Hydrogen Promoted Decomposition of Ammonium Dinitramide:an ab initio Molecular Dynamics Study
Ling-hu TnJin-hu XuLei ShiXu-rn XuGui-xing WngWei Jing
a.National Special Super fine Power Engineering Research Center,Nanjing University of Science and Technology,Nanjing 210094,China
b.School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China
I.INTRODUCTION

FIG.1 A molecule(a)and a 1×1×2 supercell(b)of ADN.Carbon,nitrogen,oxygen,and hydrogen atoms are represented by gray,blue,red,and white spheres,respectively.
High oxidizer is an important component of solid propellants,and the overall performance of propellants bene fits from the outstanding oxidizer with high density,high energy,low toxicity and pollution,and good compatibility.During the past several decades,great efforts have been devoted to designing,synthesizing,and developing novel advanced high oxidizers[1–5].Among all the high oxidizers,ammonium dinitramide(FIG.1,ADN,NH4+N(NO2)2−)[6,7]was considered as an outstanding representative with great value for future research and application. ADN,a novel energetic salt shows better energy property than the traditional oxidizer ammonium perchlorate.In order to promote the application of ADN,it is necessary to understand its structure and decomposition behavior under different conditions. In particular,thermal decomposition of solid ADN,including its initiation and combustion mechanisms,is essential for the improvement of the safety pro file during its application and disposal,and could also provide valuable guidance regarding the development of energetic salts.Therefore,it is important to clearly understand the thermal decomposition of ADN at different external conditions,and a certain amount of relevant work[8–18]has been conducted so far. Specifically,by using thermomicroscopic(TM),thermogravimetric(TG),modulated differential scanning calorimetry(MDSC),and Fourier transform infrared(FTIR)methods,Lobbeckeet al.[8]studied the thermal decomposition and the associated gas release of ADN at temperature ranging from 20°C to 275°C.The results showed that ADN melted at 91.5°C,followed by the exothermal decomposition finished at 200°C.The main decomposition products were NH4NO3,H2O and N2O,meanwhile,other gases like NH3,NO2,NO,N2and O2were also detected.Oxleyet al.[9]reported that the production of N2O during ADN decomposition was only attributed to the dinitramide,and the main decomposition paths varied from different temperatures(50−450°C):(i)T<160°C,ADN→NH4++NO3−+N2O;(ii)T>160°C,ADN→NH3+HN(NO2)2, HN(NO2)2→N2O+HNO3.Furthermore,by utilizing TG and differential scanning calorimetry(DSC)methods with the mass spectrometer(MS)(50−250°C),Vyazovkinet al. [10]demonstrated that N2O,NO and NO2were the main products during the early decomposition stage of ADN,while H2O,HONO,NH3and HNO3were produced in the following stages.They proposed two possible main thermal paths were:(i)ADN→NH4++N2O+NO3−and(ii)ADN→NH4++NO2+NNO2−. However,it should be noted that the proposed reactions in this report were completely different from that in other reports[11],which were(i)ADN→NH3+HN(NO2)2and(ii)ADN→NH3+HNO3+N2O.Moreover,Izatoetet al. [12]investigated the kinetics of thermal decomposition(30−350°C)of ADN by employing the TG-DTA-MS-IR method and found that the main gases released were NH3,H2O,N2,NO,N2O,and NO2.In addition,Rahm and Brinck studied the thermal decomposition of ADN[16]based on the chemical modeling of molecular clusters,the computed results showed that the decomposition reaction of N−NO2breaking could be favored if there is a polarized coordination of N(NO2)−by an NH4+and this reaction is the rate-determining step under atmospheric and low-pressure.Furthermore,they also used theoretical methods to investigate the thermal decomposition of dinitraminic acid HN(NO2)2[17](HDN)in gas-phase and N(NO2)−[18]in solution,it was found that the decomposition of HDN is initiated by a dissociation and formed NO2,while two decomposition mechanisms including the direct transformation into nitrate and nitrous oxide and a bond breaking into the NNO2radical anion and NO2are competitive.
Through the aforementioned studies on the decomposition of ADN in solid phase,several possible decomposition paths were proposed according to the main decomposition products detected.However,the decomposition of ADN is very fast and complex,which sometimes is challenging to be clearly understood through experiments,resulting in the inconsistence proposed mechanisms that have been reported.For instance,the proposed initial decomposition reactions and the detected decomposition products were different in three independent experimental results[9–11],which implies that the decomposition behavior and process of ADN have not been well understood.Besides,the temperature ranges in these studies were limited(30−450°C).Therefore,it is desirable to carry more studies with expanded temperature ranges,in order to further understand the decomposition chemistry of ADN.
As a high-precision theoretical method,theab initiomolecular dynamics(AIMD)method has been successfully employed to investigate the thermal decomposition of several famous CHNO energetic compounds under high temperature or pressure,including hexanitrohexaazaisowurtzitane(CL-20)[19],pentaerythritol tetranitrate(PETN)[20],1,1-diamino-2,2-dintroethylene(FOX-7)[21],and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine(HMX)[22].Through the AIMD method,it is convenient to investigate the key chemical events of decomposition,such as initial decomposition reactions,processes,intermediates,and products.This makes AIMD a promising method that could be employed to study the thermal decomposition of energetic compounds,since the laboratory operating of energetic compounds is usually much more dangerous and challenging than that of other safe compounds.Hence,in the present study,the chemical process of the decomposition of ADN under high temperature was studied systematically with the help of AIMD method.The main purpose of this work is to con firm the possible initial decomposition mechanisms,uncover the decomposition process,and investigate the effects of temperature on the thermal decomposition of ADN.
II.COMPUTATIONAL METHODS
In this study,all AIMD simulations were performed by the CASTEP code[23]using norm-conserving pseudopotentials[24]and a plane-wave expansion of the wave functions.The Perdew-Burke-Ernzerhof(PBE)[25]exchange-correlation function and a singlek-point were employed.The Tkatchenoko and Scheffer(TS)[26]was utilized to correct the missing van der Waals(vdW).The ionic temperature was controlled by using a Nosé thermostat[27].AnNV Tensemble was employed.A time step of 1.0 fs was used.Since the simulation of decomposition in the solid-phase at ambient temperature is a very slow process,AIMD simulations in the present study were carried out at a higher temperature(2000 and 3000 K)than the normal decomposition.A plane wave cutoffof 500 eV for AIMD simulations and 830 eV for geometry optimizations were used.The calculated crystal cell parameters(a=7.10˚A,b=11.67˚A,c=5.56˚A)were very close to the experimental results[28](a=6.96˚A,b=11.79˚A,c=5.61˚A),and the absolute errors were 2.8%,1.0%,and 0.9%,respectively.Both the unimolecular and multimolecular decomposition simulations were performed after 5 ps of equilibration at 300 K.The unimolecular decompositions of isolated ADN were investigated by usingNV Tensemble dynamics with a 15.0˚A cubic periodic cell,which is large enough to prevent interactions between periodic images.In order to provide appropriate statistical sampling and acquire more reliable MD results,10 independent simulations were carried out at 2000 and 3000 K for 5 ps,respectively.The multimolecular decomposition simulations at 2000 and 3000 K were performed on a 1×1×2 supercell(8 molecules,FIG.1(b)).The initial positions of the simulation supercell were taken from the experimental result of X-ray crystal structure[28].The bond-lengths criteria was employed to identify the changes in geometry in the simulations.

FIG.2 Two different initial decomposition reactions of unimolecular ADN at 2000 and 3000 K.
III.RESULTS AND DISCUSSION
A.Unimolecular decomposition

FIG.3 The snapshots of two different initial decomposition reactions of unimolecular ADN at 2000 and 3000 K.Carbon,nitrogen,oxygen,and hydrogen atoms are represented by gray,blue,red,and white spheres,respectively.
From ten independent simulations at 2000 and 3000 K, two differentinitialdecomposition reactions(IDR,FIG.2)were observed,and the corresponding snapshots were shown in FIG.3. As described,at 2000 K,ADN decomposed through intramolecular hydrogen transfer (IDR1) [9,11]and N−NO2cleavagein N(NO2)−(IDR2)[10,16–18],whichwereADN→NH3+HN(NO2)2,and ADN→NH4++NO2+N2O2,respectively.The observed number of times,i.e.the occurred number of times in 10 independent simulations,of two different decomposition reactions of unimolecular ADN at 2000 and 3000 K were counted.It can be found that the observed number of times for the two paths(IDR1,IDR2)were equal(5 times)at 2000 K,while the number of times for IDR1 increased to 7 at 3000 K while for IDR2 decreased to 3,indicating that these two reactions are competitive at 2000 K,and IDR1 became predominant along with the temperature increases.For further decomposition based on IDR1,the N−NO2bond cleavage of HN(NO2)2would generate NO2+HONNO(FIG.2),and the subsequent decomposition of HONNO would produce N2O through one of the following reactions:(i)HONNO→HO+N2O or(ii)H+HONNO→H2O+N2O(H from N−H bond cleavage).The former reaction path was predominant at 3000 K,while the later one was mostly observed at 2000 K.This may be due to that N−OH cleavage was easier to occur at higher temperature,and the hydrogen was necessary for the promotion of the HONNO decomposition at low temperature.As for the further decomposition based on IDR2,one H in NH4+would transfer into N2O2forming HONNO(FIG.2),and the HONNO would decompose into N2O via the similar path of IDR1.In all,the main initial decomposition reaction should be:ADN→NH3+N2O+NO2+OH.The production of OH and NH3could release H and O,and the active H could interact with N or O to capture O in N2O and NO2,thus generating N2and NO:H+N2O→N2+OH and H+NO2→NO+OH.Meanwhile,the active O played arole in the oxidization of NHx(x=1−3)into NO or NO2.The isolated H and O could also self-bond into H2and O2,respectively.The final decomposition products simulated via AIMD are listed in Table I.The main decomposition products were similar in independent simulations at 2000 and 3000 K,which were NO2,NO,N2O,N2,H2,O2,H2O,H,and O.These gas products from our AIMD simulation were consistent with that obtained from previous experimental investigations[8–12,16].The decomposition of ADN experienced a faster and deeper process when at 3000 K than that at 2000 K,as NO and N2was more commonly observed in all ten simulations at 3000 K.Besides,the observation of NO3−was attributed to the reactions of NO2+OH→NO3−+H,and NO2+N2O2→NO3−+N2O,which has been reported in previous experimental studies[9,10].The unstable NO3−would quickly interreact with H or NH4+to form HNO3that has also been reported as the initial decomposition product[9,11],which could further transform into other NxOy.

TABLE I The main unimolecular decomposition products of ADN at 2000 and 3000 K.
B.Multimolecular decomposition
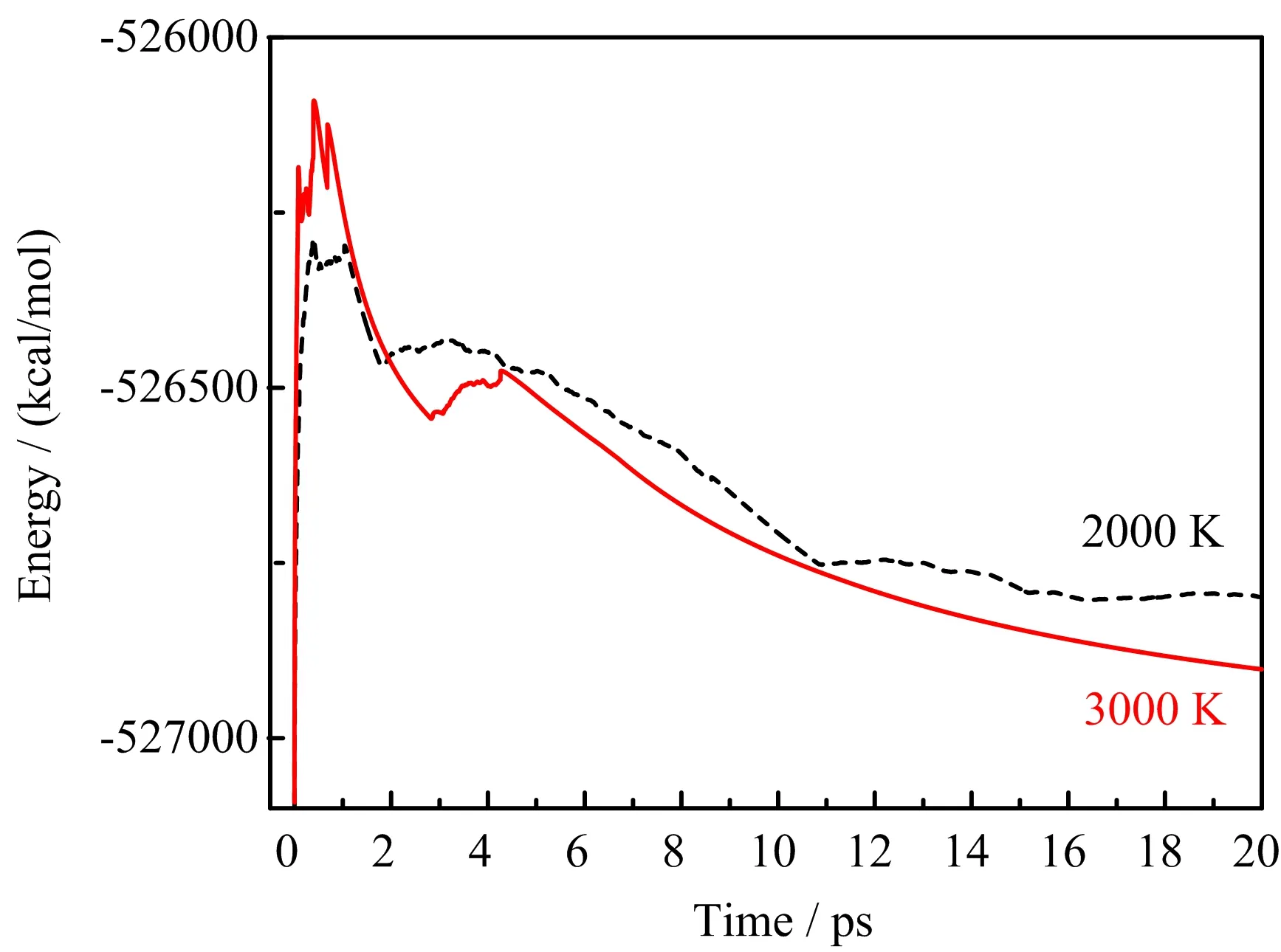
FIG.4 The time dependence of the total energy of multimolecular ADN at 2000 and 3000 K.

TABLE II The observed number of times of four different initial decomposition reactions of multimolecular ADN at 2000 and 3000 K.
In this section,AIMD method was also employed to investigate the multimolecular thermal decomposition of ADN at 2000 and 3000 K,respectively.As described in FIG.4,the energy changes at 3000 K were more obvious and faster than those at 2000 K,indicating that the decomposition at 3000 K were more drastic than that at 2000 K.Notably,the multimolecular thermal decomposition of ADN at 2000 and 3000 K were found to be more complicated than the unimolecular decomposition.In addition to the proposed two reactions of IDR1 and IDR2,another two initial decomposition mechanisms were also involved in decomposing eight ADN molecules at 2000 and 3000 K,which were the intermolecular hydrogen transfer(IDR3):the H in NH4+of one ADN molecule transfers into the O in N(NO2)2−of another ADN molecule,and the pure N−H bond breaking(IDR4):NH4+→NH3+H.The generated H in IDR4 would not suffer the intramolecular or the intermolecular transfer into N(NO2)2−,but would react with other intermediates to promote the decomposition.Table II listed the observed number of times of the four different initial multimolecular decomposition reactions of ADN at 2000 and 3000 K.The IDR1,IDR2,IDR3 and IDR4 were observed for 1,4,2 and 1 number of times at 2000 K,respectively,and the number of times were changed to 3,1,2 and 2 when at 3000 K,indicating that N−NO2cleavage(IDR2)and intramolecular hydrogen transfer(IDR1)are the dominating initial decomposition mechanisms at 2000 and 3000 K,respectively.The hydrogen-related reactions(IDR1,IDR3 and IDR4)were competitive with the N−NO2breaking(both are 4 number of times)at 2000 K,whereas they became preponderant at 3000 K(number of times:7vs.1).Since Rahm and Brinck[16]ever reported that IDR2 could be favored if there is an polarized coordination of N(NO2)−by an NH4+,it may be inferred this polarization effect reduces with the increasing of temperature,making IDR2 to be disfavored at higher temperature to some degree.These observations of multimolecular decomposition were similar to those of unimolecular decomposition of ADN.Further,after the initial decomposition,ADN would decompose into small products such as N2and H2O by hydrogen-promoted simple,fast and direct N−O,N−N,N−H and O−H cleavage and recombination(FIG.6)without forming any long catenulate,big nitrogen-rich or carbon intermediates both at 2000 and 3000 K,which was in line with the thermal decomposition of some organic energetic compounds including nitromethane[29],1,3,5-triamino-2,4,6-trinitrobenzene[30],and 3,6-di(azido)-1,2,4,5-tetrazine[31].The hydrogen-promoted decomposition reactions would be elaborated in the following section.In addition,it was found that the subsequent key events of ADN decomposition at 2000 and 3000 K were similar,and the only difference was the faster decomposition rate when at 3000 K.Therefore,the following discussions were mainly focused on the decomposition process at 2000 K.

FIG.5 Two new initial decomposition reactions of multimolecular ADN at 2000 and 3000 K.

FIG.6 The total decomposition process of multimolecular ADN at 2000 K.
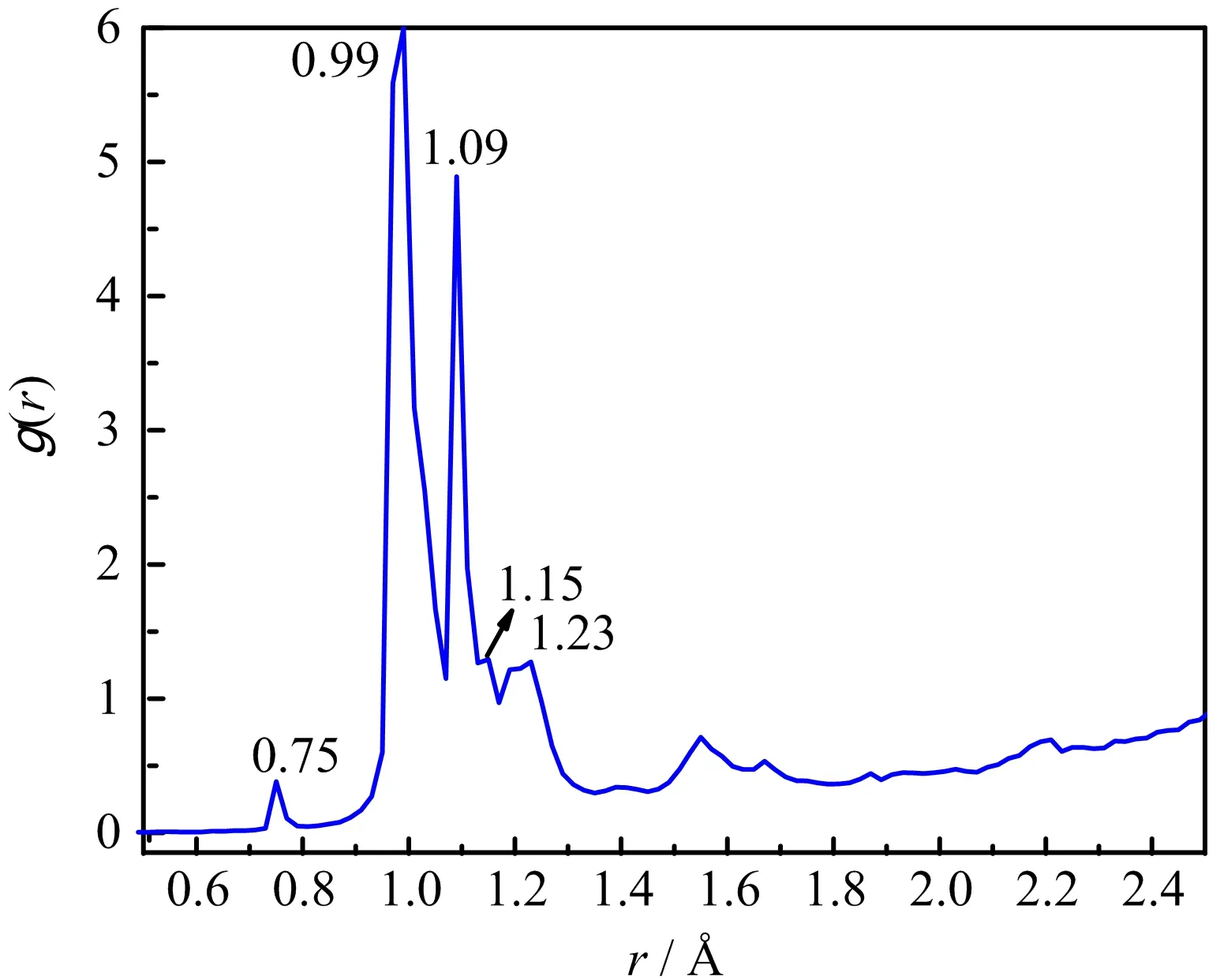
FIG.7 The radial distribution function of multimolecular ADN at 2000 K.
As described in FIG.7 for the radial distribution function(RDF)of multimolecular ADN at 2000 K,the peak at about 0.75˚A was corresponding to the H−H bond,indicating the formation of H2[11],while the peak at about 0.99˚A was associated with the O−H bond,suggesting the generation of H2O or OH.In addition,the peak at∼1.09 ˚A was related to the N≡N bond,indicating the formation of N2.The peak at∼1.15˚A could be assigned to the N=O and N−H bonds,which indicated the generation of NO and NH3.The peak at∼1.23 ˚A was related to the N−O bond in NO2.It is worthy to point that the two strongest peaks at 0.99 and 1.09˚A indicated that the H2O and N2were two main decomposition products.The RDF of multimolecular ADN at 3000 K showed similar manner to that at 2000 K,meaning that they underwent similar procedure.
FIG.8 described the time dependence of the number of four main bonds(N−H,N−O,N−N and O−H bond)in the system at 2000 K.Firstly,the number of N−H bond decreased fast to zero in 6.0 ps,and fluctuated at around 0−5.Therefore,the changing process of the weak N−H bond would be:NH4+decomposed into NH3,and H easily left N to promote the further decomposition via capturing O in N−O bond to form OH and H2O,while the rest N would combine with either N or O atoms.Secondly,the number of N−O bond decreased gradually because of the generation of N2O,NO,N2and H2O.Thirdly,the number of N−N bond deceased in the first 3.0 ps which may be due to the cleavage of N−N bond in N(NO2)−,whereas the gradual increase of the number of N−N bond in the following 17.0 ps was because of the formation of N2.Finally,the number of O−H bond increased along with the decomposition process of ADN,which was due to the formation of H2O.FIG.9 depicted the time dependence of the number of ADN molecule and the main decomposition products(NH3,NO2,NO,N2O,N2,HNO2,and H2O)in the system at 2000 K.All eight ADN molecules decomposed in about 0.6 ps,accompanied by the release of NH3[8–12],followed by its transformation into other compounds in about 6.0 ps.The formation of NO2occurred at very early stage at about 0.02 ps via the reaction:N(NO2)2−→NO2+N-NO2.Subsequently,NO2would further react with H to form NO at about 0.18 ps via the reaction:NO2+H→NO+OH.The N−NO2would react with H to release N2O at about 0.09 ps through the reaction:N-NO2+H→N2O+OH.The N2O is an active intermediate which would become N2in about 13 ps.N2formed at about 0.6 ps by the reaction:H+N2O→OH+N2.The active OH would combine with H to produce H2O:OH+H→H2O.The increasing amount of N2and H2O were formed along with the composition process of ADN,and the existence of NO and NO2at the end of the decomposition was because of the high oxygen balance of ADN.Interestingly,HNO2involved in the reaction[10]is a relatively stable product:HO+NO↔HNO2.The unstable HNO3[9,10]was observed a few times by the reaction:HO+NO2↔HNO3,and would decompose quickly.Moreover,other unstable intermediates including O2[8],O3and H2O2were also observed.The unseen of NH4NO3may be because its unstable salt form under extreme high-temperature(2000 and 3000 K in this study).Additionally,the main decomposition products of ADN were NH3,NO2,NO,N2O,N2,H2O,and HNO2,which is consistent with the previous experimental results[8–11].

FIG.8 The time dependence of the number of main chemical bonds in the system at 2000 K.
IV.CONCLUSION

FIG.9 The time dependence of the number of the ADN molecule and main decomposition products in the system at 2000 K.
In this study,the AIMD method was employed to study the thermal decomposition of a famous new high oxidizer ADN under high temperature(2000 and 3000 K).The results showed that two different initial decomposition mechanisms were involved during the unimolecular decomposition of ADN,which were the intramolecular hydrogen transfer and N−NO2breaking in N(NO2)−. The two mechanisms competed at 2000 K,and the former one was predominant at 3000 K,indicating that the initial decomposition mechanism was temperature dependent.For the multimolecular decomposition of ADN,another two new reactions were involved,which were the intermolecular hydrogen transfer and directly N−H breaking in NH4+.At 2000 K,the N−NO2cleavage competed with the other three hydrogen-related decomposition reactions,whereas hydrogen-related decomposition reactions was dominating at 3000 K,indicating that the initial decomposition reactions of multimolecular ADN were also temperature dependent.After the initial decomposition,the temperature increase from 2000 K to 3000 K could facilitate the further decomposition,while did not change the key decomposition events.ADN decomposed into small products like N2and H2O by hydrogenpromoted simple,fast and direct N−O,N−N,N−H and O−H cleavage without reforming any long or big intermediates that may impede the decomposition.The main decomposition products at 2000 and 3000 K were both NH3,NO2,NO,N2O,N2,H2O and HNO2.Our study provided another potential method for understanding the chemical process of ADN and other energetic high oxidizer with similar structure to ADN under high temperatures.
V.ACKNOWLEDGEMENTS
This work was supported by the Fundamental Research Funds for the Central Universities(No.30916011315),the Priority Academic Program Development of Jiangsu Higher Education Institutions,and the Fundamental Research Funds for the Central Universities(No.30916011317).
[1]T.T.Vo,D.A.Parrish,and J.M.Shreeve,J.Am.Chem.Soc.136,11934(2014).
[2]T.M.Klapötke,B.Krumm,S.F.Rest,M.Reynders,and R.Scharf,Eur.J.Inorg.Chem.2013,5871(2013).
[3]P.Yin,J.H.Zhang,C.L.He,D.A.Parrish,and J.M.Shreeve,J.Mater.Chem.A2,3200(2014).
[4]R.J.Buszek and J.A.Boatz,Propell.Explos.Pyrotech.39,787(2014).
[5]Q.Wu,W.H.Zhu,and H.M.Xiao,Struct.Chem.24,1725(2013).
[6]M.J.Rossi,J.C.Bottaro,and D.F.McMillen,Int.J.Chem.Kinet.25,549(1993).
[7]B.L.Fetherolf and T.A.Litzinger,Combust.Flame114,515(1998).
[8]S.Löbbecke,T.Keicher,H.Krause,and A.Pfeil,Solid State Ionics101/103,945(1997).
[9]J.C.Oxley,J.L.Smith,W.Zheng,E.Rogers,and M.D.Coburn,J.Phys.Chem.A101,5646(1997).
[10]S.Vyazovkin and C.A.Wight,J.Phys.Chem.A101,5653(1997).
[11]T.B.Brill,P.J.Brush,and D.G.Patil,Combust.Flame92,178(1993).
[12]Y.Izato,M.Koshi,A.Miyake,and H.Habu,J.Therm.Anal.Calorim.127,255(2017).
[13]H.Matsunaga,H.Habu,and A.Miyake,J.Therm.Anal.Calorim.113,1387(2013).
[14]K.Fujisato,H.Habu,and K.Hori,Propell.Explos.Pyrotech.39,714(2014).
[15]I.B.Mishra and T.P.Russell,Thermochim.Acta384,47(2002).
[16]M.Rahm and T.Brinck,J.Phys.Chem.A114,2845(2010).
[17]M.Rahm and T.Brinck,Chem.Phys.348,53(2008).
[18]M.Rahm and T.Brinck,Chem.Eur.J.16,6590(2010).
[19]O.Isayev,L.Gorb,M.Qasim,and J.Leszczynski,J.Phys.Chem.B112,11005(2008).
[20]C.J.Wu,L.E.Freid,L.H.Yang,N.Goldman,and S.Bastea,Nat.Chem.1,57(2009).
[21]Y.Liu,F.Li,and H.Sun,Theor.Chem.Acc.133,1567(2014).
[22]Q.Wu,G.L.Xiong,W.H.Zhu,and H.M.Xiao,Phys.Chem.Chem.Phys.17,22823(2015).
[23]M.D.Segall,P.J.D.Lindan,M.J.Probert,C.J.Pickard,P.J.Hasnip,S.J.Clark,and M.C.Payne,J.Phys.:Condens.Matter14,2717(2002).
[24]N.Troullier and J.L.Martins,Phys.Rev.B43,1993(1991).
[25]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev.Lett.77,3865(1996).
[26]A.Tkatchenko and M.Scheffler,Phys.Rev.Lett.102,073005(2009).
[27]S.Nosé,J.Chem.Phys.81,511(1984).
[28]R.Gilardi,J.Flippen-Anderson,C.George,and R.J.Butcher,J.Am.Chem.Soc.119,9411(1997).
[29]J.Chang,P.Lian,D.Q.Wei,X.R.Chen,Q.M.Zhang,and Z.Z.Gong,Phys.Rev.Lett.105,188302(2010).
[30]L.Z.Zhang,S.V.Zybin,A.C.T.Van Duin,S.Dasgupta,and W.A.Goddard III,J.Phys.Chem.A113,10619(2009).
[31]Q.Wu,W.H.Zhu,and H.M.Xiao,Phys.Chem.Chem.Phys.16,21620(2014).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Investigation on Preparation and Anti-icing Performance of Superhydrophobic Surface on Aluminum Conductor
- Study of Cadmium-Doped Zinc Oxide Nanocrystals with Composition and Size Dependent Band Gaps
- Glucose Isomerization into Fructose Catalyzed by MgO/NaY Catalyst
- Analysis of Solvent Effect on Mechanical Properties of Poly(ether ether ketone)Using Nano-indentation
- Direct Synthesis of Monodisperse Hollow Molecularly Imprinted Polymers Based on Unfunctionalized SiO2for the Recognition of Bisphenol A
- Agent-Based Network Modeling Study of Immune Responses in Progression of Ulcerative Colitis
