Collagen for brain repair: therapeutic perspectives
Buket Ucar, Christian Humpel
Laboratory of Psychiatry and Experimental Alzheimer’s Research, Medical University of Innsbruck, Innsbruck, Austria
Biomaterials and Brain Repair
The central nervous system (CNS) has a very limited capacity to regenerate, which makes it obligatory to search for new strategies for the treatment of CNS diseases (Tam et al., 2014). In particular the treatment of neurodegenerative conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) currently depends on symptomatic relief rather than regeneration strategies that would help to improve function or slow down the disease progression (Orive et al., 2009). The use of biomaterials provides new opportunities for protection and regeneration of the CNS(Miyata et al., 1992; Friess, 1998). Scaffolds and microspheres from various natural or synthetic biomaterials can be efficiently loaded with therapeutically active agents such as growth factors,anti-inflammatory substances, hormones, drugs or plasmids for gene delivery (Heya et al., 1994; Berthold et al., 1998; Dang and Leong, 2006; Garbayo et al., 2009). Thereby these scaffolds and micro/nanospheres provide application of relevant therapeutics in a localized manner, which minimizes unwanted side effects that are observed with systemic administration and enhances the effectiveness of the therapy (Zhong and Bellamkonda, 2008).Alternatively, they may be used to encapsulate genetically-engineered cells which secrete such molecules or stem cells that differentiate into desired specific cell types (Khaing et al., 2014).
Extracellular matrix (ECM) proteins are the key proteins for preparing biomaterials for tissue repair. ECM proteins have the advantage of interaction with cells directlyviacell surface receptors or indirectlyviaother ECM molecules. Therefore, ECM proteins play a role in cell differentiation, cell attachment, migration,survival and proliferation (Heino, 2007). Some examples of ECM proteins that are commonly used in medicine for these purposes are natural biomaterials such as collagen I, alginate, chitosan or gelatin or synthetic biomaterials such as poly(lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG). However, more and more ECM proteins are discovered and may become potent therapeutics. Such a protein is fractone, localized in the neurogenic niche, and it has been shown to capture growth factors and promote growth factor activity (Kerever et al., 2015). Further, collagen IV is the most abundant ECM protein in the basal membrane of the vasculature (50%) and considered to become an alternative biomaterial, alone or in combination with laminin(Brown and Thore, 2011).
Collagen as a Biomaterial
Collagen is classified as a hydrogel, which are polymers that have the capacity of storing large amounts of water molecules. Hydrogels are of main interest for the repair of the CNS, as they can be conveniently placed into soft nervous tissue due to their matching mechanical properties (Hoare and Kohane, 2008; Khaing et al.,2014). Collagen recently has drawn interest for the CNS repair,as it is highly biocompatible, biodegradable and non-toxic. In addition to its natural advantages, collagen already has very diverse applications in medicine and science, such as bone and cartilage reconstruction, wound dressing, tissue engineering applications,drug/gene delivery and cell encapsulation systems (Lee et al.,2001). It can be processed into various forms depending on the purpose: from films and shields to sponges, gels or nanoparticles(Friess, 1998; Lee et al., 2001). Every year several metric tons of collagen and its degradation product gelatin are used for medical applications (Olsen et al., 2003).
Collagen is present in all mammals, found both in the intracellular and extracellular compartments, and constitutes 20–30% of all body proteins in humans (Friess, 1998). Currently there are 29 different types of collagen that are characterized, nevertheless type I collagen is the most abundant and widely used (Zeugolis and Raghunath, 2011). To date, there is great effort for the commercial large scale production of recombinant human collagen from yeast, insect cells and plants, in order to prevent lot to lot differences and impurities of extracted collagen, besides eliminating concerns of pathogen transmission from animal sources (Olsen et al., 2003). Recombinant collagen production also offers the possibility of conformationally or structurally modified collagens design to better suit the target tissue (Yang et al., 2004).
