Dry needling at myofascial trigger points mitigates chronic post-stroke shoulder spasticity
Li Tang, Yan Li, , Qiang-Min Huang, Yang Yang
1 Tong Ren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2 Shanghai University of Sport, Shanghai, China
Introduction
In recent years, stroke has fallen from the third to the fourth leading cause of death but without any large decline in its incidence or case fatality (Burke et al., 2012).Kwakkel et al. (2003) demonstrated that the paretic arm remained nonfunctional in 30–66% of patients with stroke. Spasticity developed in 42.6% of patients 6 months after the first stroke (Urban et al., 2010). Similarly, Watkins et al. (2002) established that the prevalence of spasticity at 12 months after stroke was 38%. Stroke mainly damages the nervous system and leads to spasticity, which, along with a number of other factors, contributes to functional impairment. Increasing evidence shows that spastic muscle contractures can decrease the length of the muscle cells and the number of sarcomeres (Lieber et al., 2004; Salom-Moreno et al., 2014).
Fresno et al. (2004) introduced the dry needle therapy to treat incomplete spinal cord injury. There are reports of its use decreasing hypertonia, spasticity and dysfunction after nervous system damage. The use of dry needle therapy in myofascial trigger points to improve dystonia is becoming increasingly accepted. An open-label, randomized investigation found that dry needling during early rehabilitation could improve hemiparetic shoulder
pain syndrome (DiLorenzo et al., 2004). A randomized controlled trial found that a single session of dry needling decreased spasticity and widespread pressure sensitivity in individuals with post-stroke lower limb spasticity (Salom-Moreno et al., 2014). Another study found that dry needling decreased upper limb spasticity and improved the function of forearm supinators after a stroke (Ansari et al., 2015).
There are only a few reports on the application of dry needling to increase shoulder range of motion and improve post-stroke spasticity. This report aimed to show the effects of the application of dry needling on the shoulder range of motion and spasticity in the infraspinatus,teres minor, posterior deltoid, and pectoralis major after stroke.
Case Report
Patient
This is a case of a 62-year-old, right-handed man who had a sudden brain hemorrhage in the right frontal lobe 12 years previously. Relevant medical history includes hypertension and diabetes. He underwent rehabilitation treatment for 12 years, including passive motion on the hemiplegic side, activities of daily-living training and balance training in the hospital. However, the spasticity around his shoulder did not improve and had almost no active movement. Although he did not report any spontaneous pain when he presented to our hospital, there were tender trigger points around the shoulder muscles.The patient was a college graduate, and he scored 25 points in the Mini-Mental State Examination (Mai et al.,2016), showing a good cognitive level. We explained the treatment and its goals to the patient and then obtained informed consent. The patient was admitted for the dry needling treatment as an inpatient to comply with hospital policy. We obtained informed consent from the patient.
Treatment
The trigger point,i.e. the most sensitive spot in a taut band of muscles, was identified through palpation. After cleaning the surface of the skin, a dry needle (0.3 × 40-mm disposable needle: Wuxi Jiajian Medical Instrument Co., Ltd., Wuxi, China) was introduced through the skin into the subcutaneous tissue to a depth of approximately 15–20 mm. Before the needle was inserted further into the muscle tissue, the patient was reminded about the possible pain, muscle twitching, or unpleasant sensation when the needle contacts with the sensitive spots in the taut band. The muscle fibers of the taut band were explored with multiple needle insertions. A local twitch response was elicited in some insertions. The movement of the needle was “fast in–fast out” until no more local twitch responses were elicited to keep a straight track of needle insertion and avoid muscle fiber damage. This technique has been described previously (Hong, 1994),when it was used for pain healing. In our patient, upon application to the infraspinatus, teres minor, posterior deltoid, and pectoralis major, the local twitch responses were obvious. We spent approximately 45–60 seconds on each trigger point until the local twitch responses disappeared or until the patient could no longer bear the pain.The patient underwent dry needling and rehabilitation once every day, except on weekends, for a total of nine treatment sessions (Figure 1).
