Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
Jean-Sébastien Teoh, Michelle Yu-Ying Wong, Tarika Vijayaraghavan, Brent Neumann
Neuroscience Program, Monash Biomedicine Discovery Institute and Department of Anatomy and Developmental Biology, Monash University,Melbourne, Australia
Damage to the nervous system is often associated with devastating consequences that can include permanent paralysis. Despite extensive research, current treatments promoting nervous system repair frequently fail to obtain full functional recovery. The complexity of mammalian nervous systems imposes experimental limitations for understanding relevant mechanisms and processes, which can be overcome with the use of more simplified organisms. For this reason, the nematodeCaenorhabditis elegans(C. elegans) has emerged as an important model for studying regeneration of the nervous system. Findings in this system have not only supported discoveries made in mammalian systems,but have also resulted in important new insights. One exciting area of study is that of regenerative axonal fusion. This spontaneous regenerative mechanism occurs through the fusion of separated axon segments,thereby bridging the site of injury. Previously, we have shown that axonal fusion is molecularly similar to the process of apoptotic cell engulfment. A crucial early event for both processes is the exposure of recognition signals on the outer leaflet of the membrane in the form of phosphatidylserine (PS) (Figure 1).
Despite our recent characterisation of the molecules involved in axonal fusion (Neumann et al., 2015), it had remained unclear if axonal fusion restores function to damaged neurons, and if the process could be modulated. Using the mechanosensory neurons ofC.elegansas our model system, we have addressed these questions (Abay et al., 2017c). Through a combination of cell ablations and single neuron axotomies with fluorescent reporters and behavioural assays, we demonstrated that axonal fusion restores full function to transected neurons. Analyzing the frequency of axonal fusion across different ages, we observed a surprising increase in older animals. The amount of exposed PS‘save-me’ signals also increased with age and strongly correlated with the frequency of axonal fusion. These findings suggest that enhanced levels of externalized PS can increase reconnection and therefore improve the efficiency of repair by driving axonal fusion. We also observed that axonal fusion could be promoted by genetically enhancing the regenerative capacity of the neurons. Together, our findings establish axonal fusion as an age-dependent, highly efficient means of nervous system repair that is able to recover full function.
PS ‘Save-Me’ Signals
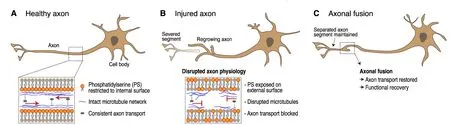
Figure 1 Injury-induced exposure of phosphatidylserine promotes axonal fusion.
PS is a membrane phospholipid that acts as a ‘saveme’ signal to initiate the process of axonal fusion inC. elegans(Neumann et al., 2015; Abay et al., 2017c).In our previous work, we identified several molecules that lie downstream to PS signaling in axonal fusion(Neumann et al., 2015). Interestingly, all of these molecules were previously implicated in the recognition of apoptotic cells. In somatic cells, the clearance of apoptotic cells requires the exposure of PS not only on the dying cell, but also on the phagocytes. This is carried out by the ABC transporter CED-7 and the transthyretin TTR-52 that promote the secretion of vesicles with externalized PS from dying cells. The apoptotic cells are then engulfed by neighboring phagocytes with the help of the transmembrane receptor CED-1/LRP1/MEGF10 and TTR-52 (Mapes et al., 2012). Curiously,we have also shown that CED-1 and the intracellular adapter CED-6/GULP act within surrounding cells to mediate engulfment of severed axon fragments (Nichols et al., 2016). In the context of axonal fusion, it is therefore plausible that exposure of PS on neighboring cells could facilitate the fusion process. PS is normally restricted to the inner leaflet of cellular membranes through the activity of flippase proteins. Inactivation of flippases, together with the activation of scramblase proteins leads to the externalization of PS.C. eleganshas provided important insights into the identity of the proteins involved in exposing PS during apoptosis.Analysis of the phospholipid scramblase proteins inC. eleganshas identified SCRM-1 as a PS scramblase(Venegas and Zhou, 2007; Wang et al., 2007). Interestingly, activation of SCRM-1 by the apoptosis-inducing factor WAH-1/AIF is required for its the scrambling activity (Wang et al., 2007). In addition, the caspase dependent scramblase CED-8/Xkr8 has also been shown to play a key role in the exposure of PS during apoptosis (Suzuki et al., 2014). Disruption of either SCRM-1/WAH-1 or CED-8 significantly reduces, but does not abolish PS exposure, indicating the likely involvement of other proteins in this process. It would be interesting to study the role of these and additional scramblase proteins in neuronal PS exposure and axonal fusion.The surprisingly close similarity between the apoptotic and axonal fusion pathways raises important questions in regards to the demarcation of the ‘eat-me’ and ‘saveme’ PS signals. Slight differences in the molecular pathways that exist between apoptotic cell recognition and axonal fusion (Neumann et al., 2015) may be sufficient to determine either the death or survival outcomes. Alternatively, proteins important for modulating the PS signal may be differentially regulated following axonal injury as opposed to apoptosis. Several other ‘eat-me’,as well as ‘find-me’ signals have been implicated in apoptosis, including calcireticulin, cell surface complex carbohydrates, intercellular adhesion molecule-3, oxidized lipids, lysophosphatidylcholine and extracellular nucleotides (Hochreiter-Hufford and Ravichandran,2013). The roles for these alternative signals remain unexplored in the context of axonal fusion. Uncovering how PS is regulated and how this signal can drive alternative cellular fates will be essential for further defining the process of regenerative axonal fusion.
Microtubule Dynamics, Axonal Transport and Functional Recovery
Axonal regeneration and fusion require specific cellular structures where fine rearrangements of the cytoskeleton are essential. Recent studies have supported the importance of microtubule dynamics in axon regeneration, whereby stabilized and growing microtubules promote regrowth. An injured axon initiates repair by developing a growth cone, which extends through persistent microtubule polymerization and senses guidance cues for directed regrowth. In our study, we observed that axonal fusion recovers active anterograde transport along microtubules, since the pooling of a kinesin motor protein at the severed tip of the axon was removed after fusion (Abay et al., 2017c).However, it remains poorly understood how microtubule dynamics and axonal transport are restored following the fusion of separated fragments. Understanding these restorative processes will be essential to gain a better understanding of functional recovery in axonal fusion. Indeed, we and others have provided supporting evidence for the complexity of functional recovery.Axonal fusion is frequently observed within 24 hours post-axotomy, but functional recovery was maximal at 48 hours post-axotomy. This delay might be explained by the length of time needed to reestablish the microtubule network, and thus axonal transport. Alternatively, this might also depend on the restoration of other cellular structures, including synapses. In addition,Basu et al. (2017) reported that aged animals undergoing axonal fusion did not always recover function.This might support the notion that the efficiency of recovering biological processes, including microtubule transport subsequent to fusion, determines functional recovery. Therefore, it will be important to explore precisely how axonal fusion enables the restoration of biological processes within the axon.
In our study, we also assessed regeneration when the axons were simultaneously severed at two different sites (Abay et al., 2017c). We found that axonal fusion at the cut site closest to the cell body improved regrowth from the second, more distal site. Fascinatingly, we even observed one fusion event at the second cut site after axonal fusion at the first site. Conversely, no fusion was observed at the furthest site in the absence of fusion at the first site. These observations indicate that the cell body is not required for the initial regenerative response, but that it is essential for persistent regrowth and axonal fusion. In future studies,it will be important to determine whether intrinsic factors are translated locally within the axon shaft, or if they are persistently delivered from the cell body. For example, one could explore how somatic players alter the expression and localization of EFF-1, the protein required for fusing the axon segments following reconnection (Ghosh-Roy et al., 2010; Neumann et al.,2015). Based on our previous imaging studies (Neumann et al., 2015), and on how it is regulated during embryonic processes (Smurova and Podbilewicz,2016), we hypothesise that active forms of EFF-1 are regulated by oscillating between endocytic vesicles and the axonal membrane before and during axonal fusion.These delicate and fine-tuned temporal and spatial movements may vary at different stages of injury, and this might determine which branches successfully fuse and which are retracted or pruned. These pathways likely act downstream of the phagocytic machinery, as overexpression of EFF-1 is capable of compensating for the loss of components of the apoptotic engulfment pathway (Neumann et al., 2015). By separating somatic and local processes in axonal regeneration, this has also probed us to pose other questions. Do cytoskeletal and membrane changes during initial regrowth utilise components recycled from disseminated axon segments or are new components locally synthesised? Furthermore, why are later stages of regrowth somatically controlled? Is it simply to meet metabolic demands or does it serve a precautionary measure to prevent excessive or inappropriate axonal fusion?
Axonal Fusion is Modulated by Age
Surprisingly, we observed a higher frequency of axonal fusion in adult animals, compared to younger ages.However, an opposite correlation was observed in regards to the average length of axonal regrowth, with a significant decrease occurring with age (Abay et al.,2017c). This indicates a switch in regenerative modalities occurs as the animals enter adulthood, with fusion the more likely method after development. Ageing inC.elegansis heavily modulated by the insulin-like growth factor receptor DAF-2, mutation of which induces a 2-fold increase in lifespan. To investigate the function of DAF-2 in mediating the age-dependent changes in axonal fusion levels, we analysed animals’ carrying a mutation in the encoding gene (Abay et al., 2017a). Loss of DAF-2 function did not consistently alter the level of axonal fusion across different ages and did not suppress the age-dependent increase (Abay et al., 2017a, b). Thus,DAF-2 is not responsible for the age-dependent modulation of axonal fusion. In the future, it will be important to uncover the genetic mechanisms impacting the time kinetics of regenerative aging. Furthermore, as neurons frequently display an array of aberrant, dynamic but often innocuous morphologies with advancing age (Chen et al., 2013), it would be interesting to determine if the pathways responsible are shared with those required for regenerative growth. If so, this might result in these phenomena competing for substrates after neuronal injury and possibly impacting neuronal recovery.
Future Directions
Axonal fusion is an extremely promising means of neuronal repair observed in a number of invertebrate species, which we now report has the potential to restore full function. Since this mechanism reestablishes the original axonal tract by fusing axon segments, it could potentially bypass major restrictions of axon regeneration in mammals. However, key factors preventing efficient recovery in mammals remain as significant challenges. Subsequent studies should focus on determining if axonal fusion can occur in higher organisms,and how the process is affected by myelinating and reactive glial cells, and the injury-induced immune response, all of which are absent inC. elegans. Due to these additional factors, translation to clinical practices will likely necessitate a multifaceted approach, requiring the promotion of intrinsic axon regeneration,blocking of the inhibitory microenvironment, and initiating axonal fusion. Nonetheless, recent studies have supported the clinical promise of axonal fusion-like mechanisms. Notably, a ‘PEG-fusion’ approach, in which microsuture techniques are combined with polyethylene glycol (PEG)-induced membrane fusion,has been used to repair severed nerves and restore function in mammalian models (Bittner et al., 2016).Understanding the underlying cellular mechanisms concerning the exposure of PS ‘save-me’ signals,age-related modulation, reestablishment of microtubules, and restoration of biological processes to mediate functional recovery, will be essential to fully understand how axonal fusion can occur. Unraveling these mechanisms and overcoming the current limitations to regeneration in mammals may contribute to clinical strategies aimed at promoting the functional recovery of injured nerves through axonal fusion.
Author contributions:All four authors wrote and edited the paper.
Conflicts of interest:None declared.
Financial support:This work was supported by National Health and Medical Research Council (NHMRC) Project Grant 1101974 to BN.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Abay ZC, Wong MYY, Neumann B (2017a) Daf-2 modulates regeneration of mechanosensory neurons I. Micropublication:biology Dataset doi: 10.17912/W2XD3R.
Abay ZC, Wong MYY, Neumann B (2017b) Daf-2 modulates regeneration of mechanosensory neurons II. Micropublication:biology Dataset doi: 10.17912/W2SM1T.
Abay ZC, Wong MY, Teoh JS, Vijayaraghavan T, Hilliard MA,Neumann B (2017c) Phosphatidylserine save-me signals drive functional recovery of severed axons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 114:E10196-10205.
Basu A, Dey S, Puri D, Das Saha N, Sabharwal V, Thyagarajan P,Srivastava P, Koushika SP, Ghosh-Roy A (2017) let-7 miRNA controls CED-7 homotypic adhesion and EFF-1-mediated axonal self-fusion to restore touch sensation following injury.Proc Natl Acad Sci U S A 114:E10206-10215.
Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP (2016) The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res 94:207-230.
Chen CH, Chen YC, Jiang HC, Chen CK, Pan CL (2013) Neuronal aging: learning from C. elegans. J Mol Signal 8:14.
Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD (2010)Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30:3175-3183.
Hochreiter-Hufford A, Ravichandran KS (2013) Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 5:a008748.
Mapes J, Chen YZ, Kim A, Mitani S, Kang BH, Xue D (2012)CED-1, CED-7, and TTR-52 regulate surface phosphatidylserine expression on apoptotic and phagocytic cells. Curr Biol 22:1267-1275.
Neumann B, Coakley S, Giordano-Santini R, Linton C, Lee ES,Nakagawa A, Xue D, Hilliard MA (2015) EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 517:219-222.
Nichols ALA, Meelkop E, Linton C, Giordano-Santini R, Sullivan RK, Donato A, Nolan C, Hall DH, Xue D, Neumann B, Hilliard MA (2016) The apoptotic engulfment machinery regulates axonal degeneration in C. elegans neurons. Cell Rep 14:1673-1683.
Smurova K, Podbilewicz B (2016) RAB-5- and DYNAMIN-1-mediated endocytosis of EFF-1 fusogen controls cell-cell fusion. Cell Rep 14:1517-1527.
Suzuki J, Imanishi E, Nagata S (2014) Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J Biol Chem 289:30257-30267.
Venegas V, Zhou Z (2007) Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell 18:3180-3192.
Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, Shi Y,Kobayashi T, Shi Y, Mitani S, Xie XS, Xue D (2007) C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol 9:541-549.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
- Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention

