End-to-end and end-to-side neurorrhaphy between thick donor nerves and thin recipient nerves: an axon regeneration study in a rat model
Tohru Tateshita , Kazuki Ueda, Akiyoshi Kajikawa
1 Department of Plastic and Reconstructive Surgery, St. Marianna Medical University, Kawasaki City, Japan
2 Department of Plastic and Reconstructive Surgery, Fukushima Medical University, Fukushima Perfecture, Japan
Introduction
Neurorrhaphy conducted under a surgical microscope is an established neurosurgical procedure (Viterbo et al., 1994;Yoleri et al., 2000; Hayashi et al., 2004; Frey et al., 2006;Ueda et al., 2007). However, the procedure can be challenging when nerve stumps of different cross-sectional areas need to be sutured together, and postoperative results from such procedures have considerable variation. Connecting a thick nerve to a thin nerve is particularly challenging. For example, a sural nerve jump graft to treat facial paralysis(May et al., 1991; Ueda et al., 2007) requires connecting the thick sural nerve with a considerably thinner buccal branch of the facial nerve on the paralyzed side. Similar challenges are also encountered in cross facial nerve grafting and surgical nerve transplantation following malignant craniofacial tumor resection.
In these scenarios, end-to-end neurorrhaphy leads to an excess of regenerated axons that extend uselessly outside of the suture surface, and the distal recipient axons undergo Wallerian degeneration within 3 days after injury. End-toend neurorrhaphy is also associated with worse functional outcome when suturing a thick nerve to a thin nerve. For example, end-to-end neurorrhaphy between the nerve to the rectus femoris muscle and the thick buccal branch of the facial nerve showed significantly better muscle strength,axonal area, and axon number compared to suturing to the thin marginal mandibular branch (MacQuillan and Grobbelaar, 2008). End-to-side neurorrhaphy, which was originally described in the late 19thcentury (Letievant, 1873; Hoffman,1884), has recently gained attention because of the finding that axon elongation occurs even if nerve stumps are not directly attached to each other (Viterbo et al., 1994). In particular, by suturing the end of the donor nerve to the side surface of the recipient nerve, an “in flow type” of nerve regeneration (regenerated axons extend through an epineural window into the windowed nerve) is established (Okouchi et al., 2008, 2009). In inflow type end-to-side neurorrhaphy, 52.8% of axon fibers regenerated, compared to 89% in out flow type end-to-side neurorrhaphy (regenerated axons extend in the opposite direction).
We hypothesized that the larger contact area between donor axons and recipient axons in end-to-side neurorrhaphy compared to end-to-end neurorrhaphy when suturing a thick nerve to a thin nerve reduces the number of regenerated axons that are misdirected or deviated and, as a result,achieves better functional recovery (Figure 1). In the current study, we created an in flow-type end-to-side and endto-end neurorrhaphy model with nerve stumps of different thicknesses using a rat facial nerve, and we investigated axon regeneration by measuring the number and diameter of regenerated axons in the two models.
Materials and Methods
Neurorrhaphy model
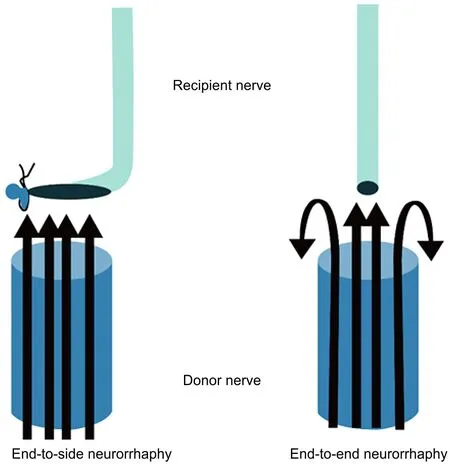
Figure 1 Schematic diagram of end-to-side and end-to-end neurorrhaphy showing a larger axon contact area between donor and recipient nerves in the end-to-side neurorrhaphy model.