Assessment
Spasticity was assessed with the Modified Ashworth Scale before and after the treatment. This scale is probably the most widely used test for the measurement of muscle spasticity in research and clinical practice, and it has been demonstrated to be moderately reliable (Bohannon and Smith, 1987; Pandyan et al., 1999; Gregson et al., 2000).The degrees of passive range of motion (flexion, extension, abduction and horizontal adduction) were assessed in the shoulder joints using a bubble goniometer before and after the treatment. Each motion was assessed three times, and the mean value was reported.
Treatment effect
The primary outcome measure was the severity of spasticity of the target muscle group, which was assessed using the Modified Ashworth Scale. A clinically significant improvement in spasticity was reported for all muscles treated (Table 1). After the first dry needling treatment,the spasticity of the shoulder flexors, extensors, abductors, and horizontal adductors decreased from grade 3 to grade 2. Before and after the ninth dry needling treatments, the spasticity of the shoulder flexors, extensors,abductors and horizontal adductors remained in grade 2,showing no change.
The other outcome measure was the degree of shoulder passive range of motion (Table 2). There was a notable improvement in shoulder passive range of motion after the treatment. Furthermore, the patient reported that turning over on the bed became easier, that he could shrug his shoulder slightly, and that his shoulder generally felt better.
Discussion
The myofascial trigger point is a sensitive spot in a taut band of muscle fibers (Hong, 1994). The shoulder of our patient, whose stroke occurred 12 years previously, had no movement. The physical therapist noted that passive movement of the patient’s shoulder was severely limited.Initially, the patient did not report any pain in the shoulder, but palpation of the infraspinatus, teres minor, posterior deltoid and pectoralis major brought about pain.
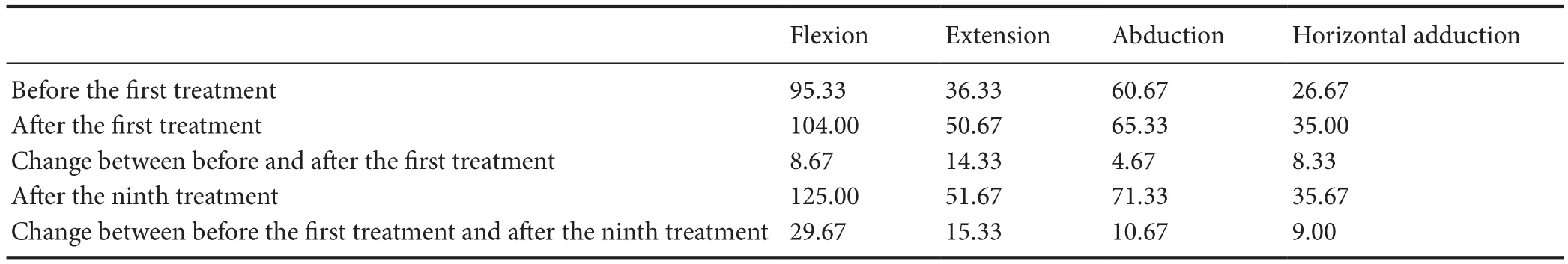
Table 2 Change in the degree of shoulder passive range of motion before and after the dry needling treatment in a patient with chronic stroke
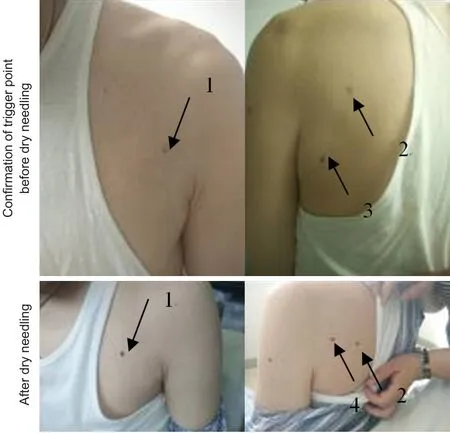
Figure 1 Dry needle treatment.
Furthermore, local twitch responses were evoked during the dry needling treatment, which implies the trigger points were present in these muscles (Hong, 1994).
Clinically, dry needling of the myofascial trigger point has been used as a rehabilitation technique, along with articular mobilization, manipulation, and exercise therapy,for other pathological muscle conditions (Rainey, 2013;Pavkovich, 2015a, b). A study reported that dry needling of the trigger points of the infraspinatus was effective in improving conditions with restricted movement and lessening shoulder pain (Hsieh et al., 2007). Dry needling of the trigger points of the soleus led to a notable improvement in ankle dorsiflexion (Grieve et al., 2011). Osborne and Gatt (2010) reported an improvement in shoulder range of motion, function, and pain condition of four elite volleyball players after dry needling of the trigger points of the infraspinatus and teres minor. Ingber (2000)reported a long-term alleviation of pain (2 years) in three cases of shoulder impingement in tennis/racquetball players who underwent dry needling of the trigger pointsof the subscapularis.
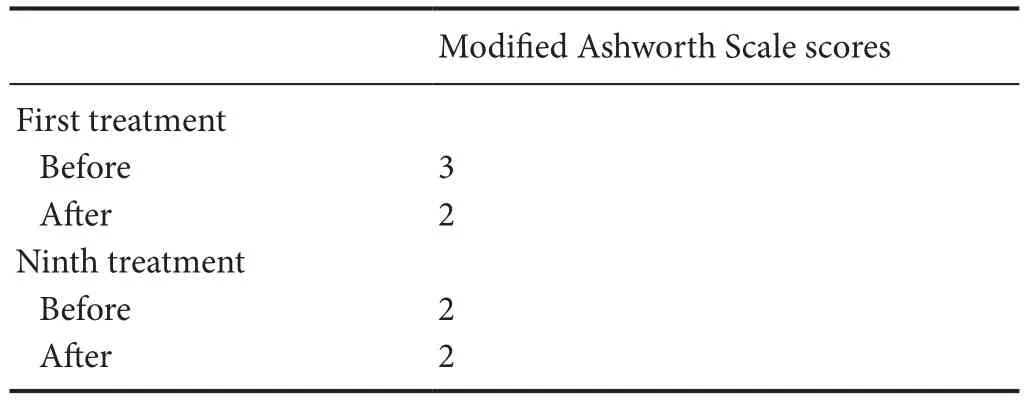
Table 1 Modified Ashworth Scale scores before and after the first and ninth dry needling treatments in a patient with chronic stroke
These studies show that dry needling of trigger points mainly alleviates pain and improves the range of motion of related joints. The latter improvements are in accordance with our results. After the first dry needling treatment to the trigger points, the degree of shoulder flexion,extension, abduction, and horizontal adduction improved(8.67°, 14.33°, 4.67°, and 8.33°, respectively). The change in degrees of range of motion was noted immediately after treatment, which implies that dry needling of the trigger points of the infraspinatus, teres minor, posterior deltoid and pectoralis major can immediately increase shoulder passive range of motion. After the first treatment, the classification of spasticity changed from grade 3 to grade 2 in each of the shoulder flexor, extensor, abductor, and horizontal adductor muscles. Our patient did not report autonomous pain, except when his shoulder moved. We infer that a full stretch range of motion is limited by pain. A passive range of motion restriction may be caused by trigger points, perpetuated by spasticity.Lieber et al. (2004) reported that spasticity can change the structure of the muscle by increasing the stiffness in muscle cells and tissues. We believe that dry needling changes the integrity of local soft tissues and muscle fibers, thus decreasing resistance when stretching the muscle. This could explain the improvement in related muscle spasticity and shoulder passive range of motion in our case.
Our findings suggest that dry needling of the trigger points may have a prolonged effect. Spasticity is a sensorimotor disorder, and we believe that during dry needling, the mechanical sensory stimulation evokes a sensory transduction that not only affects the needling areas, but the nervous system as well. Encouragingly, after the ninth treatment, the patient was able to shrug his shoulder.
In conclusion, dry needling of the trigger points improves the spasticity of related muscles that in turn gives the nerve control of the relatively undamaged muscles,allowing movement of the shoulder.
Author contributions:LT wrote this paper and treated the patient.YL and QMH revised this paper, and YY was in charge of measurement. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support:None.
Declaration of patient consent:The authors certify that they have obtained the appropriate patient consent form. In the form, the patient gave his consent for his images and other clinical information to be reported in the journal. The patient understand that his name and initial will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Min Cheol Chang, Asan Medical Center, Korea.
Ansari NN, Naghdi S, Fakhari Z, Radinmehr H, Hasson S (2015)Dry needling for the treatment of poststroke muscle spasticity: a prospective case report. NeuroRehabilitation 36:61-65.
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206-207.
Burke JF, Lisabeth LD, Brown DL, Reeves MJ, Morgenstern LB(2012) Determining stroke’s rank as a cause of death using multicause mortality data. Stroke 43:2207-2211.
DiLorenzo L, Traballesi M, Morelli D, Pompa A, Brunelli S, Buzzi MG, Formisano R (2004) Hemiparetic shoulder pain syndrome treated with deep dry needling during early rehabilitation: a prospective, open-label, randomized investigation. J Musculoskeletal Pain 12:25-34.
Fresno MJ, Mediavilla P, Mayoral O (2004) Dry needling of miofascial trigger points for hypertonia spastica in incomplete spinal cord injuries. Report of two cases. J Musculoske Pain 12:75.
Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL (2000) Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 29:223-228.
Grieve R, Clark J, Pearson E, Bullock S, Boyer C, Jarrett A (2011)The immediate effect of soleus trigger point pressure release on restricted ankle joint dorsiflexion: A pilot randomised controlled trial. J Bodyw Mov Ther 15:42-49.
Hong CZ (1994) Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response.Am J Phys Med Rehabil 73:256-263.
Hsieh YL, Kao MJ, Kuan TS, Chen SM, Chen JT, Hong CZ (2007)Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil 86:397-403.
Ingber RS (2000) Shoulder impingement in tennis/racquetball players treated with subscapularis myofascial treatments. Arch Phys Med Rehabil 81:679-682.
Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ (2003) Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34:2181-2186.
Lieber RL, Steinman S, Barash IA, Chambers H (2004) Structural and functional changes in spastic skeletal muscle. Muscle Nerve 29:615-627.
Mai LM, Sposato LA, Rothwell PM, Hachinski V, Pendlebury ST(2016) A comparison between the MoCA and the MMSE visuoexecutive sub-tests in detecting abnormalities in TIA/stroke patients. Int J Stroke 11:420-424.
Osborne NJ, Gatt IT (2010) Management of shoulder injuries using dry needling in elite volleyball players. Acupunct Med 28:42-45.
Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H (1999) A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil 13:373-383.
Pavkovich R (2015a) The use of dry needling for a subject with acute onset of neck pain: a case report. Int J Sports Phys Ther 10:104-113.
Pavkovich R (2015b) The use of dry needling for a subject with chronic lateral hip and thigh pain: a case report. Int J Sports PhysTher 10:246-255.
Rainey CE (2013) The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther 8:145-161.
Salom-Moreno J, Sanchez-Mila Z, Ortega-Santiago R, Palacios-Ceña M, Truyol-Domínguez S, Fernández-de-las-Peñas C(2014) Changes in spasticity, widespread pressure pain sensitivity, and baropodometry after the application of dry needling in patients who have had a stroke: a randomized controlled trial. J Manipulative Physiol Ther 37:569-579.
Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, Bauermann T, Weibrich C, Vucurevic GD, Schneider A, Wissel J (2010) Occurence and clinical predictors of spasticity after ischemic stroke.Stroke 41:2016-2020.
Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK (2002) Prevalence of spasticity post stroke. Clin Rehabil 16:515-522.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration

