Micellar Exchange Kinetics of Quaternary Ammonium Type Gemini Surfactants Monitored by Nuclear Magnetic Resonance
Jun LiuYou-ru DuShi-zhen MoMi-li Liu
a.State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics,Wuhan Institute of Physics and Mathematics,Chinese Academy of Sciences,Wuhan 430071,China
b.National Engineering Laboratory of Wheat&Corn Further Processing,Henan University of Technology,Zhengzhou 450000,China
I.INTRODUCTION
The kinetic and dynamic molecular exchange between monomers in the bulk solution and those in the micelle,and the disintegration and reformation of the micelle are the key factors that affect the properties of surfactants[1].The two distinguishable relaxation processes are the fast process(molecular exchange)with the relaxation time,T1,generally on the order of microseconds and the slow process(disintegration and reformation)with the relaxation time,T2,generally on the order of milliseconds[2−4].
Nuclear magnetic resonance(NMR)spectroscopy has been used to detect the first class of relaxation dynamic process,i.e.,the molecular exchange kinetics[5].Huc and Oda[6] first reported the slow exchange of 14-2-14 at the NMR time scale,and quantitatively obtained the exchange characteristic time through 1D line shape analysis,which proved that NMR is an effective tool for the study of micellar exchange kinetics in aqueous solution.Afterwards,1D line shape analysis and 2D EXSY have been applied to the qualitative and quantitative analysis of exchange kinetics of some Gemini and conventional surfactants in single surfactant or mixed surfactant systems[7−10].In comparison with other experimental techniques available for studying the surfactant mixtures,NMR has special advantages of nonperturbation and invasion of the sample,as well as a small dosage of sample[11−15].Surfactant monomers in the bulk solution and those in the micelle are treated as two states in NMR methods.
Gemini surfactants are a new class of surfactants[16,17].They exhibit superior physicochemical properties compared to their analogous conventional surfactants[18−21].In spite of the wide application,kinetic processes of Gemini surfactants are not fully researched[22].The relaxation kinetics of quaternary ammonium type Gemini surfactants,12-s-12,has been investigated by Zanaet al.[23]using the pressure-jump relaxation method,where 12-s-12 was found to exhibit especially longer relaxation time ofT1andT2compared to their corresponding monomeric counterparts and the twostep exchange mechanism was proposed at the first time for explanation.Recently,the exchange kinetic process of 14-s-14(s=2,3 and 4)has been investigated by Cuiet al.,Jianget al.and Liuet al.using NMR methods of the line shape analysis and EXSY,whereas the exchange characteristic time of 14-s-14 at 298 K in the single surfactant as well as the mixed surfactant solution was obtained[8−10].However,the exchange rate constant and the exchange mechanism of 14-s-14 were not further investigated.Afterwards,Jianget al.[24]reported the molecular exchange kinetics of 12-s-12 in the mixed surfactant solution with conventional surfactants.Unfortunately,quantitative results of exchange kinetic parameters for 12-s-12 were lack.
In this study,we applied DNMR to quantitatively measure the molecular exchange rate constant between monomers in the bulk solution and those in the micelle of Gemini quaternary ammonium surfactants,14-s-14 as well as 12-s-12(s=2,3 and 4).Moreover,the exchange mechanism of Gemini surfactants was also further investigated through measurements of the activation energy and the micro-polarity by temperature variation and fluorescence probe experiments.
II.EXPERIMENTS
The Gemini quaternary ammonium surfactants,14-s-14(s=2,3 and 4)and 12-s-12(s=2,3 and 4),were synthesized and supplied by the Southwest Petroleum University(Chengdu,China).D2O with a deuteration of 99.9%and 2,2,3,3-d4-3-(trimethylsilyl)propionic acid sodium(TMSP)with a deuteration of 98%were obtained from Cambridge Isotope Laboratories.Pyrene with a purity of 98%was from ACROS ORGANICS.The reagents were used as received,without any further puri fication.The structures and proton numbering of the surfactants used in this study are shown in Scheme 1.
Stock solutions of 14-s-14(s=2,3,4)(1.0 mmol/L),12-2-12(2.0 mmol/L),12-3-12(2.4 mmol/L),12-4-12(3.0 mmol/L)in D2O were prepared initially.For 14-s-14,sample solutions at 2 cmc(critical micelle concentration)for 1D line shape analysis were prepared by diluting the stock solution until resonance peak areas for the monomer and the micelle are the same,and sample solutions below cmc were prepared by further dilution until the micelle resonance peaks well disappeared and the half-height line width of monomer resonance peaks no longer changed.For 12-s-12,their stock solutions were stepwise diluted to get a series of sample solutions with concentrations respectively at well below and above cmc for measurement of their cmc values.And their sample solutions at 2 cmc and below cmc for 1D line shape analysis were prepared by dilution of the stock solutions according to the obtained cmc values.
NMR measurements were performed on a Bruker AVANCE-500 with a proton frequency of 500.13 MHz and Bruker AVANCE-600 with a proton frequency of 600.13 MHz.For all 1D1H NMR spectra,a smallangle pulse of 30°was used rather than 90°in the conventional single-pulse sequence to save time.An external standard of TMSP(0.03 mmol/L)in a capillary was added in sample solutions of 14-s-14.Water suppression was performed with presaturation and the chemical shift of TMSP was calibrated to 0 ppm.Residue HOD was used as the internal standard in sample solutions of 12-s-12. No water suppression was performed and the chemical shift of HDO was calibrated to 4.70 ppm.The fluorescence emission spectra of pyrene were recorded using a Horiba Fluoromax-4 spectro- fluorometer at 298 K,operated at an excitation wavelength of 335 nm,with an excitation slit of 5 nm and an emission slit of 1 nm.The concentration of pyrene added in micellar solutions of each surfactant was 10−6mol/L.

Scheme 1 Molecular structures and proton numberings of 12-s-12(s=2,3,and 4)and 14-s-14(s=2,3,and 4).
III.RESULTS AND DISCUSSION
To well display the exchange process of 14-s-14 and 12-s-12 at the NMR time scale,resonance signals of H6(N-methyl proton)of 14-3-14 and 12-3-12 at different solution concentrations and different temperatures are shown in FIG.1.It is obvious that two sets of resonance peak for 14-s-14 appeared.Resonance signals of micelles,expressed as H6′in the spectra,appeared at the lower field corresponding to those of the free monomers in the bulk solution,expressed as H6 in the spectra.This shows that the exchange of 14-s-14 is slow at the NMR time scale.As for 12-s-12,only one coalesced signal is observed,showing that the exchange process is fast at the NMR time scale.So,different approaches should be considered to treat the experimental data.
According to the pseudo- first-order chemical exchange process(the two-site exchange reaction)of two states,the exchange equilibrium can be described by Eq.(1).


FIG.1 The resonance peaks of N-methyl proton,H6 for 14-3-14 and 12-3-12,(a)at different concentrations of 14-3-14 and 294 K,(b)at different concentrations of 12-3-12 and 288 K(The red lines are spectra at 2 cmc and below cmc for 1D line-shape analysis),(c)at different temperatures of 14-3-14 and 2 cmc,(d)at different temperatures of 12-3-12 and 2 cmc.
Where,SmicandSmonare the surfactant molecules respectively sited at the micelle state and the free monomer state in the bulk solution,k+andk−are the rate constants of the molecule entering from the bulk solution into the micelles and that of the molecule escaping from the micelles into the bulk solution.When the two molecule states,SmicandSmonexchange slowly at the NMR time scale,two sets of NMR signals respectively corresponding to the micellar and the monomeric states are observed as FIG.1(a)shows.Based on twosite exchange equation developed by Gutowsky and Holm[25],the NMR line width broadening at half height of the exchanging singlet compared to the nonexchanging reference singlet can be used to determinek+by the equation:

where ΔW1/2is the width broadening,which can be determined as the half-height line width(W1/2)difference of the resonance peak of free monomers respectively at the concentration above cmc and below cmc.At the concentration of 2 cmc,the statistical proportions of surfactant molecules at the free monomer state and the micelle state are identical,andk−is equal tok+and can be thus determined.
When the two states,SmicandSmon,exchange fast at the NMR time scale,only one set of their coalesced signal can be observed as FIG.1(b)shows.The observed resonance frequency(νobs)is actually between the resonance peak of free monomers and that of the micelles,expressed as:

whereνmonandνmicare the resonance frequencies of free monomers and micelles,respectivly.PmonandPmicare the statistical proportions of surfactant molecules sited at the two states.The half-height line width could be approximately given by Meiboom’s equation[26],

whereT2is the transverse relaxation time,τis the average residence time.The relationships ofτandk+,k−can be expressed as:

At the concentration of 2 cmck−is equal tok+and can be determined as 1/2τ.Therefore the half-height line width broadening at 2 cmc is analyzed to measurek−by the equation:

where ΔW1/2is the exchanging broadening of halfheight line width of the coalesced peak at 2 cmc compared to the resonance peak of free monomers below cmc.And Δνis the frequency difference betweenνmonandνmic,i.e.(νmic−νmon).νmonandνmiccan be determined from the chemical shift(Hz)variation curves as a function of the reciprocal of concentration,i.e.the cmc determination curves[7],where they-intercept values respectively of the linear fitted plot below cmc and of the linear fitted plot above cmc are deemed asνmonand
Values of cmc of 14-s-14 and 12-s-12 measured by the NMR method[7,9,10]in this study are listed in Table S1(supplementary materials).The calculatedk−values andτmic(the residence time in the micelle)of 14-s-14 determined according to Eq.(2)at 298 K are listed in Table I.Wherein,k−is determined ask+/αto eliminate the concentration deviation from 2 cmc(α=1)ask+is in direct proportion toαat certain concentration range(shown as FIG.S1 in supplementary materials).
Values ofk−andτmicof 12-s-12 were determined according to Eq.(5)and listed in Table II.
As Tables I and II show,the magnitude orders ofk−for 14-s-14 and 12-s-12 are respectively 10 and 103s−1,correspondingly,their residence time in micelles are respectively 0.07−0.51 s with the time scale of 10−1s and 0.19−0.54 ms with the time scale of 10−1ms.This shows that 12-s-12 exchanges nearly 103times faster than 14-s-14 in spite of having the same spacer length.It suggests that surfactants with the longer alkyl chains experience the stronger hydrophobic interactions which prevents them escaping from micelles,i.e.a longer residence time in micelles.
The variations ofk−of 12-s-12 and 14-s-14 with the spacer length are plotted in FIG.2.It shows that the values ofk−of 12-s-12 and 14-s-14 both increase withthe spacer length growing,andk−of 12-s-12 increases slower as compared to that of 14-s-14.

TABLE I Values of ΔWH6′,ΔWTMSP,ΔWH6and accordingly calculated values of k+,k− and τmicfor 14-s-14 at 298 K.
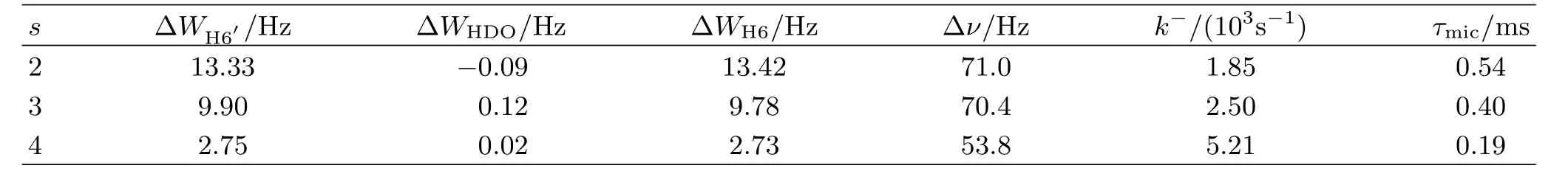
TABLE II Values of ΔWH6′,ΔWHDO,ΔWH6,Δν and accordingly calculated values of k− and τmicfor 12-s-12 at 298 K.
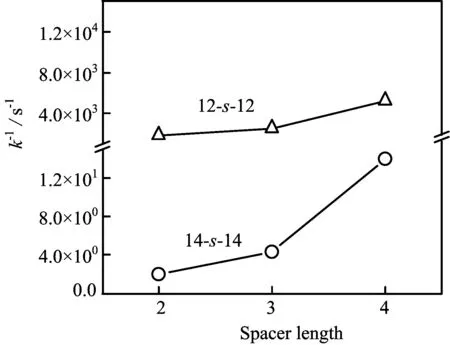
FIG.2 The variations of k−of 14-s-14 and 12-s-12 with the spacer length at 298 K.
Temperature variation experiments of the exchange process were performed.Tables S2 and S3(supplementary materials)listand accordingly calculated values ofk−of 14-s-14 and 12-s-12 at different temperatures.Results show thatk−values of 14-s-14 and 12-s-12 increased with temperature.Consequently,Ea−could be detected according to the Arrhenius equation:
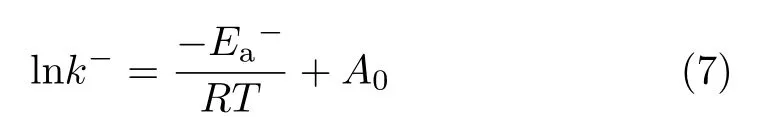
Curves of lnk−were fitted as a function of 1/Twith the linear correlation coefficients all above 0.95.Values ofEa−were obtained and are listed in Table III.As Table III shows,Ea−of 14-s-14 and 12-s-12 obviously decreases with the spacer length growing,which well explains the increase ofk−with the spacer length.
Generally speaking,the micro-polarity inside micelles can be a measure of its hydrophobicity inside the micelle core.The micro-polarity of these surfactant micelles was measured using fluorescence emission spectra.Pyrene(10−6mol/L)was added into the micellar solution with surfactant concentrations at 2 cmc,which wassupposed to be all dissolved in the micelle core.After being excited at 335 nm, fluorescence emission spectra of pyrene were recorded.As well known,the ratio of intensities for the first(I1)and the third(I3)electronic vibration peaks of pyrene are strongly dependent on the micro-polarity of its surrounding environment.The value ofI1/I3is high in pure aqueous solution(1.81 in current study).It would drastically fall when pyrene is dissolved in the hydrophobic core of micelles.The tighter the molecules are packed,the stronger the hydrophobicity and the weaker the micro-polarity in the micelle core,and the lower theI1/I3value would be.

TABLE III The values of Ea−(kJ/mol)for 14-s-14 and 12-s-12.
ExperimentalI1/I3values of pyrene in the micelles of 12-s-12 and 14-s-14 are listed in Table IV.
To better figure out the relationship between the hydrophobicity andEa−,the variation tendencies ofEa−(red symbols and lines)andI1/I3values(black symbols and lines)of 14-s-14 and 12-s-12 with the spacer length are depicted in the FIG.3.
It clearly shows that,Ea−of 12-s-12 and 14-s-14 almost linearly decreases withsincreasing.However,I1/I3increases less whensincreases from 3 to 4,which accordingly indicates that the hydrophobicity of the micelle core decreases less withsincreasing from 3 to 4.Besides,the micro-polarity of 14-s-14 is lower than that of 12-s-12 with the same spacer length,which suggests that the hydrophobicity inside the micelle core of 14-s-14 is higher than that in 12-s-12 micelles.It should be noted that the hydrophobicity difference between the micelle of 14-s-14 and 12-s-12 with the same spacer length actually gets larger as the spacer length increases.In contrast,the increment ofEa−of 14-s-14compared to 12-s-12 gets smaller.It suggests thatEa−does not totally originate from the intermolecular hydrophobic interactions in the micellar core.Some other factors should be considered.
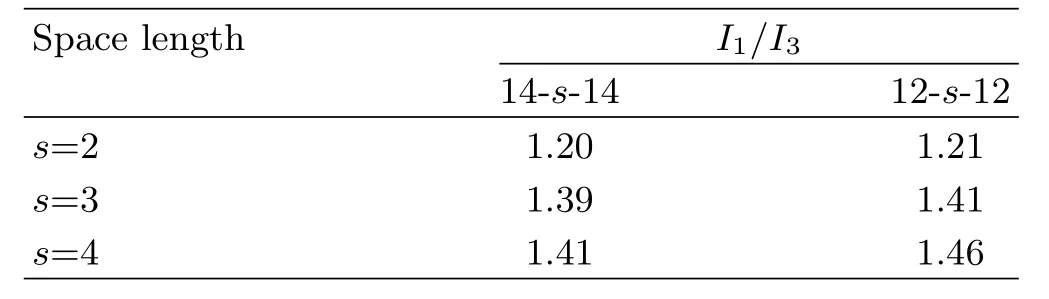
TABLE IV The I1/I3values of the pyrene probe inside the micelles of 12-s-12(s=2,3 and 4),and 14-s-14(s=2,3 and 4).

FIG.3 The Ea−(red colour)and I1/I3values(black colour)of 12-s-12(s=2,3 and 4)and 14-s-14(s=2,3 and 4).
The Gemini surfactant molecules have to adoptcisconformation inside the micelles due to high hydrophobic interaction. However,leaving from the micelle,thetrans-form should favor the molecule to overcome the intra-hydrophobic interaction between the two alkyl chains.The energy of transformation fromcis-form totrans-form in the micelle depends not only on the intra-molecular hydrophobic interactions but also on the steric effects inside the micelle core.Consequently,Gemini surfactant molecules have to overcome an extra steric hindrance effects inside the micelle core.The longer the spacer length,the looser the hydrophobic chain packed,consequently,the weaker the steric effect inside the micelle.So,the increment in the activation energy for Gemini surfactant to overcome the steric hindrance effect with longer spacer length becomes smaller,which should account for the additional decrease ofEa−in addition to the non-linear weakening of the hydrophobicity inside the micelle with the spacer length increasing.

FIG.4 Possiblemolecularexchangemodelsbetween monomers in the bulk solution and in the micelle for Gemini surfactants.
This conclusion actually well supports the two-step exchange model of 12-s-12 put forward by Zanaet al.[23],that is one alkyl chain of Gemini surfactant molecule exit first and then the other when escaping from the micelle.As FIG.4 shows,there should simultaneously exist the two-step exchange and one-step exchange mechanisms for Gemini surfactants,as free monomers in the bulk solution should adopt thetransas well as thecis-conformations in a view of statistic thermodynamics.With the spacer length growing,the statistical proportion of free monomers adopting thetrans-conformation decreases,and so would the proportion of Gemini surfactants to adopt the two-step exchange mechanism.Therefore,the energy for conformation changes of Gemini surfactant molecules to escape from the micelles gets smaller,which should be another factor accounting for the activation energy decrease with the spacer length.
IV.CONCLUSION
Quantitative kinetic parameters,k−andEa−,of the molecular exchange process between monomers in the bulk solution and those in the micelle for Gemini quaternary ammonium surfactants were acquired by the DNMR method.The exchange mechanism of Gemini surfactants was further discussed throughEa−and the micro-polarity variations with the spacer length.It was indicated that the conformation change is supposed to be experienced by the Gemini surfactant molecule when it escapes from the micelle,which well supports the possible two-step molecular exchange model for Gemini surfactants.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21375145).
[1]A.Patist,J.R.Kanicky,P.K.Shukla,and D.O.Shah,J.Colloid Interface Sci.245,1(2002).
[2]E.A.G.Aniansson and S.N.Wall,J.Phys.Chem.78,1024(1974).
[3]E.A.G.Aniansson and S.N.Wall,J.Phys.Chem.79,857(1975).
[4]E.A.G.Aniansson,S.N.Wall,M.Almgren,H.Hoff-mann,I.Kielmann,W.Ulbricht,R.Zana,J.Lang,and C.Tondre,J.Phys.Chem.80,905(1976).
[5]B.D.Nageswara Rao,Methods Enzymol.176,279(1989).
[6]I.Huc and R.Oda,Chem.Commun.2025(1999).
[7]X.H.Cui,Y.Jiang,C.S.Yang,X.Y.Lu,H.Chen,S.Z.Mao,M.L.Liu,H.Z.Yuan,P.Y.Luo,and Y.R.Du,J.Phys.Chem.B114,7808(2010).
[8]X.H.Cui,X.Y.Yang,H.Chen,A.H.Liu,S.Z.Mao,M.L.Liu,H.Z.Yuan,P.Y.Luo,and Y.R.Du,J.Phys.Chem.B112,2874(2008).
[9]Y.Jiang,X.Y.Lu,H.Chen,S.Z.Mao,M.L.Liu,P.Y.Luo,and Y.R.Du,J.Phys.Chem.B113,8357(2009).
[10]J.Liu,Y.Jiang,H.Chen,S.Z.Mao,Y.R.Du,and M.L.Liu,J.Phys.Chem.B116,14859(2012).
[11]M.Frindi,B.Michels,H.Levy,and R.Zana,Langmuir10,1140(1994).
[12]J.Lang,J.J.Auborn,and E.M.Eyring,J.Colloid Interface Sci.41,484(1972).
[13]D.Schaeffel,A.Kreyes,Y.Zhao,K.Landfester,H.J.Butt,D.Crespy,and K.Koynov,ACS Macro Lett.3,428(2014).
[14]K.Takeda and T.Yasunaga,J.Colloid Interface Sci.40,127(1972).
[15]T.Zinn,L.Willner,V.Pipich,D.Richter,and R.Lund,ACS Macro Lett.4,651(2015).
[16]F.M.Menger and C.A.Littau,J.Am.Chem.Soc.115,10083(1993).
[17]M.J.Rosen and D.J.Tracy,J.Surfactants Deterg.1,547(1998).
[18]M.Hajy Alimohammadi,S.Javadian,H.Gharibi,A.R.Tehrani-Bagha,M.R.Alavijeh,and K.Kakaei,J.Chem.Thermodyn.44,107(2012).
[19]M.A.Mir,N.Gull,J.M.Khan,R.H.Khan,A.A.Dar,and G.M.Rather,J.Phys.Chem.B114,3197(2010).
[20]U.S.Siddiqui,F.Khan,I.A.Khan,and Kabir-ud-Din,J.Solution Chem.41,1133(2012).
[21]Z.Jiang,J.Liu,K.Sun,J.F.Dong,X.F.Li,S.Z.Mao,Y.R.Du,and M.L.Liu,Colloid Polym.Sci.292,739(2014).
[22]C.Groth,M.Nydén,K.Holmberg,J.R.Kanicky,and D.O.Shah,J.Surfactants Deterg.7,247(2004).
[23]W.Ulbricht and R.Zana,Colloids Surf.A183-185,487(2001).
[24]Y.Jiang,H.Chen,S.Z.Mao,P.Y.Luo,Y.R.Du,and M.L.Liu,J.Phys.Chem.B115,1986(2011).
[25]H.S.Gutowsky and C.H.Holm,J.Phys.Chem.25,1228(1956).
[26]N.M.Sergeev,Russ.Chem.Rev.42,339(1973).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Investigation on Preparation and Anti-icing Performance of Superhydrophobic Surface on Aluminum Conductor
- Study of Cadmium-Doped Zinc Oxide Nanocrystals with Composition and Size Dependent Band Gaps
- Glucose Isomerization into Fructose Catalyzed by MgO/NaY Catalyst
- Analysis of Solvent Effect on Mechanical Properties of Poly(ether ether ketone)Using Nano-indentation
- Direct Synthesis of Monodisperse Hollow Molecularly Imprinted Polymers Based on Unfunctionalized SiO2for the Recognition of Bisphenol A
- Agent-Based Network Modeling Study of Immune Responses in Progression of Ulcerative Colitis