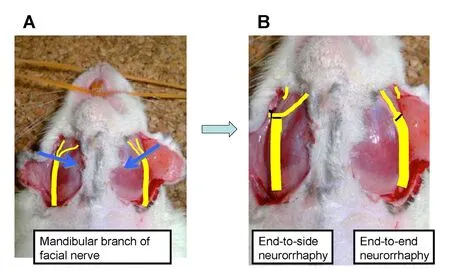
Figure 2 Experimental model showing (A) end-to-side and (B)end-to-end neurorrhaphy model in the rat facial nerve.

Figure 3 Schematic of surgical technique used in (A) end-to-end neurorrhaphy, and (B) end-to-side neurorrhaphy.
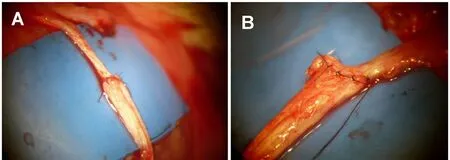
Figure 4 Appearance of nerve repair immediately following (A)end-to-end neurorrhaphy and (B) end-to-side neurorrhaphy.

Figure 5 Histological appearance of cross-section of donor nerve at 12 weeks under (A) low and (B) high magnification (Toluidine Blue stain, original magnification 200×).
Fourteen 9-week-old male Sprague-Dawley (SD) rats (Japan SLC, Inc., Hamamatsu, Japan), weighing 334 ± 15 g, were used to create an experimental neurorrhaphy model using the mandibular branch of the facial nerve. Anesthesia was induced using ether and maintained with intraperitoneal administration of Nembutal (Sumitomo Dainippon Pharma,Osaka, Japan). Next, the rat was fixed in a supine position,and the region extending from the face to the lower jaw was shaved, disinfected, and prepared for surgery.
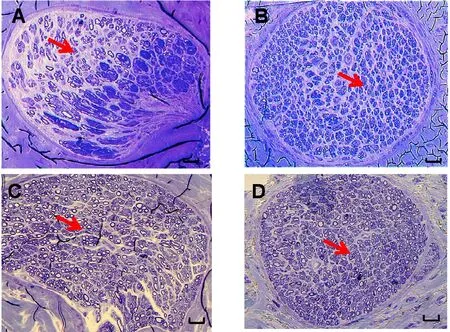
Figure 6 Histological appearance of cross-section of recipient nerves at (A, B) 6 weeks and (C, D) 12 weeks, following (A, C) end-to-side neurorrhaphy and (B, D) end-to-end neurorrhaphy (original magnification, 200×).
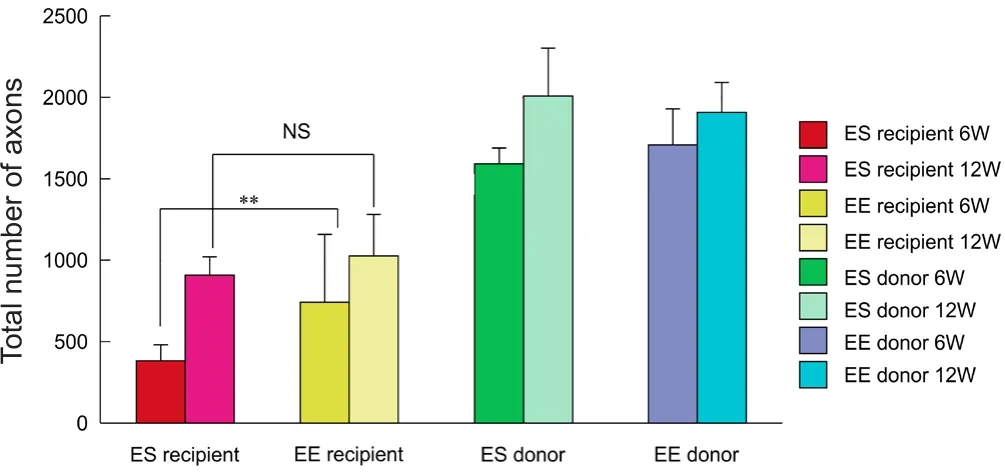
Figure 7 Total number of myelinated axons.
