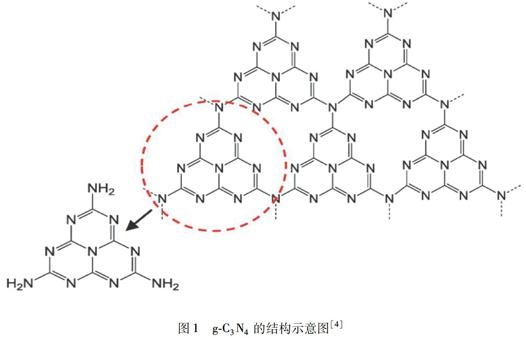
氮化碳是继碳纳米管、石墨烯等碳系材料后的新兴材料.氮化碳分为α相、β相、立方相、准立方相以及类石墨相这5种结构.其中石墨相氮化碳(g-C3N4)的片层结构更类似于石墨烯,这一发现引起了各界的关注.g-C3N4由大量的碳和氮元素组成(图1),具有聚合物特征,其表面化学性质能够通过表面修饰来进行调节.g-C3N4中的碳和氮是通过sp2杂化的,具有π-共轭电子结构[1].g-C3N4具有适度的带隙,其带隙为2.7~2.8 eV,导致约450~460 nm的可见光吸收[2].由于g-C3N4的结构具有芳族C-N杂环,所以g-C3N4是所有C3N4结构中最稳定的一种.通过热重分析(TGA)可知g-C3N4在600 ℃空气中仍然能保持热稳定性[3].此外,g-C3N4化学性质很稳定,不溶于酸、碱或有机物溶剂,这些性质使其在各种环境条件下都能成为可靠的材料.优异的光学性能和良好的生物相容性使得g-C3N4在光催化降解、生物传感器、生物成像等方面有着广泛的应用.
图1g-C3N4的结构示意图[4]
1制备方法
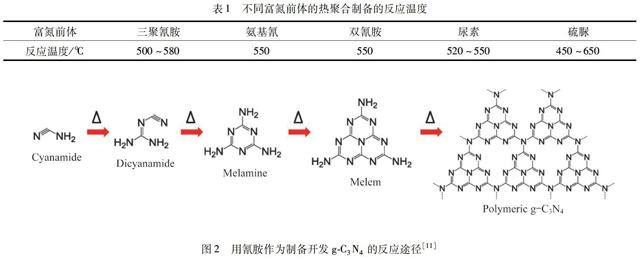
g-C3N4易于通过富氮前体的热聚合制备,如三聚氰胺[5]、双氰胺[6]、氨基氰[7]、尿素[8-9]、硫脲[10]等.其中,不同的富氮前体需要不同的反应参数才能制备得到g-C3N4(表1).基于Wang等[11]的开创性工作,采用氨基氰作为g-C3N4的前体.通过TGA和X射线衍射(XRD)技术的组合来表征反应中间体化合物.其中氰胺分子在约203 ℃和234 ℃下凝结成双氰胺和三聚氰胺.之后是除去氨的凝結阶段.当温度在335 ℃左右时,基本上都会存在三聚氰胺.进一步加热至约390 ℃导致三聚氰胺发生重排,形成三-三嗪单元.最后,在约520 ℃时形成聚合物g-C3N4.通过进一步凝结,可以得到产物.当升高温度达到600 ℃以上,g-C3N4就会变得不稳定.超过700 ℃,g-C3N4将分解产生氮和氰基碎片,最终消失殆尽,没有任何产物残留(图2).
表1不同富氮前体的热聚合制备的反应温度
图2用氰胺作为制备开发g-C3N4的反应途径[11]
2石墨烯的功能化
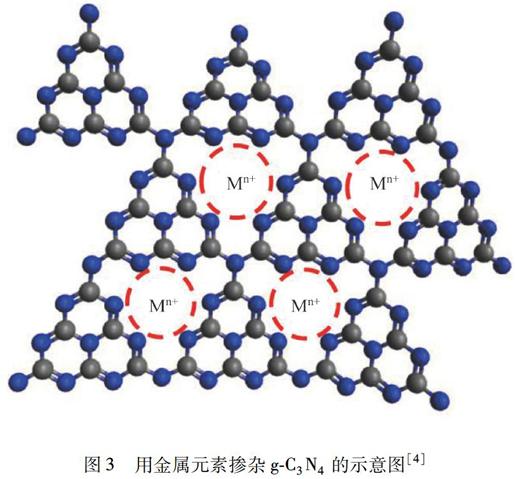
迄今,国内外已发表了大量关于修饰g-C3N4的文献.通过使用诸如Zn、Ni、Cu、Fe等金属元素[12-16]以及O、C、P、S、B、I、F等非金属元素[17-32]的掺杂来有效地增加光吸收,降低带隙,改进g-C3N4的光学和电子性能,提高电荷迁移率和延长电荷载体的寿命,这些都能显著提高光催化活性(图3).
图3用金属元素掺杂g-C3N4的示意图[4]
Wang等[33]首次报道了Zn2+和Fe2+掺杂在g-C3N4的骨架中.科研人员发现含金属的g-C3N4不仅扩大了光吸收范围,而且金属/g-C3N4纳米混合物随着金属含量的增加,其吸收峰缓慢地发生红移(较低的能量),这表明在金属掺杂剂和g-C3N4之间形成了主-客体相互作用.Huang等[34]通过用H2O2处理三聚氰胺来制备具有高度多孔网络的O掺杂的g-C3N4,产生氢键诱导的超分子聚集体,然后在550 ℃,N2流下热煅烧.O元素的掺杂使得g-C3N4的带隙变小,C原子处的电子密度显着降低,产生了对电子-空穴分离有利的内部电场.Hu等[35]利用双氰胺作为g-C3N4前体和磷酸氢二铵作为磷源,制备了磷掺杂的g-C3N4.磷原子掺杂到g-C3N4晶格中,产生P-N键.
与此同时,不少文献报道了通过稀土元素的掺杂来增强g-C3N4的光学性质和催化降解效果.Zhao等[36]在200 ℃的微波水热条件下合成g-C3N4/tz-Bi0.92Gd0.08VO4异质结.这异质结能够形成自组装球,具有优异的紫外光响应的n型半导体(图4).g-C3N4的引入可以形成羟基,然后捕获光孔并将大量光电子注入到四方相BiVO4(tz-BiVO4)中.这样能有效促进光生电子-空穴对的分离和迁移,产生活性基团,随后迅速矿化罗丹明B(RhB)分子.而Gd3+诱导效应影响晶体的转变,从单斜相(ms-BiVO4)到tz-BiVO4,使得降解性质得到了提升,提高了矿化效果.Zhao等[37]首次通过使用壳聚糖作为绿色交联剂的热蒸发方法,成功地制造了具有高光学透明度和机械稳定性的下转换和上转换发光g-C3N4纳米微粒(图5).借助N-羟基琥珀酰亚胺/1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC/NHS),将羧酸修饰过的多色稀土(物质的量分数:78%Y+20%Yb+2%Er)上转换纳米粒子(cit-UCNPs)与氨基封端的超薄g-C3N4纳米片进行化学偶联,形成下转换和上转换荧光发射的双重模式.
图4g-C3N4/tz-Bi0.92Gd0.08VO4异质结提出的矿化机理[36]
图5负载上转换纳米粒子(UCNP)的g-C3N4 的光动力治疗机理[37]
3应用领域
g-C3N4是一种无金属的聚合物半导体,具有良好的光学性质、较高的比表面积、良好的生物相容性等.这使得g-C3N4在光学、电学、生物医学等诸多领域的应用潜力非常广泛.
3.1光催化领域的应用
虽然g-C3N4在Wang等[10]的开创性报告已被证明能够在可见光照射下,通过分解水和有机污染物产生H2.然而,因为受到光电子-空穴对的快速复合,块状g-C3N4仍然具有低光催化活性.所以为了改进这一点,更多的研究者开始在Wang等[10]研究的基础上,深入对g-C3N4结构的探究并进行不同修饰和改进.这样使得g-C3N4在光催化领域的潜在应用被挖掘出来,提高了其光学性能.
Xu等[38]首次通过简单的化学剥离块状g-C3N4成功地获得了单原子层g-C3N4纳米片.与块状g-C3N4相比,单层g-C3N4纳米片表面积更大,光电载流子传输性能优异.因此,单层g-C3N4纳米片的光催化活性和光电流明显增强.与块状氮化碳相比,单层氮化碳纳米片作為光催化剂,能够催化产生3倍量的H2.Tong等[39]制备g-C3N4/TiO2(二氧化钛)纳米复合材料,在可见光和模拟阳光照射下,该材料对于RhB的催化降解,比单纯的TiO2、g-C3N4及其混合物具有更高的降解效率.其中g-C3N4占质量分数为25.9%的g-C3N4/TiO2纳米片表现出最高的光催化效率.在模拟太阳光的照射下,可以在50 min内降解几乎所有的RhB.
另外,Jiang等[40]成功地制备了一种具有不同无定形五氧化二钽(Ta2O5)溶胶/g-C3N4质量比的新型可见光反应型纳米片复合材料(ATCN).ATCN复合材料的这种显著增强的光催化活性可以主要归因于g-C3N4和无定形Ta2O5之间的协同效应,这种协同效应导致光生电子-空穴对更有效的分离、增大BET表面积以及增强可见光吸收.其中,加入体积为10 mL无定形Ta2O5为最佳材料.该光催化剂具有最高的可见光光催化活性(99.14%),降解速率常数为2.0055 h-1,分别约为纯g-C3N4、无定形Ta2O5的6.2倍和14.9倍.值得一提的是,即使在4个循环之后,ATCN样品也具有良好的再利用性和稳定性.
Zhang等[41]则是将稀土元素应用到了光催化领域.将等离子体激发和上转换效应结合,之后应用在光催化中,借此大幅度扩大光响应范围并且增强光催化的活性.该实验首次将Au纳米粒子、上转换纳米微球(NYF)和g-C3N4纳米片巧妙地整合到单个纳米结构体系中.这里的NYF微球用到了稀土元素Y、Yb、Tm.与纯g-C3N4相比,这种纳米复合材料在甲基橙(MO)的光催化降解中表现出优异的活性,以及极好的稳定性.值得一提的是,在优化实验的过程中,最佳方案的催化剂为1 %Au-NYF/g-C3N4(每1 mg催化剂的催化速率为0.032 h-1,即负载质量分数为1%的Au纳米粒子).该催化剂的催化速率远远超过NYF/g-C3N4(每1 mg催化剂的催化速率为0.009 h-1)和g-C3N4(每1 mg催化剂的催化速率为0.009 h-1).Au-NYF/g-C3N4纳米复合材料在不同光照下的高性能归因于明显促进的电荷的分离和抑制复合,以及这些组分中载流子和能量的有效转移.通过光电化学测量进一步证实了促进的电荷的分离和转移.利用同样的原理,梁瑞钰等[42]用Ce掺杂g-C3N4,随后对亚甲基蓝进行催化降解.实验表明Ce掺杂质量分数为0.21%时,材料的催化性能最优,是纯g-C3N4的4.9倍,并经过5次循环后仍保持96%的催化活性.同时,该课题组通过实验还发现,Ce的掺杂量决定了材料的催化性能.如若Ce的掺杂量过高,材料的体系中就会产生高浓度的Ce4+,Ce4+将会捕获大量的光生电子,进而间接引起光生电子-空穴复合效应.李婷婷等[43]则是利用研磨-焙烧法来制备g-C3N4-SmVO4.并通过实验得出材料在450 ℃、g-C3N4的质量分数为70%时,材料对RhB的催化降解活性是最优的.此外,材料不仅具有良好的稳定性,对于亚甲基蓝也有很好的催化作用.
3.2生物医学领域的应用
近年来g-C3N4在生物医学领域的应用取得了很大的进展.利用g-C3N4所具有的良好的生物相容性,科研人员在生物医学中开展了大量的研究,进而探索出g-C3N4在该领域的许多应用,包括生物成像、药物和基因载体、光学治疗等.
3.2.1生物成像
Zhang等[44]首次从含有大量块状g-C3N4的水中,通过在溶液中剥离的方法,成功制备超薄g-C3N4纳米片.该超薄纳米片的尺寸分布范围为70~160 nm,高度为约2.5 nm,约为7个C-N层(图6).与块状g-C3N4相比,超薄纳米片显示增强的光吸收和光响应,而且其量子产率高达19.6%.受益于高量子产率、高稳定性、良好的生物相容性以及无毒性,水溶性超薄g-C3N4纳米片有望用于生物成像,可进一步扩展到生物标记和生物医学应用.
图6剥离后的g-C3N4 纳米片的原子力显微镜(AFM)图(a)和透射电子显微镜(TEM)图(b)[41]
Zhang等[45]在g-C3N4上修饰了MnO2纳米粒子,通过对体内谷胱甘肽的响应来进行荧光成像.Xie等[46]制备了单层g-C3N4量子点,用于细胞核的双光子荧光成像.该单层g-C3N4量子点尺寸分布在2~6 nm,具有稳定且强烈的双光子荧光以及良好的生物相容性,能作为与4,6-联脒-2-苯基吲哚(DAPI)相当的绿色、经济、安全的荧光探针.Ma等[47]开发了基于Fe(III)掺杂的二维C3N4的线粒体靶向纳米平台.Fe(III)的掺杂导致过氧化物酶模拟材料在癌细胞中对H2O2具有优异的催化性能并产生O2.因为肿瘤的缺氧问题被克服,所以光动力治疗(PDT)的效率得到改善.同时材料具有有效的T1加权的体内磁共振成像(MRI)能力.在对荷瘤小鼠静脉注射12 h后,肿瘤区域的信号强度有明显增强.通过增强的渗透性和保留(EPR)效应,24 h后整个肿瘤面积变得更加明亮,这有利于提供最佳的治疗时间.该设计也是首次将多个不同的功能纳入基于梭型Fe掺杂的C3N4纳米片的单一纳米级纳米平台.
图7材料在小鼠体内的造影效果[49]
Feng等[48]则是将稀土元素与g-C3N4结合,此举也在生物成像方面起到了非常优异的效果.该课题组制备了一种UCNP纳米粒子,其核-壳结构是由NaGdF4∶Yb/Tm @ NaGdF4∶Yb @ NaNdF4∶Yb上转换发光(UCL)芯和光敏g-C3N4介孔壳组成.这种结构设计有着大比表面积和高负载量的优点,还具备了UCL、MRI和CT三模式成像的创新性.而在此之后,该课题组又进一步通过在超顺磁性氧化铁纳米球表面涂覆介孔石墨相碳氮化物(g-C3N4),然后用超小型UCNP来进行修饰,最后借助聚乙二醇(PEG)分子来进行改性[49].Fe3O4核与UCNPs之间的氮化碳层可以显著降低Fe3O4对UCNPs发射强度的淬灭效应.纳米平台表现出特定的磁性靶向性能,可以同时通过体外和体内T1/T2加权双模态MRI来监测(图7).
3.2.2分子载体
Liu等[50]在g-C3N4/Fe3O4纳米片上载了药物RhB,载药量高达108.6 mg·g-1,证明了其在靶向药物递送中具有很大的潜在应用.Li等[51]首次报道了g-C3N4纳米片可以作为纳米载体以抑制金属离子诱导的β-淀粉样蛋白(Aβ)聚集并分解预制的Aβ-Cu2+聚集体.
3.2.3生物检测
Hu等[52]验证了g-C3N4具有对各种染料标记的ssDNA荧光团的高荧光猝灭能力,进而设计了一种新的多色荧光纳米探针,用于多重序列特异性DNA检测(图8).Deng等[53]基于超薄氮化碳纳米片(C3N4)负载的钴(II)原卟啉IX(CoPPIX)制备了高效仿生催化剂.制备的纳米催化剂通过在C3N4的边缘平面上的多种胺与链霉抗生物素蛋白进一步缀合以便于标记.使用生物素化的分子信标作为捕获探针,通过电化学还原H2O2作为共反应物,开发出敏感的基于电化学发光的DNA测定方法,显示线性范围为10-15~10-10 mol/L,检测限为3.7×10-16 mol/L.
图8g-C3N4 纳米探针检测ssDNA机理图[52]
3.2.4光动力治疗
Chen等[54]设计开发了一种由负载DOX的g-C3N4@ZIF-8纳米颗粒组成的新型纳米尺度核-壳平台.制备的g-C3N4@ZIF-8纳米颗粒具有良好的生物相容性,可以有效地产生单线态氧,可用于光动力治疗.
Feng等[55]利用Tm3+激活的UCNPs进行光动力治疗,通过触发无机光敏剂在组织穿透近红外(NIR)光照射下产生细胞毒性活性氧(ROS)来有效消除肿瘤细胞.由于稀土元素的加入,使得UCNPs芯上涂覆介孔g-C3N4层在连接超小型Au25纳米團簇和PEG分子(命名为UCNPs@g-C3N4-Au25-PEG)之后,形成了一种新型双光敏剂纳米平台.来自UCNPs的紫外-可见(UV-vis)光和强烈的NIR发射可以分别激活g-C3N4和激发Au25纳米团簇以产生ROS,从而实现两种光敏剂的同时激活,增强单个NIR光激发介导的PDT效率.值得一提的是,实验通过高温热解法获得核壳结构的UCNP合成β-NaYF4∶Yb,Tm核,然后通过顺序外延生长法将外壳(β-NaGdF4∶Yb)涂覆在表面上.这也是制备UCNP的经典方法.在此之前,该课题组还在制备UCNP的时候掺杂了Nd元素[50].通过808 nm NIR光激发,发射的UV-vis光可以激活g-C3N4产生大量的ROS,并且掺杂的Nd3+离子能产生明显的热效应,这导致该材料能通过光动力治疗和光热治疗两种手段来对肿瘤进行治疗和干预.
4总结
如今,g-C3N4在光学、生物医学、电化学等领域的相关研究和应用已经取得了明显的成果.g-C3N4的制备方法也有了简单稳定的操作手段,但是现有的g-C3N4的制备方法都或多或少存在缺陷,比如如何大规模、短时间、高产率的制备高质量的g-C3N4仍然是研究者们探索的问题之一.在光催化方面,g-C3N4通过元素掺杂、化学修饰等方式,来解决光生电子-空穴对的快速复合的问题,使得g-C3N4提高了其在光催化方面的稳定性和光催化效率.而在生物医学领域,g-C3N4良好的生物相容性使得该材料在此领域有很好的发展潜力.剥离后的g-C3N4本身所具有的荧光能在生物成像、生物检测方面得以应用,其良好的光动力性质使其在肿瘤的光动力治疗方面具有良好的应用前景.但是g-C3N4的探索还需更加深入,如何能将其实际应用于生物医学领域,这还需要进行更多实验去研究.
參考文献:
[1]Maeda K,Wang X,Nishihara Y,et al.Photocatalytic activities of graphitic carbon nitride powder for water reduction and oxidation under visiblelight [J].Journal of Physical Chemistry C,2009,113(12):4940-4947.
[2]Dong F,Zhao Z W,Xiong T,et al.In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis [J].Applied Materials & Interfaces,2013,5(21):11392-11401.
[3]Wang X C,Blechert S,Antonietti M.Polymeric graphitic carbon nitride for heterogeneous photocatalysis [J].Acs Catalysis,2012,2(8):1596-1606.
[4]Ong W J,Tan L L,Ng Y H,et al.Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation:are we a step closer to achieving sustainability? [J].Chemical Reviews,2016,116(12):7159-7329.
[5]Yan S C,Li Z S,Zou Z G.Photodegradation performance of g-C3N4 fabricated by directly heating melamine [J].Langmuir,2009,25:10397-10401.
[6]Bai X J,Yan S C,Wang J J,et al.A simple and efficient strategy for the synthesis of a chemically tailored g-C3N4 material [J].Journal of Materials Chemistry A,2014,2(41):17521-17529.
[7]Wang X C,Maeda K,Chen X F,et al.Polymer semiconductors for artificial photosynthesis:hydrogen evolution by mesoporous graphitic carbon nitride with visible light [J].Journal of the American Chemical Society,2009,131(5):1680-1681.
[8]Ong W J,Tan L L,Chai S P,et al.Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane [J].Chemical Communications,2014,51(5):858-861.
[9]Zhang Y W,Liu J H,Wu G,et al.Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production [J].Nanoscale,2012,4(17):5300-5303.
[10]Zhang G G,Zhang J S,Zhang M W,et al.Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts [J].Journal of Materials Chemistry,2012,22(16):8083-8091.
[11]Wang X C,Maeda K,Thomas A,et al.A metal-free polymeric photocatalyst for hydrogen production from water under visible light [J].Nature Materials,2009,8(1):76-80.
[12]Pan H,Zhang Y W,Shenoy V B,et al.Ab initio study on a novel photocatalyst:functionalized graphitic carbon Nitride Nanotube [J].Acs Catalysis,2011,1(1):99-104.
[13]Ding G,Wang W,Jiang T,et al.Highly selective synthesis of phenol from benzene over a vanadium-doped graphitic carbon nitride catalyst [J].Chemcatchem,2013,5(1):192-200.
[14]Yue B,Li Q Y,Iwai H,et al.Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light [J].Science & Technology of Advanced Materials,2011,12(3):034401.
[15]Tonda S,Kumar S,Kandula S,et al.Fe-doped and mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight [J].Journal of Materials Chemistry A,2014,2(19):6772-6780.
[16]Ye X,Cui Y,Qiu X,et al.Selective oxidation of benzene to phenol by Fe-CN/TS-1 catalysts under visible light irradiation [J].Applied Catalysis B Environmental,2014,152-153(1):383-389.
[17]Guo S N,Zhu Y,Yan Y Y,et al.Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity [J].Applied Catalysis B Environmental,2016,185:315-321.
[18]Bu Y Y,Chen Z Y.Effect of oxygen-doped C3N4 on the separation capability of the photoinduced electron-hole pairs generated by O-C3N4@TiO2 with quasi-shell-core nanostructure [J].Electrochimica Acta,2014,144(144):42-49.
[19]Dong G H,Ai Z H,Zhang L Z.Efficient anoxic pollutant removal with oxygen functionalized graphitic carbon nitride under visible light [J].Rsc Advances,2014,4(11):5553-5560.
[20]She X,Liang L,Ji H,et al.Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4,nanosheets with highly efficient photocatalytic H2,evolution from water under visible light [J].Applied Catalysis B Environmental,2016,187(5):144-153.
[21]Dong G H,Zhao K,Zhang L Z.Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4 [J].Chemical Communications,2012,48(49):6178-6180.
[22]Zhang P,Li X H,Shao C L,et al.Hydrothermal synthesis of carbon-rich graphitic carbon nitride nanosheets for photoredox catalysis [J].Journal of Materials Chemistry A,2015,3(7):3281-3284.
[23]Zhang L G,Chen X F,Guan J,et al.Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity [J].Materials Research Bulletin,2013,48(9):3485-3491.
[24]Lan D H,Wang H T,Chen L,et al.Phosphorous-modified bulk graphitic carbon nitride:Facile preparation and application as an acid-base bifunctional and efficient catalyst for CO2,cycloaddition with epoxides [J].Carbon,2016,100:81-89.
[25]Xu C Y,Han Q,Zhao Y,et al.Sulfur-doped graphitic carbon nitride decorated with graphene quantum dots for an efficient metal-free electrocatalyst [J].Journal of Materials Chemistry A,2015,3(5):1841-1846.
[26]Ma X G,Lyu Y H,Xu J,et al.A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements:a first-principles study [J].Journal of Physical Chemistry C,2016,116(44):23485-23493.
[27]Lin S,Ye X X,Gao X M,et al.Mechanistic insight into the water photooxidation on pure and sulfur-doped g-C3N4,photocatalysts from DFT calculations with dispersion corrections [J].Journal of Molecular Catalysis a Chemical,2015,406:137-144.
[28]Lu C H,Chen R Y,Wu X,et al.Boron doped g-C3N4,with enhanced photocatalytic UO2+2,reduction performance [J].Applied Surface Science,2016,360(Part B):1016-1022.
[29]Raziq F,Qu Y,Zhang X L,et al.Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons [J].The Journal of Physical Chemistry C,2016,48(2):31-41.
[30]Pan H Z,Zhang H Y,Liu H M,et al.Interstitial boron doping effects on the electronic and magnetic properties of graphitic carbon nitride materials [J].Solid State Communications,2015,203(203):35-40.
[31]Hu S Z,Ma L,Xie Y,et al.Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions [J].Dalton Transactions,2015,44(48):20889-20897.
[32]Han Q,Hu C G,Zhao F,et al.One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting [J].Journal of Materials Chemistry A,2015,3(8):4612-4619.
[33]Wang X C,Chen X F,Thomas A,et al.Metal-containing carbon nitride compounds:a new functional organic-metal hybrid material [J].Advanced Materials,2010,21(16):1609-1612.
[34]Huang Z F,Song J,Pan L,et al.Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution [J].Nano Energy,2015,12:646-656.
[35]Hu S Z,Ma L,You J G,et al.A simple and efficient method to prepare a phosphorus modified g-C3N4 visible light photocatalyst [J].Rsc Advances,2014,4(41):21657-21663.
[36]Zhao C C,Tan G Q,Huang J,et al.Preparation of self-assembled spherical g-C3N4/tz-Bi0.92Gd0.08VO4 heterojunctions and their mineralization properties [J].Acs Applied Materials & Interfaces,2015,7(43):23949-23957.
[37]Zhao Y F,Wei R Y,Feng X,et al.Dual-mode luminescent nanopaper based on ultrathin g-C3N4 nanosheets grafted with rare-earth up-conversion nanoparticles [J].Acs Applied Materials & Interfaces,2016,8(33):21555-21562.
[38]Xu J,Zhang L W,Shi R,et al.Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis [J].Journal of Materials Chemistry A,2013,1(46):14766-14772.
[39]Tong Z W,Dong Y,Xiao T X,et al.Biomimetic fabrication of g-C3N4/TiO2,nanosheets with enhanced photocatalytic activity toward organic pollutant degradation [J].Chemical Engineering Journal,2015,260(260):117-125.
[40]Jiang Y H,Liu P P,Liu Y,et al.Construction of amorphous Ta2O5/g-C3N4,nanosheet hybrids with superior visible-light photoactivities for organic dye degradation and mechanism insight [J].Separation & Purification Technology,2016,170:10-21.
[41]Zhang J Z,Deng J J,Xu Z H,et al.High-efficiency broadband C3N4 photocatalysts:synergistic effects from upconversion and plasmons [J].ACS Catalysis,2017,7:6225-6234.
[42]梁瑞鈺,徐冬冬,查文莹,等.铈掺杂石墨相氮化碳的合成及可见光光催化性能 [J].高等学校化学学报,2016,37(11):1953-1959.
Liang R Y,Xu D D,Zha W Y,et al.Preparation of Ce-doped graphitic carbon nitride with enhanced visible-light photocatalytic activity [J].Chemical Journal of Chinese Universities,2016,37(11):1953-1959.
[43]李婷婷.钒酸钐基催化剂的制备及其光催化性能的研究 [D].金华:浙江师范大学,2013.
Li T T.Preparation and photocatalytic activities of vanadate samarium based catalysts [D].Jinghua:Zhejiang Normal University,2013.
[44]Zhang X D,Xie X,Wang H,et al.Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging [J].Journal of the American Chemical Society,2013,135(1):18-21.
[45]Zhang X L,Zheng C,Guo S S,et al.Turn-On fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet-MnO2 sandwich nanocomposite [J].Analytical Chemistry,2014,86:3426-3434.
[46]Zhang X D,Wang H X,Wang H,et al.Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus [J].Advanced Materials,2014,26(26):4438-4443.
[47]Ma Z F,Zhang M C,Jia X D,et al.Fe(III)-doped two-dimensional C3N4 nanofusiform:a new O2-evolving and mitochondria-targeting photodynamic agent for MRI and Enhanced Antitumor Therapy [J].Small,2016,12(39):5477-5487.
[48]Feng L L,He F,Liu B,et al.g-C3N4 coated upconversion nanoparticles for 808 nm near-infrared light triggered phototherapy and multiple imaging [J].Chemistry of Materials,2016,28(21):7935-7946.
[49]Feng L L,Dan Y,Fei H,et al.A core-shell-satellite structured Fe3O4@g-C3N4-UCNPs-PEG for T1/T2-weighted dual-modal MRI-guided photodynamic therapy [J].Advanced Healthcare Materials,2017,6(18):1700502.
[50]Liu C G,Wu X T,Li X F,et al.Synthesis of graphene-like g-C3N4/Fe3O4 nanocomposites with high photocatalytic activity and for drug delivery [J].Rsc Advances,2014,4(107):62492-62498.
[51]Li M,Guan Y J,Ding C,et al.An ultrathin graphitic carbon nitride nanosheet:a novel inhibitor of metal-induced amyloid aggregation associated with alzheimer′s disease [J].Journal of Materials Chemistry B,2016,4(23):4072-4075.
[52]Hu K,Zhong T M,Huang Y,et al.Graphitic carbon nitride nanosheet-based multicolour fluorescent nanoprobe for multiplexed analysis of DNA [J].Microchimica Acta,2015,182(5-6):949-955.
[53]Deng S Y,Yuan P X,Ji X B,et al.Carbon nitride nanosheet-supported porphyrin:a new biomimetic catalyst for highly efficient bioanalysis [J].Acs Applied Materials & Interfaces,2015,7(1):543-552.
[54]Chen R,Zhang J F,Wang Y,et al.Graphitic carbon nitride nanosheet@metal-organic framework core-shell nanoparticles for photo-chemo combination therapy [J].Nanoscale,2015,7(41):17299-17305.
[55]Feng L L,He F,Dai Y L,et al.A versatile near infrared light triggered dual-photosensitizer for Synchronous bioimaging and photodynamic therapy [J].Acs Applied Materials & Interfaces,2017,9(15):12993-13008.