Collagen Scaffolds (CollScaff): Preparation and Application
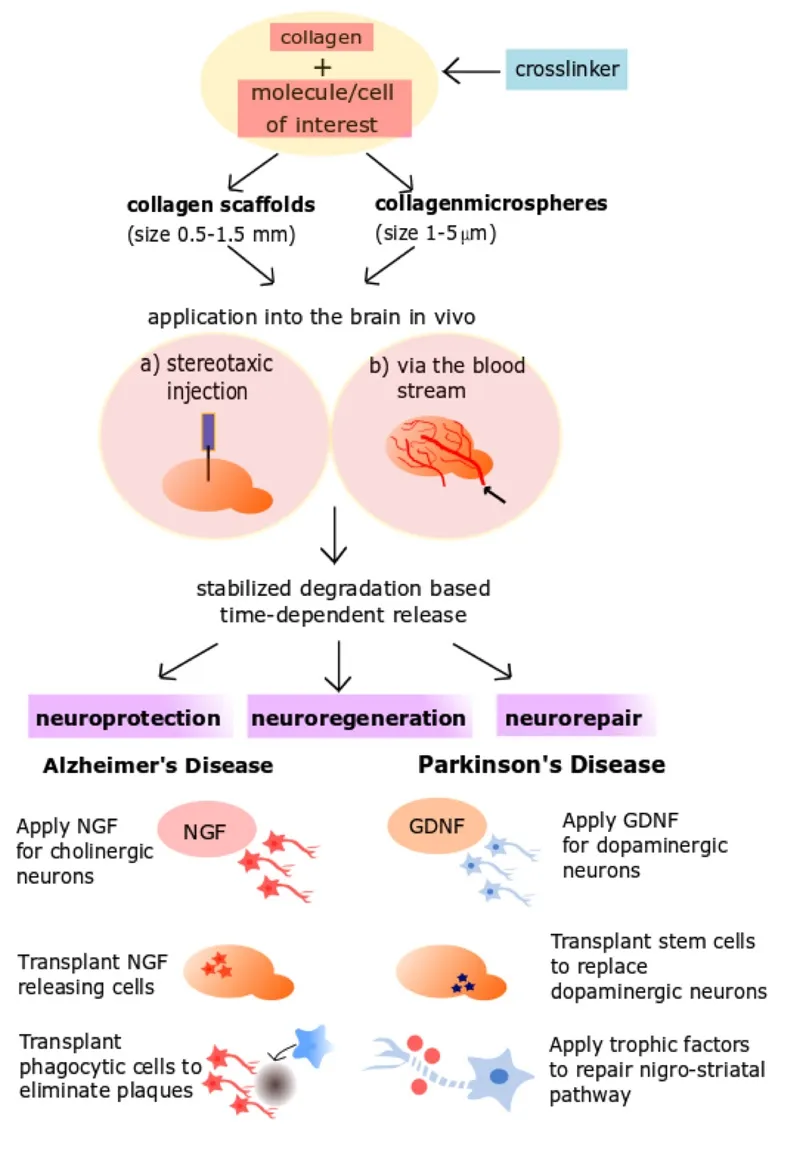
Figure 1 Therapeutic strategies to apply collagen scaffolds in Alzheimer’s and Parkinson’s diseases.
CollScaff are easily and rapidly generated using different chemical crosslinkers (Figure 1) such as glutaraldehyde, formaldehyde or polyethyleneglycole (PEG), as well as physical treatment with dry heat and ultraviolet irradiation (Friess, 1998; Caliari and Burdick,2016). CollScaff can be efficiently loaded with therapeutically active substances and release them in a degradation based fashion,in which the substance is released along with the degradation of the collagen (Foidl et al., 2018). Furthermore, these CollScaff can be modified to have desired properties such as thermorespondency, pH-respondency and increased or decreased rate of drug release depending on the degree of cross-linking (Hoare and Kohane, 2008; Caliari and Burdick, 2016). One of the major advantages of CollScaff is that they are injectable, which provides direct application to the target site of action rather than a systemic administration route (Figure 1). These scaffolds then are formed in situ due to increased temperature in the body. As the bioavailability of many drugs to the brain is greatly restricted by the blood-brain barrier (BBB), injectable CollScaff may be a minimally invasive method of administration that provides local,controlled and prolonged release of the drugs with less systemic side effects. In addition to difficulties in passage through the BBB,most therapeutic molecules are unstable in the body and degrade rapidly before they reach their target (Popovic and Brundin,2006). Application of these substances within CollScaff will prevent rapid degradation of the drugs before they reach the brain(Figure 1). CollScaff can also be used as cell delivery systems that encapsulate progenitor stem cells which can differentiate into desired cell types or act as localized sources of growth factors to promote endogenous protection and repairing capacity of the CNS. Encapsulation of the cells with a matrix provides immobilization of the transplanted cells and provides more optimal microenvironment for the cells, which enhances their survival within the tissue (Khaing et al., 2014). Moreover, this method prevents tumor formation caused by uncontrolled growth of transplanted cells and does not require repeated interventions to refill the drug source (Popovic and Brundin, 2006).
Micro/Nanospheres
Hollow spheres of collagen can be prepared following different methods in micro or even nano-scales (Figure 1). Microspheres allow larger amounts of drug encapsulation as they have a larger surface/volume ratio and they may be easier to applyin vivoby stereotaxic surgery into a precise location in the brain owing to their smaller size. One of these methods of production is the template method, in which collagen forms a shell around a stable microtemplate such as polystyrene or silica with the help of a crosslinker. The template is then removed chemically, leaving hollow collagen spheres with a uniform size, which can be loaded with molecules of interest. Collagen hollow spheres are useful to load large amounts of growth factors, such as nerve growth factor(NGF) (Kraskiewicz et al., 2013) or vascular endothelial growth factor (VEGF) (Nagai et al., 2010) without causing any toxicityin vitroandin vivo. Alternatively, collagen microspheres carrying oligodendrocyte progenitor cells support the cell growth and differentiation of progenitors into mature oligodendrocytes that can provide myelinationin vitro(Yao et al., 2013). These results indicate that collagen microspheres can also be used as cell carriers in the nervous system.
Another promising approach for drug delivery to the brain is surface modified nanospheres that are administered to the blood stream, which provides passage through BBB without needing stereotaxic surgery. Surface modification in these spheres may provide recognition of the specific cell surface receptors that permits their transcytosis through BBB or modify physiological characteristics of the spheres (such as hydrophobicity and surface charge) in order to facilitate their passage over BBBviaadsorption by endothelial cells (Wohlfart et al., 2012). By using this method, intravenously administered NGF was successfully delivered to brain within surfactant-coated nanospheres and provided improvement of cognitive functions in an AD model and motor functions in a PD model (Kurakhmaeva et al., 2009).
As the CNS injury and neurodegenerative conditions comprise of various complex pathophysiological events, release of multiple therapeutics from the same system at specific time points with individual releasing rates can be a valuable method. Microsphere/scaffold composites is an effective approach for achieving this goal, in which one therapeutic is encapsulated in microspheres and these microspheres are dispersed into a scaffold together with a second therapeutic. It was shown that in such a composite neurotrophin encapsulated into PLGA microspheres showed a long lasting slow release profile, while a neurotrophic factor loaded into PEG scaffold had a rapid release (Burdick et al., 2006). This method is very convenient for control of releasing properties for multiple drugs, in that the release rates can be adjusted by changing properties of microspheres or the scaffold, as well as changing concentration of loaded therapeutics or microspheres.
CollScaff and Neuroprotection
Brain damage may occur due to acute events such as stroke and traumatic brain injury or progressive degeneration over long time periods due to neurodegenerative diseases. Although arising from distinctive etiologies and taking place at different times, all these conditions share certain common pathophysiologic phenomena,which eventually leads to cell death: oxidative stress, inflammation and apoptotic processes (Bamberger and Landreth, 2002;Bramlett and Dietrich, 2004; Dexter and Jenner, 2013). In these cases CollScaff can be used for preventing inflammation, excitotoxicity and oxidative stress with the aim of neuroprotection(Figure 1). Besides, neurodegenerative diseases in general cause selective degeneration of specific populations of neurons in the brain; for instance while PD causes degeneration of dopaminergic neurons in substantia nigra, AD leads to degeneration of cholinergic neurons in nucleus basalis of Meynert (nBM). This provides an opportunity in terms of application of neuroprotective substances at a specific location with close proximity to the related brain area. Glial cell line-derived neurotrophic factor (GDNF),brain-derived growth factor (BDNF) and NGF are promising neuroprotective proteins in preclinical and clinical trials for PD and AD, respectively (Williams et al., 1986; Lin et al., 1993; Allen et al., 2013). Phase I–II study of intraventricularly injected GDNF over 8 months to patients with PD did not show any efficacy,besides caused a wide range of adverse events following GDNF administration; most likely because GDNF failed to reach the target regions of the brain (Nutt et al., 2003). On the contrary, in a phase I trial of NGF gene therapy for 10 patients with AD, application of NGF secreting autologous fibroblasts directly to the nBM provided improvement in the disease progression, as well as clear demonstration of a response to NGF with post-mortem analysis of enrolled patients (Tuszynski et al., 2005, 2015). These results highlight the importance of novel drug delivery methods in order to provide a localized administration of trophic factors to the target regions.
Furthermore, it was noted that the most significant effect was observed when GDNF was delivered continuously in PD (Popovic and Brundin, 2006). Although this can be achieved by osmotic pumps, these systems require invasive surgery for implementation and regular replacement of the drug reservoir (Jain et al., 2006).Therefore, the use of CollScaff that provide sustained release of such growth factors over extended periods of time would be beneficial in neurodegenerative diseases. Current research in our laboratory is in the scope of the EU project BrainMatTrain and focuses on the neuroprotective effects of GDNF releasing CollS-caff on dopaminergic neurons in PD. In AD models, we have previously shown that NGF releasing CollScaff protect cholinergic neurons in anex vivomodel (Foidl et al., 2018). Alternatively,CollScaff loaded with beta-amyloid degrading enzymes may be potent to eliminate the toxic plaques in the brain (Figure 1).
CollScaff and Neuroregeneration
Currently collagen is widely used in FDA approved nerve conduits (NeuraGen®, NeuroFlex®and NeuroMend®) and nerve cuffs (NeuroWrap®and NeuroMend®) with different degredation rates ranging from months to years for peripheral nerve tissue repair. They were shown to successfully support and guide neuroregeneration across nerve gaps up to 2–3 centimeters in the peripheral nervous system injuries (Taras et al., 2011; Khaing et al., 2014). Furthermore, hydrogels can be used as bulk scaffolds to fill in lesions in the CNS that are formed due to tissue damage after traumatic brain injury or spinal cord injury in order to provide mechanical support and a favorable microenvironment for axon sprouting and cell infiltration (Zhong and Bellamkonda,2008; Orive et al., 2009). Injectable agarose gel loaded with BDNF releasing lipid microtubules was shown to support axon growth through an irregular shaped cavity and reduced inflammatory response in spinal cord injury (Jain et al., 2006). However, agarose has a lower gelling temperature (17°C) and requires a cooling system when applied to the body (Jain et al., 2006). In this respect collagen can be superior, as it can form a gel in situ at the body temperature (Pakulska et al., 2012).
In PD dopamine cell death in the substantia nigra leads to degeneration of nigro-striatal axons. Therefore, regeneration of this nigro-striatal pathway (Figure 1) is of interest in Parkinson´s disease, where collagen microspheres may be a useful method of drug delivery. Indeed, firstly dopamine loaded microspheres injected to striatum were shown to provide fiber growth in striatum and correlating functional recovery (McRae and Dahlström,1994). More recently Jollivet et al. (2004) demonstrated that intra-striatal implantation of GDNF releasing microspheres promoted sprouting in the dopaminergic nerve fibers, besides motor function recovery in a rat model of Parkinson’s disease. Similarly in AD, Péan et al. (2000) showed that microspheres loaded with NGF enhanced the survival of axotomized cholinergic neurons.Thus, collagen microspheres can be used in a similar manner to provide safe and efficient delivery of these growth factors to the brain tissue to promote neuroregeneration but are also considered as a regenerative approach in spinal cord lesions (Joosten et al., 1995). Furthermore, GDNF loaded CollScaff encapsulating ventral mesencephalon grafts improved dopaminergic cell survival 5-fold and striatal re-innervation 3-fold in a rat model of Parkinson’s disease, as well as providing significant functional recovery (Moriarty et al., 2017). CollScaff functioned to retain the GDNF in striatum to support dopaminergic cell survival, formed a physical barrier against host immune system and facilitated cell adhesion (Moriarty et al., 2017). This strategy holds the promise of improving graft survival for stem cell transplants in the future for cell replacement therapies in neurodegenerative disorders, for which collagen is an ideal candidate of biomaterial.
Conclusions
Better understanding of collagen interactions with other ECM molecules and cell receptors, as well as the cellular pathways they take part in, will lead to better regulation of cellular processes and precise control over interactions of the material with the environment (An et al., 2016).
CollScaff and collagen microspheres loaded with therapeutic factors have a great potential in the safe and efficient treatment of various neurodegenerative conditions and other CNS disorders such as stroke and traumatic brain injury. This method of drug delivery may be used for application of multiple drugs, such as combinations of growth factors and anti-oxidant substances against neurodegeneration. Additionally, surface modified collagen nanospheres can be systemically administered and used to target the brain without an invasive severe stereotaxic surgery. It is certain that despite the excellent natural properties of collagen,side effects of these systems such as possible gliosis must be considered and evaluated carefully in preclinical trials before entering clinical trials. Nevertheless, collagen as a natural biomaterial presents remarkable opportunities in terms of both repair and regeneration of the CNS, which are worth to be investigated.
Author contributions:BU and CH both wrote and corrected the manuscript.
Conflicts of interest:None declared.
Financial support:This work has been supported by The BrainMatTrain project, which is funded by the European Union Horizon 2020 Programme(H2020-MSCA-ITN-2015) under the Marie Skłodowska-Curie Initial Training Network and Grant Agreement No. 676408.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer:Mercedes Fernandez, University of Bologna, Bologna, Italy.
Comments to authors:This paper deals with the use of collagen for brain repair. Authors describe the state of the art in the field of neurorepair using scaffolds and other types of biomaterials.
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF,NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155-175.
An B, Lin YS, Brodsky B (2016) Collagen interactions: Drug design and delivery. Adv Drug Del Rev 97:69-84.
Bamberger ME, Landreth GE (2002) Inflammation, apoptosis, and Alzheimer’s disease. Neuroscientist 8:276-283.
Berthold A, Cremer K, Kreuter J (1998) Collagen microparticles: carriers for glucocorticosteroids. Eur J Pharm Biopharm 45:23-29.
Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24:133-150.
Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37:56-74.
Burdick JA, Ward M, Liang E, Young MJ, Langer R (2006) Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 27:452-459.
Caliari SR, Burdick JA (2016) A practical guide to hydrogels for cell culture.Nat Methods 13:405-414.
Dang JM, Leong KW (2006) Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev 58:487-499.
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132-144.
Foidl BM, Ucar B, Schwarz A, Rebelo AL, Pandit A, Humpel C (2018)Nerve growth factor released from collagen scaffolds protects axotomized cholinergic neurons of the basal nucleus of Meynert in organotypic brain slices. J Neurosci Methods 295:77-86.
Friess W (1998) Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm 45:113-136.
Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS,Blanco-Prieto MJ (2009) Effective GDNF brain delivery using microspheres--a promising strategy for Parkinson’s disease. J Control Release 135:119-126.
Heino J (2007) The collagen family members as cell adhesion proteins. Bioessays 29:1001-1010.
Heya T, Mikura Y, Nagai A, Miura Y, Futo T, Tomida Y, Shimizu H, Toguchi H (1994) Controlled release of thyrotropin releasing hormone from microspheres: evaluation of release profiles and pharmacokinetics after subcutaneous administration. J Pharm Sci 83:798-801.
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: Progress and challenges. Polymer 49:1993-2007.
Jain A, Kim YT, McKeon RJ, Bellamkonda RV (2006) In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 27:497-504.
Jollivet C, Aubert-Pouessel A, Clavreul A, Venier-Julienne MC, Remy S,Montero-Menei CN, Benoit JP, Menei P (2004) Striatal implantation of GDNF releasing biodegradable microspheres promotes recovery of motor function in a partial model of Parkinson’s disease. Biomaterials 25:933-942.
Joosten EA, Bar PR, Gispen WH (1995) Collagen implants and cortico-spinal axonal growth after mid-thoracic spinal cord lesion in the adult rat. J Neurosci Res 41:481-490.
Kerever A, Yamada T, Suzuki Y, Mercier F, Arikawa-Hirasawa E (2015)Fractone aging in the subventricular zone of the lateral ventricle. J Chem Neuroanat 66-67:52-60.
Khaing ZZ, Thomas RC, Geissler SA, Schmidt CE (2014) Advanced biomaterials for repairing the nervous system: what can hydrogels do for the brain? Mater Today 17:332-340.
Kraskiewicz H, Breen B, Sargeant T, McMahon S, Pandit A (2013) Assembly of protein-based hollow spheres encapsulating a therapeutic factor.ACS Chem Neurosci 4:1297-1304.
Kurakhmaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS, Kreuter J, Gelperina S, Begley D, Alyautdin RN(2009) Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J Drug Target 17:564-574.
Lee CH, Singla A, Lee Y (2001) Biomedical applications of collagen. Int J Pharm 221:1-22.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons.Science 260:1130-1132.
McRae A, Dahlström A (1994) Transmitter-loaded polymeric microspheres induce regrowth of dopaminergic nerve terminals in striata of rats with 6-OH-DA induced parkinsonism. Neurochem Int 25:27-33.
Miyata T, Taira T, Noishiki Y (1992) Collagen engineering for biomaterial use. Clin Mater 9:139-148.
Moriarty N, Pandit A, Dowd E (2017) Encapsulation of primary dopaminergic neurons in a GDNF-loaded collagen hydrogel increases their survival, re-innervation and function after intra-striatal transplantation.7:16033.
Nagai N, Kumasaka N, Kawashima T, Kaji H, Nishizawa M, Abe T (2010)Preparation and characterization of collagen microspheres for sustained release of VEGF. J Mater Sci Mater Med 21:1891-1898.
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr., Lozano AM, Penn RD, Simpson RK Jr, Stacy M, Wooten GF, ICV GDNF Study Group. Implanted intracerebroventricular, Glial cell line-derived neurotrophic factor (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60:69-73.
Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perala M,Hamalainen ER, Jarvinen M, Polarek J (2003) Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev 55:1547-1567.
Orive G, Anitua E, Pedraz JL, Emerich DF (2009) Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci 10:682-692.
Péan JM, Menei P, Morel O, Montero-Menei CN, Benoit JP (2000) Intraseptal implantation of NGF-releasing microspheres promote the survival of axotomized cholinergic neurons. Biomaterials 21:2097-2101.
Pakulska MM, Ballios BG, Shoichet MS (2012) Injectable hydrogels for central nervous system therapy. Biomed Mater 7:024101.
Popovic N, Brundin P (2006) Therapeutic potential of controlled drug delivery systems in neurodegenerative diseases. Int J Pharm 314:120-126.
Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS (2014) Regenerative therapies for central nervous system diseases: a biomaterials approach.Neuropsychopharmacology 39:169-188.
Taras JS, Jacoby SM, Lincoski CJ (2011) Reconstruction of digital nerves with collagen conduits. J Hand Surg Am 36:1441-1446.
Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, Pay MM, Masliah E,Conner JM, Kobalka P, Roy S, Nagahara AH (2015) Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease.JAMA Neurol 72:1139-1147.
Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J (2005) A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 11:551-555.
Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A,Gage FH (1986) Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A 83:9231-9235.
Wohlfart S, Gelperina S, Kreuter J (2012) Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release 161:264-273.
Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW (2004) The application of recombinant human collagen in tissue engineering. Biodrugs 18:103-119.
Yao L, Phan F, Li Y (2013) Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res Ther 4:109.
Zeugolis DI, Raghunath M (2011) 2.215 - Collagen: materials analysis and implant uses A2 - ducheyne, Paul. Compr Biomater 2:261-278.
Zhong Y, Bellamkonda RV (2008) Biomaterials for the central nervous system. J R Soc Interface 5:957-975.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
- Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention