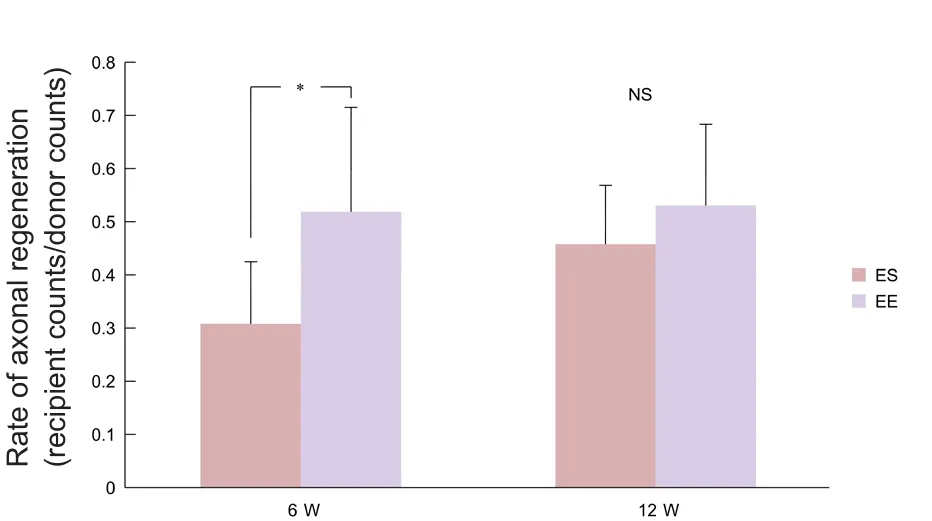
Figure 8 Rate of axonal regeneration.
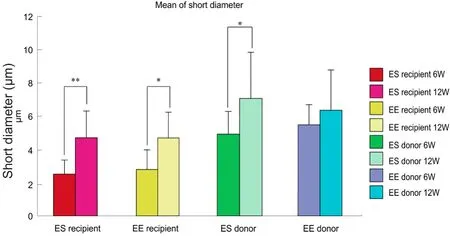
Figure 9 Minor axis diameter of regenerated axons.
A U-shaped incision was made, and the mandibular branch of the facial nerve was identified on both sides. The nerve was carefully dissected until its bifurcation into two branches. After carefully exposing the bifurcation region,two neurorrhaphy models were produced using an operating microscope (BX53, Olympus, Tokyo, Japan) (Figure 2). On each side, an approximately 1-mm wide region of the mandibular branch of the facial nerve that included the bifurcation point was excised. The central stump was confirmed to be approximately twice the diameter of the peripheral stumps. Next, the central stump (thicker donor nerve)and the medial strand of the peripheral stump (thinner recipient nerve) were stitched using an end-to-end or end-to side technique under the operating microscope.
(1) End-to-end neurorrhaphy group (EE): On the left side, the central and peripheral recipient nerve stumps were stitched end-to-end with a 10-0 black nylon suture. The nerve membrane and peripheral membrane were sutured with four stitches.
(2) End-to-side neurorrhaphy group (ES): On the right side, the central and peripheral recipient nerve stumps were stitched end-to-side with a 10-0 black nylon suture. The peripheral nerve stump was ligated with a 6-0 nylon suture,and an epineural window was created in the recipient nerve by completely excising the epineural membrane and exposing the perineural membrane. To the epineural window and four points of the central stump (a→a’, b→b’, c→c’, d→d’)(Figure 3), the nerve membrane and peripheral membrane were sutured.
In both models, 2–4 supplemental stitches were used so as not to expose the thicker nerve fiber bundles on the central stump (Figure 3). After the repairs were performed (Figure 4), the skin incision was then closed in layers. After 6–12 weeks, rats were evaluated for nerve regeneration. The study followed the guidelines of the Japanese Association for Laboratory Animal Science (JALAS) and was approved by the institutional animal ethics committee (Institute for Animal Experimentation, St Marianna University Graduate School of Medicine, No. 2013053004).
Evaluation of nerve regeneration
Nerve regeneration was assessed at 6 weeks (n= 7 rats) and 12 weeks (n= 7 rats) after neurorrhaphy. Rats were re-anesthetized, and the facial nerve repair regions were exposed.The repair region, including 10 mm of the peripheral nerve and 10 mm of the central nerve, was retrieved, and the rats were subsequently euthanized. Then, 3 mm samples from the retrieved nerves were Epon-embedded, sectioned,stained with Toluidine Blue (Sigma-Aldrich, St. Louis, MO,USA), and scanned (BX53 light miscroscope, Olympus, Tokyo, Japan).
The total number of myelinated axons and the average minor axis diameter of the myelinated axons in the central and peripheral sections of the end-to-side and end-to-end neurorrhaphy specimens were calculated using image analysis software (WinROOF ver. 5.2.1, Mitani Corp., Fukui, Japan)(Figure 5). The axon regeneration rate (peripheral section myelinated count/central section myelinated count) was also calculated.
Statistical analysis
The total number of myelinated axons, axon regeneration rate, and average minor axis diameter of the myelinated axons were compared between groups using the Wilcoxon(examining two related groups, non-parametric) test. Statistical comparisons were performed using WinROOF ver.5.2.1 software, and differences were considered statistically significant atP< 0.05.
Results
Myelinated axon co unt
At 6 weeks, the total number of regenerated axons on the distal side was significantly higher for end-to-end neurorrhaphy than for end-to-side neurorrhaphy (P< 0.01) (Figure 6A, B). However, at 12 weeks, there were no significant differences between the two groups (Figures 6C, D and 7).
Axon regeneration rate
At 6 weeks, the axon regeneration rate (distal counts/proximal counts) was significantly higher for end-to-end neurorrhaphy than for end-to-side neurorrhaphy (P< 0.05).However, at 12 weeks, there were no significant differences between the two groups (Figure 8).
Axon diameter
There was a significant increase in minor axis diameter of axons at 12 weeks in both end-to-end and end-to-side neurorrhaphy groups compared with at 6 weeks (P< 0.05 orP<0.01). However, there were no significant differences in minor axis diameter of axons between the two groups at either time point (Figure 9).
Discussion
Our results show that compared to end-to-side neurorrhaphy, end-to-end neurorrhaphy between a thick donor nerve and a thin recipient nerve results in a significantly higher number of regenerated myelinated axons and axon regeneration rate at 6 weeks. While there were no differences in nerve count and axon regeneration rate at 12 weeks or axon diameter at either time point, the early improvement in axon regeneration in the end-to-end neurorrhaphy model suggests that end-to-end neurorrhaphy is more effective and favorable than end-to-side neurorrhaphy. Because faster axonal regeneration potentially reduces muscle atrophy in the target organ, end-to-end neurorrhaphy may be preferable when suturing a thick nerve to a thin nerve.
The absence of significant differences in axon regeneration between end-to-end and end-to-side neurorrhaphy at 12 weeks suggests that end-to-side neurorrhaphy might be slower but not completely inferior to end-to-end neurorrhaphy in terms of axonal regeneration ability, and equivalent results may be obtained given a prolonged duration of healing. It should be recognized that axon regeneration and wound healing abilities in rodent and human facial nerves may not be identical, and the absence of significant differences at 12 weeks could also be a result of the “blow through effect” commonly encountered in rodent models, wherein early significant differences become difficult to distinguish later on (Brenner et al., 2008). We speculate that slower axon regeneration in end-to-side neurorrhaphy is due to regenerated axons potentially deviating and getting misdirected towards the ligated end side, instead of being directed into the recipient nerve’s target organ side.
Additionally, we observed increases in regenerated axon number and short diameter in both recipient and donor nerves in both end-to-side and end-to-end neurorrhaphy models. These increases are likely the result of collateral sprouting and proliferation of nerve fiber lateral buds from the traumatic neuroma that is formed by Schwann cells and connective tissues at the repair site. In out flow type end-toside neurorrhaphy, lateral sprouting of regenerated axons occurs from the node of Ranvier proximal to the stump on the donor nerve and may traverse the epineurium and extend into the recipient nerve (Cao et al., 1997; Sterne et al., 1997; Zhao et al., 1997; Lutz et al., 1998; Tham and Morrison, 1998; Zhang et al., 1999, 2000, 2001; Hayashi et al.,2004). While the exact mechanisms underlying such collateral sprouting after end-to-side neurorrhaphy are unclear,growth factors and neurotrophic factors such as insulin-like growth factor and neurotrophin-3 at the nerve connection site likely play an important role (Sterne et al., 1997) and need additional investigation.
The study has several limitations. First, we investigated nerve regeneration by measuring nerve counts and axon diameters, but we did not investigate underlying mechanisms or changes in neuronal and muscle function. Future studies that investigate local changes in neurotrophic and growth factors, as well as evaluate neuromuscular junction characteristics (Cheever et al., 2011), would help in clarifying the precise mechanisms and effects of axonal regeneration in the two models. In addition, muscle size and function could be evaluated by assessing vibrissal movement and eye blink reflex (Beahrs et al., 2010). Second, we used a normalized outcome axon regeneration rate (distal counts/proximal counts) to adjust for size differences in proximal donor and distal recipient nerves in the two models. Additional parameters, such as the g-ratio (ratio of the inner axonal diameter to the total outer diameter), could also be utilized as functional and structural indexes of optimal axonal myelination(Perrot et al., 2007). Lastly, the study lacked an uninjured control model that would have allowed comparisons among native injured and repaired scenarios.
To conclude, end-to-end suturing of a larger diameter nerve to a smaller diameter nerve (ratio of sutured nerve diameter, 2:1) resulted in a significantly higher number of regenerated axons and rate of regeneration than end-to-side suturing at 6 weeks following neurorrhaphy; however, there were no significant differences between the two groups at 12 weeks. There were also no differences in axon diameter at 6 or 12 weeks. Though end-to-side suture and end-to-end suturing appear to result in equivalent axon regeneration at 12 weeks, earlier regeneration in end-to-end suturing may reduce target organ muscle atrophy. End-to-end suturing may therefore be more desirable when suturing a thick nerve to a thin nerve.
Author contributions:TT was responsible for data acquisition, analysis and interpretation and manuscript writing. KU was responsible for conceptualization and design of experiments, data interpretation, and manuscript writing. AK were responsible for data interpretation and manuscript writing. All authors approved the final version of this paper.Conflicts of interest:There are no conflicts of interests to declare.
Research ethics:The study followed the guidelines of the Japanese Association for Laboratory Animal Science (JALAS) and was approved by the institutional animal ethics committee (Institute for Animal Experimentation, St. Marianna University Graduate School of Medicine No.2013053004).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Jayant P. Agarwal, University of Utah, USA;Donald Albert Godfrey, University of Toledo College of Medicine and Life Sciences, USA.
Additional file:Open peer reviewer reports 1 and 2.
Ballance CA, Ballance HA, Stewart P (1903) Remarks on the operative treatment of chronic facial palsy of peripheral origin. Br Med J 1:1009-1013.
Beahrs T, Tanzer L, Sanders VM, Jones KJ (2010) Functional recovery and facial motoneuron survival are influenced by immunodeficiency in crush-axotomized mice. Exp Neurol 221:225-230.
Brenner MJ, Moradzadeh A, Myckatyn TM, Tung TH, Mendez AB,Hunter DA, Mackinnon SE (2008) Role of timing in assessment of nerve regeneration. Microsurgery 28:265-272.
Cao X, Shidao H, Yu J (1997) Experimental study on the collateral sprouting after end-to-side anastomosis of nerve trunk. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 11:321-324.
Cheever TR, Olson EA, Ervasti JM (2011) Axonal regeneration and neuronal function are preserved in motor neurons lacking ss-actin in vivo. PLoS One 6:e17768.
Frey M, Giovanoli P, Michaelidou M (2006) Functional upgrading of partially recovered facial palsy by cross-face nerve grafting with distal end-to-side neurorrhaphy. Plast Reconstr Surg 117:597-608.
Hayashi A, Ynai A, Komuro Y, Nishida M, Inoue M, Seki T (2004)Collateral sprouting occurs following end-to-side neurorrhaphy.Plast Reconstr Surg 114:129-137.
Hoffman P (1884) Einige Falle von Nervenlahmung end Nervennaht.Griefswald: Mitt a d Chir Klin z.
Letievant E (1873) T’raite des Sections Nerveuses. Paris: J B Bailliere.
Lutz BS, Chuang DCC, Hsu JC, Wei FC (1998). End-to-side neurorrhaphy; functional and double-labeling study in rat upper limb. J Reconstr Microsurg 14:590.
MacQuillan AH, Grobbelaar AO (2008). Functional muscle transfer and the variance of reinnervating axonal load: part 1. The facial nerve. Plast Reconstr Surg 121:1570-1577.
May M, Sobol SM, Mester SJ (1991) Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy.Otolaryngol Head Neck Surg 104:818-825.
Okouchi H, Ueda K, Kajikawa A, Tateshita T, Okouchi M, Asai E,Sakaba T, Tachi K, Momiyama M, Gorai K, Katusragi Y, Imanishi M(2008) Experimental study on in flow type end-to-side neurorrhaphy:changes in neuronal axon in flow due to the presence or absence of recipient nerve disorder. J J S Reconstr Microsurg 21:195-196.
Okouchi H, Ueda K, Kajikawa A, Tateshita T, Okouchi M, Hirose T,Sakaba T, Tachi K, Momiyama M, Katusragi Y (2009) Experimental study on inflow type end-to-side neurorrhaphy (second report):effects from the extent of the recipient nerve disorder. J J S Reconstr Microsurg 22:90-91.
Perrot R, Lonchampt P, Peterson AC, Eyer J (2007) Axonal neurofilaments control multiple fiber properties but do not influence structure or spacing of nodes of Ranvier. J Neurosci 27:9573-9584.
Sterne GD, Coulton GR, Brown RA, Green CJ, Terenghi G (1997)Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J Cell Biol 139:709-715.
Tham SK, Morrison WA (1998) Motor collateral sprouting through an end-to-side nerve repair. J Hand Surg Am 23:844-851.
Ueda K, Kajikawa A, Suzuki Y, Ohkouchi M, Hirose T, Asai E, Tateshita T (2007) Combination of hypoglossal-facial nerve jump graft by end-to-side neurorrhaphy and cross-face nerve graft for the treatment of facial paralysis. J Reconstr Microsurg 23:181-187.
Viterbo F, Trindade JC, Hoshino K, Mazzoni AN (1994) End-to-side neurorrhaphy with removal of the epineurial sheath: an experimental study in rats. Plast Reconstr Surg 94:1038-1047.
Yoleri L, Songur E, Yoleri O, Vural T, Cağdaş A (2000) Reanimation of early facial paralysis with hypoglossal/facial end-to-side neurorrhaphy: a new approach. J Reconstr Microsurg 16:347-355.
Zhang Z, Soucacos PN, Beris AE, Bo J, Ioachim E, Johnson EO (2000)Long-term evaluation of rat peripheral nerve repair with end-to-side neurorrhaphy. J Reconstr Microsurg 16:303-311.
Zhang Z, Soucacos PN, Bo J, Beris AE (1999) Evaluation of collateral sprouting after end-to-side nerve coaptation using a fluorescent double-labeling technique. Microsurgery 19:281-286.
Zhang Z, Soucacos PN, Bo J, Beris AE, Malizos KN, Ioachim E, Agnantis NJ (2001) Reinnervation after end-to-side nerve coaptation in a rat model. Am J Orthop 30:400-406.
Zhao JZ, Chen ZW, Chen TY (1997) Nerve regeneration after terminolateral neurorrhaphy: experimental study in rats. J Reconstr Microsurg 13:31-37.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration

