Exendin-4 inhibits high-altitude cerebral edema by protecting against neurobiological dysfunction
Zhong-Lei Sun , Xian-Feng Jiang , Yuan-Chi Cheng , Ying-Fu Liu, Kai Yang, Shuang-Long Zhu Xian-Bin Kong, Yue Tu ,Ke-Feng Bian, Zhen-Lin Liu , Xu-Yi Chen
1 Affiliated Hospital of Logistics University of Chinese People’s Armed Police Forces, Institute of Traumatic Brain Trauma and Neurological of CAPF, Neurotrauma Repair Key Laboratory of Tianjin, Tianjin, China
2 Jinzhou Medical University, Jinzhou, Liaoning Province, China
3 Tianjin Medical University, Tianjin, China
4 Central Hospital of Fengxian District of Shanghai, Shanghai, China
5 Logistics University of People’s Armed Police Force, Tianjin, China
6 The No. 2 Hospital of Nanjing, Nanjing, Jiangsu Province, China
7 Tianjin University of Traditional Chinese Medicine, Tianjin, China
Introduction
Each year, millions of people travel from places close to sea level to destinations greater than 2500 m above sea level(MASL) (Plant and Aref-Adib, 2008) and millions more permanently live at altitudes of 2500 to 5100 MASL (West,2002). As such, plateau-related disease, and particularly high-altitude cerebral edema (HACE), is a significant epidemiological problem; although the incidence of HACE is very low, its high mortality makes it worthy of study.
Hypoxia-induced inflammation increases brain water content and blood-brain barrier permeability, which are two primary causes of cerebral edema and lead to both brain tissue and vascular endothelial damage (Patir et al., 2012; Rodrigues and Granger, 2015). Fukuhara et al. (2005) reported that second messenger cyclic adenosine monophosphate (cAMP)-mediated inflammatory protective mechanisms function through activation of exchange protein directly activated by cAMP 1(EPAC1) in vascular endothelial cells (VECs). Activation of EPAC1 leads to suppression of cytokine signaling-3 (SOCS-3) gene expression (Sands et al., 2006), which can effectively inhibit interleukin-6 (IL-6) and nuclear factor-kappa B (NF-κB) signaling pathways (Bode et al., 2001; Hovsepian et al.,2013; Mahony et al., 2016). The aquaporin family of proteins also plays a key role in controlling cellular water content, with aquaporin-4 (AQP4) playing a key role in brain conditions such as cerebral edema, blunt force trauma, and Alzheimer’s disease (Benga and Huber, 2012; Badaut et al., 2014).
The glucagon-like peptide-1 (GLP-1) receptor agonist exenatide is synthetic analogue of exendin-4 (Ex-4), a peptide originally isolated from the salivary secretions of the Heloderma suspectum (Gila monster) lizard. Ex-4 has been shown to pass the blood-brain barrier (BBB) (Sandoval and Sisley, 2015) and increase cAMP expression (Li et al., 2015).GLP-1 analogues have demonstrated protective effects on the central nervous system, including effects on synapses,cell repair, and reduction of chronic inflammatory responses (Holscher, 2014). The receptor for GLP-1 is present on pancreatic beta cells and also widely distributed on neurons throughout the brain and peripheral nervous system (Li et al., 2015). Ex-4 elicits protective effects against atherosclerosis (Zhao et al., 2014) and reperfusion injury (Kang et al., 2015) by increasing vascular endothelial growth factor(VEGF) expression to promote the proliferation of vascular endothelial cells (VECs). Ex-4 can also inhibit VEC apoptosis (Erdogdu et al., 2013), thus serving to maintain the integrity of vascular endothelium. Ex-4 has exhibited neuroprotective effects within the CNS in studies of geriatric disorders (Holscher, 2014) and spinal cord injury (Chien et al.,2015; Li et al., 2016). However, whether Ex-4 inhibits HACE remains poorly understood. To investigate the effects of Ex-4, we performed biochemical, pathological, and animal behavioral tests in rat models of HACE.
Materials and Methods
Animals
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Logistics, College of Chinese People’s Armed Police Forces, China (approved No. 2017-0004.2).All efforts were made to minimize the number and suffering of animals and all experimental procedures described here were performed in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals. Two-hundred and eighty-eight male Sprague-Dawley rats (SPF level), aged 8 weeks and weighing 190–220 g, were provided by the Experimental Animal Center of the Academy of Military Medical Sciences [license No. SCXK (Army) 2012-0004)]. Rats were kept at a constant temperature of 22 ± 1°C under a 12-hour dark/light cycles with adlibitumaccess to food and water.
Model exploration of HACE
We assessed the presence and severity of HACE in our modelsviathe Chinese diagnosis standards of acute mountain sickness (AMS) and the international standard diagnosis of Lake Louise for AMS, which we have described previously(Savourey et al., 1995; West, 2010). However, there is no precise method for modeling HACE in animals (Botao et al.,2013; Huang et al., 2015). As such, we sought to verify our own models. To investigate the effects of drugs on HACE,we exposed rats to a simulated elevation of 6000 or 7000 MASL. We evaluated brain water content and vascular leakage, two key indicators of cerebral edema occurrence (Patir et al., 2012), to determine the optimal altitude and hypoxia treatment duration to achieve a robust and valid HACE model.
Rats were randomly divided into three groups: control (sea level;n= 8), simulated 6000 MASL (n= 40; low pressure 47.2 kPa and hypoxia (O247.46%), and simulated 7000 MASL(n= 40; low pressure 41.3 kPa and hypoxia 123.16 g/m3).Animals in the simulated 6000 and 7000 MASL groups were placed in an experimental chamber to simulate a high-altitude, low-pressure and oxygen environment. Humidity was maintained at 50 ± 2% and fresh air- flow was 5 L/h in the experimental chamber. Rats in each group were further divided into six subgroups based on the time that they remained in the experimental chamber: 12, 24, 48, 72, and 96 hours. Animals in the hypoxia groups (12, 24, 48, 72, and 96 hours) were exposed to simulated 6000 or 7000 MASL (n=8 per subgroup). At the end of all experiments, rats were euthanized by chloral hydrate overdose. Brains were weighed to measure wet/dry ratios and assess for Evans blue leakage.Severity of cerebral edema was evaluated at each time point to determine optimal experimental conditions. Finally, animals exposed to high altitude environments were evaluated by measurements of glutathione (GSH), malondialdehyde(MDA), and superoxide dismutase (SOD) values.
Animal grouping and Ex-4 intervention
To investigate the effects of Ex-4 on animal models of HACE(7000-m altitude), fi ve groups were used: control, HACE, HACE+ 2 μg Ex-4, HACE + 10 μg Ex-4, and HACE + 100 μg Ex-4.Within each group, five subgroups (A–E;n= 8/subgroup)were used for the following analyses: A, wet/dry weight ratio; B, Evans blue leakage; C, behavioral evaluation; D, western blot and ELISA; E, pathological examination of brain tissue.
Ex-4 was administered in advance of experiments as the experimental chamber was a closed environment. Rats in the HACE + 2 μg Ex-4, HACE + 10 μg Ex-4, and HACE + 100 μg Ex-4 groups were administered Ex-4 (Cayman Chemical Company, Michigan, USA) intraperitoneally once daily for 3 days prior to experimentation to maintain blood drug concentration. Rats in the control and HACE groups received equal amounts of physiological saline. Following this, rat behavioral and then biochemical analyses were performed.
Behavioral evaluation
The open field test is used to evaluate animal locomotion and anxiety-like behavior (Desikan et al., 2014). The open-field test chamber used here was a custom-built black, wooden box(100 cm × 100 cm × 50 cm), with a generalized central area (30 cm × 30 cm). Each animal was tested for 6 minutes. The apparatus was cleaned with ethanol between animals. This assay is based on the premise that rats will naturally tend towards the periphery of the open field where they are less exposed and thus less vulnerable to potential dangers (e.g. predation)than they would be in the center of the field. Total distance traveled, time spent in the center of the open field, and the number of animal rearings, defined as times that the rat supported itself on its hind legs alone, were assessed.
The tail suspension test was carried out as previously described (Skolnick et al., 2015). Briefly, rats were suspended from a metal rod mounted at a distance of 40 cm above the ground by the tail, which was secured to the rod with adhesive tape. The duration of this test was 6 minutes. The absence of limb or body actions, except those caused by breathing, was defined as resting. Resting time data were collected by the Xmaze video analysis system.
Arterial blood oxygen saturation
On the day of testing, blood samples (approximately 200 μL)were collectedviaarterial cannulae. After sample collection,the catheter was fl ushed with heparin saline. Samples were analyzed with an ABL Flex 800 blood gas analyzer (Radiometer Medical ApS, Åkandevej, Denmark).
Body weight
Rat body weight was measured before and after all experimentation.
White blood cell (neutrophil) counts
On the day of testing, approximately 200 μL of rat caudal venous blood was collected and samples were analyzed by routine blood tests (Affiliated Hospital of Logistics, College of Chinese People’s Armed Police Forces).
Tissue preparation and hematoxylin-eosin staining
Rats were intraperitoneally injected with chloral hydrate (100 mg/kg) and perfused with physiological saline and 4% paraformaldehyde (dissolved in 1 M phosphate-buffered saline,pH 7.4)viacardiac puncture. Brains were harvested, fixed with 4% formaldehyde, embedded in paraffin, sliced into 5-μm-thick coronal sections, and stained with hematoxylin-eosin (Sigma Aldrich, St. Louis, MO, USA) (Alawa et al.,2015). Areas of interest were photographed with a digital camera (Nikon, Tokyo, Japan) coupled to a light microscope(Olympus, Tokyo, Japan).
Brain water content
Wet/dry weight ratios were used to measure brain water content (Park et al., 2009). All rats in the experimental groups were intraperitoneally administered an appropriate dosage of Ex-4 30 minutes prior to hypoxia. Rats in the control and HACE groups were intraperitoneally administered equal amounts of physiological saline. At the end of the experiment, rats in each group were anesthetized by intraperitoneal injection of chloral hydrate (300 mg/kg body weight)and brains stripped of their meninges at the brain stem level were taken for wet weight measurement. Samples were then dried at 110°C for 24 hours and the dry weight was measured by analytical balance (Sartorius, Germany). Water content of brain tissue was calculated as the ratio of wet/dry weight (W/D).
Evans blue extravasation
Evans blue dye-bound albumin in the blood can enter the parenchyma through a damaged BBB, ultimately being detectable in brain tissue (Cangalaya et al., 2016). Thirty minutes before the end of hypoxia exposure, rats were removed from the hypobaric chamber, injected with 2% Evans blue (Sigma-Aldrich, St. Louis, MO, USA; 4 mL/kg body weight)viathe tail vein, and then replaced into the hypobaric chamber until the end of hypoxia. Rats were anesthetized by intraperitoneal chloral hydrate (Sigma-Aldrich; 300 mg/kg body weight) before brains were removed. Left and right hemispheres were separated along the sagittal suture. After weighing, both hemispheres were soaked in formamide (1 mL/100 mg) at 60°C for 24 hours. The content of dye extracted from each brain was determined spectrophotometrically (at 620 nm). Quantitative calculation of dye content was based on an external standard dissolved in the same solvent (Liu et al., 2013).
ELISA
MDA, GSH, and SOD contents, as well as interleukin-6 (IL-6), tumor necrosis factor-α (TNFα), and cyclic adenosine monophosphate (cAMP) levels in brain homogenates were measured by ELISA according to kit instructions (R&D Systems, Minneapolis, MN, USA) (Wyatt et al., 2010).
Western blot analyses
Occludin and ZO-1 are classic proteins for use in the assessment of blood-brain barrier integrity (Liu et al., 2014).SOCS-3, a key protein parameter in this experiment, plays an important role in the anti-inflammatory response (Hovsepian et al., 2013). AQP4 is an important marker of cere-bral edema (Iacovetta et al., 2012). NF-κB is a prominent indicator of inflammation (Himadri et al., 2010; Benga and Huber, 2012). VEGF levels have recently been used to indicate vascular permeability (Dewhirst and Ashcraft, 2016).Rats subjected to hypoxia were anesthetized by an intraperitoneal injection of chloral hydrate (300 mg/kg body weight)before collecting brains, which were homogenized on ice.Homogenate protein levels were determinedviabicinchoninic acid (BCA) assay (Li et al., 2016). Protein samples(30 μg) were mixed with sample buffer [0.0625 M Tris-HCl,pH 6.8, 2% (w/v) sodium dodecyl sulfate (SDS), 5% (w/v)β-mercaptoethanol, 10% (v/v) glycerin, and 0.002% (w/v)bromophenol blue] for 10% SDS-polyacrylamide gel electrophoresis (Zanotto et al., 2013). After separation, protein samples were transferred onto polyvinylidene difluoride membranes for incubation at room temperature for 1 hour with rabbit anti-rat occludin polyclonal antibody (Abcam),rabbit anti-rat ZO-1 polyclonal antibody (Abcam), rabbit anti-rat SOCS-3 polyclonal antibody (Abcam), rabbit anti-rat VEGF polyclonal antibody (Abcam), rabbit anti-rat EPAC1 polyclonal antibody (Abcam), rabbit anti-rat NF-κB polyclonal antibody (Abcam), and rabbit anti-rat AQP4 polyclonal antibody (Abcam) (all diluted to 1:5000). After three saline washes, protein samples were incubated with anti-rabbit or anti-mouse peroxidase-conjugated immunoglobulin (IgG, diluted at 1:10,000) at room temperature for 1 hour (Desoubeaux et al., 2017). Chemiluminescence signal was detected using an ECL Kit (Beyotime Institute of Biotechnology, Nanjing, Jiangsu, China) and analyzed using ScionImage software (ScionImage, Chicago, IL, USA). Rabbit anti-rat GAPDH polyclonal antibody (1:5000; Abcam)was used as an internal reference.
Statistical analyses
All data are presented as the mean ± standard deviation(SD) and were analyzed using one-way analysis of variance(ANOVA) and Tukey’spost hocmultiple comparison tests with SPSS 16.0 software (SPSS, Chicago, IL, USA). AP-value< 0.05 was considered statistically significant.
Results Verification of HACE animal models
In the simulated 6000 MASL environment for 72 hours condition, water content and Evans blue leakage levels in the brain tissue of rats were significantly different compared control animals (water content:P< 0.05; Evans blue leakage:P< 0.05). There were no significant differences between the other experimental groups (Figure 1A & B). There were significant differences in water content and Evans blue leakage level in the brain tissue of rats subjected to the simulated 7000 MASL with hypoxia environment, especially after 72 hours, compared with controls (water content:P< 0.001;Evans blue leakage:P< 0.001; Figure 1C & D). SOD, GSH,and MDA values in the brain tissue of rats subjected to a simulated 7000 MASL environment were also significantly different from controls, especially in the 72 hours of hypoxia group (SOD:P< 0.01; GSH:P< 0.01; MDA:P< 0.001; Figure 1E–G).
Ex-4 improved behavioral deficits in rats with HACE
Results of the open field test showed that rat behaviors significantly improved in the HACE + 2 μg Ex-4, HACE + 10 μg Ex-4, and HACE + 100 μg Ex-4 groups compared with the untreated HACE group. Distance traveled by rats increased with increasing dose of Ex-4 (2 μg Ex-4:P< 0.05; 10 μg Ex-4:P< 0.01), but was decreased in the HACE + 100 μg Ex-4 group compared with the HACE + 10 μg Ex-4 group (P< 0.05; Figure 2A). Time spent in the center of the open field also increased with increasing dosage of Ex-4 (2 μg Ex-4:P< 0.05; 10 μg Ex-4:P< 0.01; Figure 2B). Similarly, distance traveled was reduced in the HACE + 100 μg group compared with the HACE + 10 μg Ex-4 group (P< 0.05). While rearing count increased with increasing dosage of Ex-4 (2 μg Ex-4:P< 0.05; 10 μg Ex-4:P< 0.01), it was also reduced in the HACE+ 100 μg Ex-4 group compared with the HACE + 10 μg Ex-4 group (P< 0.05; Figure 2C). The tail suspension test indicated that Ex-4 pretreatment effectively reduced immobility time. The amount of time that animals spent immobile was gradually reduced with an increasing dosage of Ex-4 (2 μg Ex-4:P< 0.05; 10 μg Ex-4:P< 0.01), but was not shorter in the HACE + 100 μg Ex-4 group than it was in the HACE +10 μg Ex-4 group (P< 0.05; Figure 2D).
Ex-4 inhibited BBB injury in HACE rats
Cerebral edema was more significantly inhibited in the Ex-4-pretreated groups than in the HACE group (Figure 3A). 10 μg Ex-4 pretreatment in particular effectively reduced brain water content (P< 0.01). Ex-4 pretreatment effectively inhibited HACE-associated vascular leakage (Figure 3B). While the inhibitory effect of Ex-4 increased from 2 to 10 μg, 10 μg Ex-4 pretreatment most effectively alleviated cerebral vascular leakage (P< 0.001). Collectively, these results suggest that a moderate dosage of Ex-4 can effectively alleviate high altitude hypoxia-mediated BBB injury and reduce cerebral edema.
To identify changes in BBB-related protein in rats with HACE, we assessed expression of occludin, ZO-1, and AQP4 proteins using western blot analysis. Occludin and ZO-1 protein expression decreased, but was significantly higher in the 10 μg Ex-4-pretreated groups than in the HACE group (occludin:P< 0.01; ZO-1:P< 0.01; Figure 3C–E). At the same time, the 10 μg Ex-4-pretreated group evidenced significantly decreased AQP4 protein expression compared with the HACE group (P< 0.01; Figure 3F).
Ex-4 inhibited oxidative stress and inflammatory responses in rats with HACE
GSH, MDA, and SOD expression detected by ELISA indicated decreased GSH expression in the HACE group compared with the control group, and significantly increased GSH in the 10 μg Ex-4-pretreated group compared with the HACE group (P< 0.01; Figure 4A). In contrast, MDA expression was increased in the HACE group compared with the control group, and decreased significantly in the 10 μg Ex-4-pretreated group compared with the HACE group (P< 0.01; Figure 4B). SOD expression was decreased in the HACE group compared with the control group, and significantly increased in the 10 μg Ex-4-pretreated group compared with the HACE group (P< 0.01; Figure 4C).

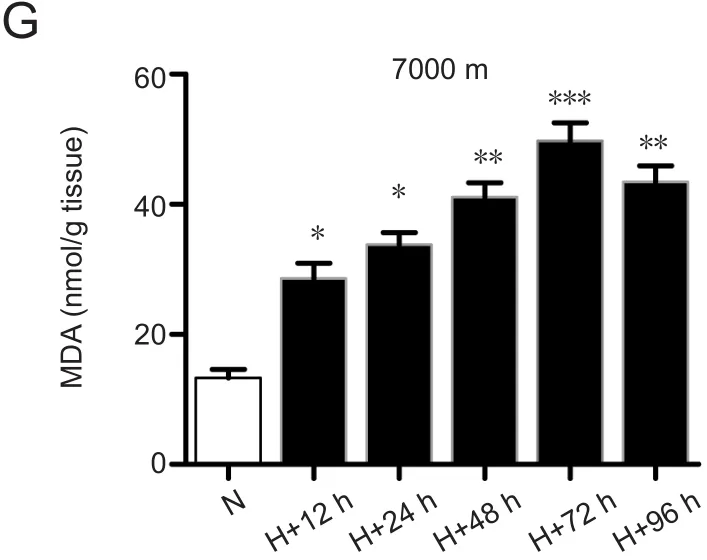
Figure 1 7000-m altitude 72-hour hypoxia was the best experiment condition.
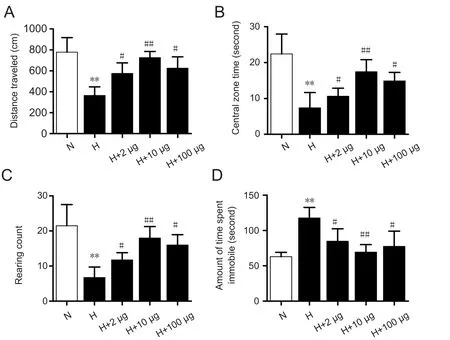
Figure 2 Exendin-4 effectively improved behaviors of rats with cerebral edema exposed to a simulated 7000-m altitude environment and 72 hours of hypoxia.

Figure 3 Exendin-4 (Ex-4) alleviated BBB injury effects mediated by high-altitude cerebral edema in rats.
IL-6, TNF-α, and NF-κB expression was also detected.IL-6 and TNF-α expression was increased in the HACE group compared with the control group and decreased in the 10 μg Ex-4-pretreated groups compared with the HACE group (IL-6:P< 0.01; TNF-α:P< 0.01; Figure 4D& E). Western blot analysis showed that NF-κB expression increased in the HACE group, but were decreased significantly in the 10 μg Ex-4-pretreated group compared with the HACE group (P< 0.01; Figure 4G & H). Finally, using GAPDH as a reference, we performed western blot analyses to detect SOCS-3 and EPAC1 protein expression levels.While SOCS-3 expression was decreased in the HACE group, it was increased significantly in the 10 μg Ex-4-pretreated group compared with the HACE group (P< 0.01;Figure 4G & I). Additionally, EPAC1 expression was significantly decreased in the HACE group compared with the control group and was significantly increased in the 10 μg Ex-4-pretreated group compared with the HACE group (P<0.01; Figure 4G & J). ELISA results indicated that exposure to high-altitude hypoxia inhibited cAMP expression, which could be significantly alleviated by 10 μg Ex-4 pretreatment(P< 0.01; Figure 4F).
Ex-4 alleviated cerebral edema and reduced perivascular space in HACE rats
Hematoxylin-eosin staining was performed to confirm the inhibitory effects of Ex-4 on cerebral edema. Under 400×magnification, cells and vascular morphology were compared between groups. When compared with control group(Figure 5B), HACE group animals exhibited increased peripheral vessel space and widening, obvious cell swelling, irregular nuclei, expanded intercellular space, and disordered stromal cells (Figure 5C). Cerebral edema was inhibited to different degrees in the Ex-4 pretreated groups. In particular, the HACE + 10 μg Ex-4 group showed the most obvious inhibitory effects, as manifested by slightly dilated perivascular space. These results suggest that Ex-4 exerts protective effects on cerebral edema (Figure 5D–F). Our results demonstrate that the 10 μg Ex-4 pretreated group evidenced decreased perivascular space dosage-dependently when compared with the HACE group (P< 0.01; Figure 5G).
Ex-4 improved additional parameters in HACE rats
The body weight of rats with HACE was significantly lower than that of control rats of the same age. Furthermore,Ex-4-pretreatment resulted in a significantly increased body weight, especially in the 10 μg Ex-4 dose group (P< 0.01;Figure 6A). Partial pressure of O2in arterial blood (PaO2)was significantly lower in the HACE group compared with the control group (P< 0.01). PaO2was also lower in the Ex-4-pretreated groups than in the control group, but was significantly higher than that in the HACE group, especially at the 10 μg Ex-4 dose level (P< 0.01; Figure 6B). Exposure to high-altitude hypoxia led to an increase in white blood cells that could effectively be reduced by in particular 10 μg Ex-4 pretreatment (P< 0.01; Figure 6C).
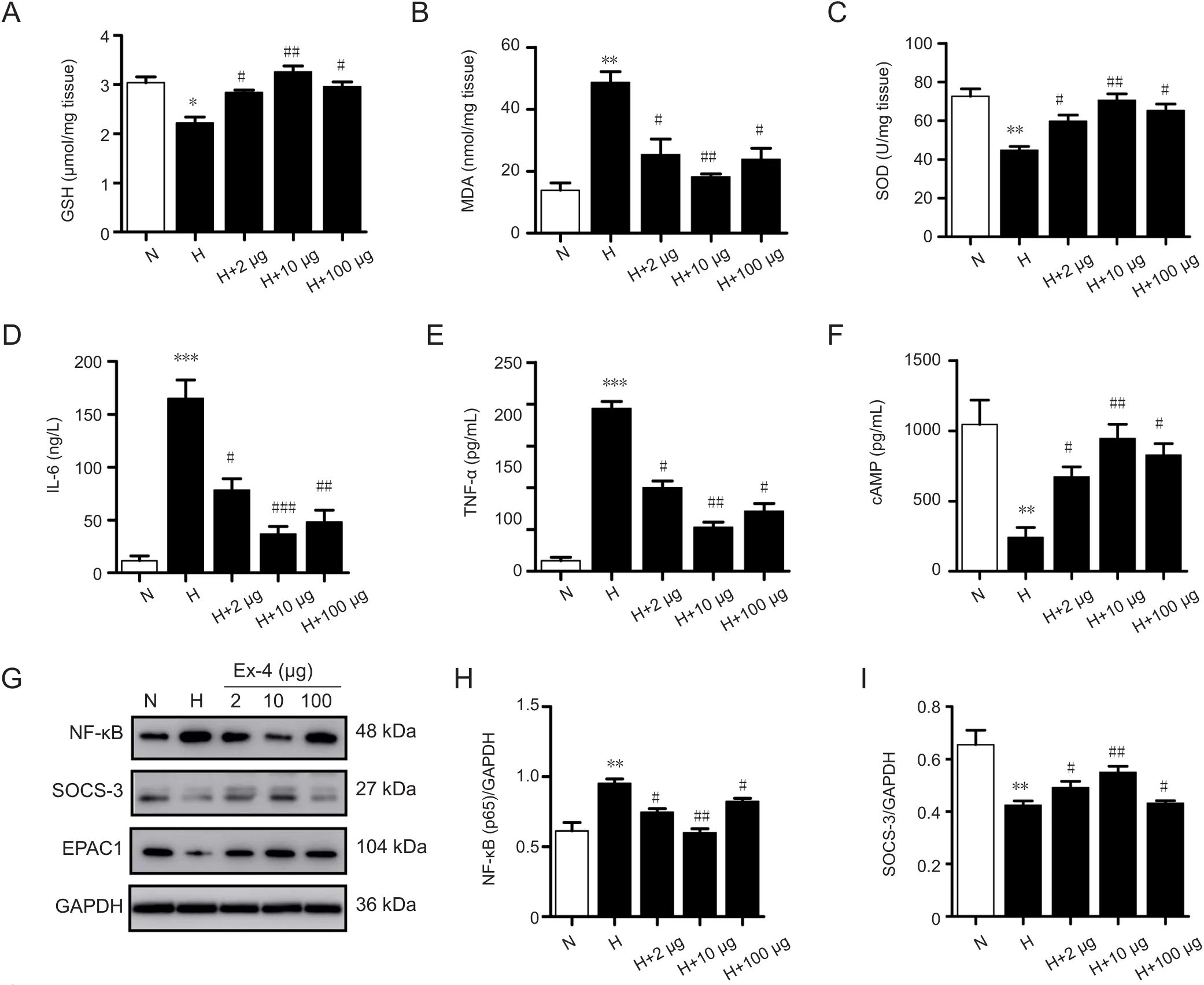
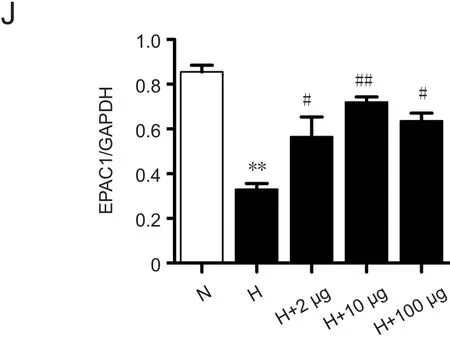
Figure 4 Exendin-4 pretreatment inhibited oxidative stress and inflammatory response in rats with high-altitude cerebral edema.
Peak expression of VEGF is earlier than the peakpermeability of BBB in HACE rats pre-treated with Ex-4
Western blot analysis performed to detect VEGF expression demonstrated increased expression in HACE and Ex-4-pretreated groups. Increased protein expression occurred in a dose-dependent manner and was higher in the 100 μg Ex-4-pretreated group compared with the HACE group (P< 0.001; Figure 7A). However, peak expression of VEGF,which occurred at 48 hours, precedes peak BBB permeability, which occurs at 72 hours (Figures 1D, 7B & C).
Discussion
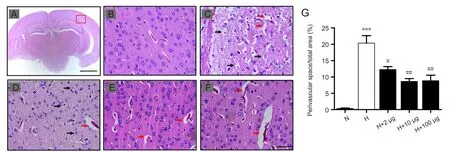
Figure 5 Exendin-4 (Ex-4) alleviated cerebral edema and reduced perivascular space in rats with high-altitude cerebral edema (HACE).
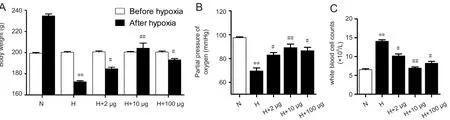
Figure 6 Physio-biochemical parameters in rats with high-altitude cerebral edema after Exendin-4 pretreatment.

Figure 7 Peak expression of VEGF precedes peak permeability of the BBB.
As the most fatal component of altitude sickness, HACE is a medical problem requiring emergent treatment. Therefore,understanding its underlying mechanisms and develop-ing new treatment methods is of the utmost importance.However, uniform standard parameters for creating HACE animal models do not exist. Altitude selection (6000, 7000,7500, 8000 or 10,000 m) and duration of hypoxia (12, 24, 48 or 72 hours) vary between studies (Botao et al., 2013; Huang et al., 2015). To determine optimal experimental conditions for rat models of HACE, the current study examined exposure to a simulated altitude of 6000 MASL and various durations of hypoxia. Resulting brain water content and vascular leakage were measured to evaluate cerebral edema and demonstrated that HACE was not successfully induced at 6000 MASL. We next selected an altitude of 7000 MASL with 72-hours of hypoxia and found that HACE was successfully induced, as confirmed by brain water content and vascular leakage, as well as levels of oxidative stress markers(GSH, SOD, and MDA).
We selected Ex-4 to further examine the effects of drug treatment on HACE as it has been shown previously to protect pancreatic β cells from pro-inflammatory and pro-oxidant insults (Carlessi et al., 2015). A previous study demonstrated that a double-hit model of plateau hypoxia and oxidative stress caused the accumulation of oxygen free radicals, resulting in unstable membrane lipid peroxidation, inflammatory system activation, and BBB dysfunction(Lafuente et al., 2016). Our results demonstrated that Ex-4 pretreatment effectively reduced HACE oxidative stress by increasing expression of GSH and SOD and decreasing expression of MDA. Inflammation and neurodegenerative diseases increase vascular permeability and organ dysfunction, leading to severe pathological consequences (Kumar et al., 2009). The endothelial inflammatory cytokines IL-6 and NF-κB play an important role in the development of HACE(Himadri et al., 2010; Benga and Huber, 2012). Our results show that Ex-4 pretreatment effectively reduced the inflammatory response with HACE by decreasing IL-6, TNF-α,and NF-κB expression levels.
As a negative regulator of inflammatory signaling, SOCS-3 has been shown to inhibit acute and chronic inflammation of VECsin vivoby exerting a negative feedback mechanism on the expression of signal transduction receptors on various cell surfaces (Dalpke et al., 2008; White et al., 2011).In addition, upon activation by EPAC1, SOCS-3 reduced inflammatory responses by inhibiting both NF-κB (Dhar et al., 2013) and IL-6 (Yarwood et al., 2008) inflammatory pathways and TNF-α expression. Activation of SOCS-3 can be mediated by at least three signaling pathways: cAMP/EPAC1 (Li et al., 2015), cAMP/protein kinase A (PKA)/c-Jun N-terminal kinase (JNK) (Gorentla et al., 2009), and the cAMP/PKA/extracellular signal-related kinase (ERK)(Woolson et al., 2009). In this study, we analyzed the cAMP/EPAC1 signaling pathway. MacDonald et al. (2003) reported that the GLP analogue Ex-4 can activate the cAMP/EPAC1 signaling pathway. In alignment with this, our results showed that Ex-4 pretreatment increased cAMP, EPAC1,and SOCS-3 expression. We propose that Ex-4 pretreatment inhibited the occurrence of HACE in our model by increasing SOCS-3 expression, inhibiting endothelial inflammatory responses, and maintaining BBB integrityviathe cAMP/EPAC1 signaling pathway. cAMP signaling pathway effects on endothelial permeability are based on two factors: (i)inactivation of contractile machinery and relaxation of endothelial cells, which release the centripetal force provided by tension in endothelial contractile machinery, mainlyviaPKA activation (Goeckeler and Wysolmerski, 2005), and(ii) strengthening of cell–cell adhesion by the stabilization of adherens junctionsviathe Epac pathway (Birukova et al.,2007; Aslam et al., 2010). Collectively, these findings suggest that Ex-4 prevents inflammation by inhibiting oxidative stress and protecting BBB integrity.
To determine whether Ex-4 can inhibit the development of HACE, we further analyzed related parameters in rats with HACE. Our results demonstrate that Ex-4 reduced brain water content and BBB leakage, supporting the notion that Ex-4 inhibits both the occurrence and development of HACE. Next, we assessed tight junction proteins that regulate BBB permeability. These results demonstrated that Ex-4 effectively increased expression of the tight junction proteins occludin and ZO-1. Subsequent detection of AQP4 expression on the BBB surface showed that Ex-4 increased AQP4 expression. Other affected parameters included blood oxygen content, white blood cell (neutrophil) counts, and animal body weight. Changes in these parameters suggests that Ex-4 inhibits the occurrence and development of HACE. Finally, we performed hematoxylin-eosin staining to analyze the neuropathological effects of Ex-4 and found that Ex-4 reduced void areas in brain tissues. Our results also showed that a 10 μg dose of Ex-4 elicited better inhibitory effects than did 2 μg or 100 μg Ex-4 doses. Previous reports demonstrating that Ex-4 exhibits protective effects on spinal cord injury in a linear, dose-dependent manner (Li et al.,2016) are not applicable for HACE.
HACE is associated with a number of behavioral changes,such as reduced mobility, unresponsiveness, drowsiness,and coma (Okiyama et al., 1997; Quigley and Zafren, 2016).As behavioral changes in our rat model of HACE were also found in the open field test, we considered using further behavioral evaluation methods to indirectly measure the degree of rat cerebral edema. Although few studies have explored animal behavioral in HACE models, rats with HACE have previously been shown to exhibit decreased autonomous activity and reduced autonomous exploratory consciousness (Christakis et al., 2012). Based on these findings, we selected the classic open field and tail suspension tests to evaluate behavioral changes in our model. We found that exposure to high altitude and hypoxia elicited an obvious alteration in these behaviors, which were rescued by Ex-4 pretreatment. This provides a novel method for further evaluating HACE models and the prevention of deleterious HACE-associated phenotypes.
Our results indicate that obvious increases in VEGF expression in the plateau environment are further increased after Ex-4 pretreatment. Previous studies have demonstrated that Ex-4 affects reperfusion injury, ischemic encephalopathy, and cardiovascular sclerosis by increasing VEGF expression and promoting angiogenesis (Zhao et al., 2014;Li et al., 2016). However, an increase in VEGF expression increases vascular permeability throughout the whole body in a dose-dependent manner (Dobrogowska et al., 1998).Therefore, VEGF is often used as a standard to evaluate vascular permeability.
These results suggest that, although increased VEGF expression does not support protection of BBB integrity by Ex-4, it does not alter the effective, therapeutic action of Ex-4 in the occurrence and development of HACE. While increased VEGF expression can increase vascular endothelial cell activity, inhibit endothelial cell apoptosis, promote angiogenesis, and behave in a neuroprotective way, its effect on vascular permeability leads to both favorable and unfavorable consequences (Reischl et al., 2014). An increase in VEGF expressionin vivois often accompanied by an inflammatory response, which can harm the BBB (Song et al., 2016). As the role of increased endogenous VEGFin vivois unknown,we hypothesized here that modest increases in endogenous VEGF could promote protective mechanism (i.e. VEGF at the levels observed here is not sufficient to impact BBB integrity). Future studies may seek to determine this relationship more definitively.
Although the outcomes presented here are promising,several limitations exist which must be addressed. First,higher doses of Ex-4 were not protective against the neurobiological effects of HACE. Instead, moderate doses were found to be more effective. This is not consistent with previous findings in spinal cord injury (Li et al., 2016). This may be related to the optimum concentration range for drug effectiveness. Thus, the most effective dose of Ex-4 should be further explored. Second, we examined Ex-4 as a preventive drug, not a therapeutic drug, meaning that it was treated prior to insult rather than after the insult had occurred.Thus, whether Ex-4 exhibits similar utility in the prevention of HACE effects as a therapeutic agent should be investigated in future studies. Third, we only performed the open field and tail suspension tests to evaluate for CNS dysfunction,leaving some uncertainty with regard to other behavioral phenotypes implicated in our HACE model. Additional behavioral results of CNS dysfunction in HACE need to be further quantified. Fourth, our finding that a modest increase in simple endogenous VEGF does not destroy BBB integrity should be validated further.
This paper is the first to demonstrate that Ex-4 exhibits inhibitory effects on HACE by increasing SOCS3 expression and inhibiting inflammatory responses, at least in part through the cAMP/EPAC1 signaling pathway. At the same time, we report that the time course of HACE and peak time for VEGF levels, as well as the permeability of the BBB in this model, are not consistent. Collectively, our findings suggest that Ex-4 may serve as a relevant target for the prevention of acute HACE.
Author contributions:ZLS, XFJ, and YCC designed the experiment and wrote the paper. YFL, KY, XBK, and KFB collected the data. SLZ, YT, ZLL,and XYC analyzed the data. All authors approved the final version of the paper.
Conflicts of interest:All authors declare that no conflict of interest exists.
Financial support:This work was financially supported by the National Key Research and Development Plan of China, No. 2016YFC1101500; the National Natural Science Foundation of China, No. 11672332, 11102235,31200809, 81772018; the Key Science and Technology Support Foundation of Tianjin City of China, No. 17YFZCSY00620; the Natural Science Foundation of Tianjin City of China, No. 15JCYBJC28600, 17JCZDJC35400. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Ethical approval:All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Logistics, College of Chinese People’s Armed Police Forces, China(approval No. 2017-0004.2). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Murat Sahin, University of Amasya, Turkey.
Additional file:Open peer review report 1.
Alawa JN, Gideon GO, Adetiba B, Alawa CB (2015) Comparative tissue stainability of Lawsonia inermis (Henna) and eosin as counterstains to hematoxylin in brain tissues. Microsc Microanal 21:343-350.
Aslam M, Härtel FV, Arshad M, Gündüz D, Abdallah Y, Sauer H, Piper HM, Noll T (2010) cAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: role of CPI-17.Cardiovasc Res 87:375-384.
Badaut J, Fukuda AM, Jullienne A, Petry KG (2014) Aquaporin and brain diseases. Biochim Biophys Acta 1840:1554-1565.
Benga O, Huber VJ (2012) Brain water channel proteins in health and disease. Mol Aspects Med 33:562-578.
Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR,Birukov KG (2007) Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313:2504-2520.
Bode JG, Ludwig S, Freitas CA, Schaper F, Ruhl M, Melmed S, Heinrich PC,Häussinger D (2001) The MKK6/p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biol Chem 382:1447-1453.
Botao Y, Ma J, Xiao W, Xiang Q, Fan K, Hou J, Wu J, Jing W (2013) Protective effect of ginkgolide B on high altitude cerebral edema of rats. High Alt Med Biol 14:61-64.
Cangalaya C, Bustos JA, Calcina J, Vargas-Calla A, Suarez D, Gonzalez AE, Chacaltana J, Guerra-Giraldez C, Mahanty S, Nash TE, García HH,Cysticercosis Working Group in Peru (2016) Perilesional inflammation in neurocysticercosis - relationship between contrast-enhanced magnetic resonance imaging, Evans blue staining and histopathology in the pig model. PLoS Negl Trop Dis 10:e0004869.
Carlessi R, Lemos NE, Dias AL, Oliveira FS, Brondani LA, Canani LH, Bauer AC, Leitao CB, Crispim D (2015) Exendin-4 protects rat islets against loss of viability and function induced by brain death. Mol Cell Endocrinol 412:239-250.
Chien CT, Jou MJ, Cheng TY, Yang CH, Yu TY, Li PC (2015) Exendin-4-loaded PLGA microspheres relieve cerebral ischemia/reperfusion injury and neurologic deficits through long-lasting bioactivity-mediated phosphorylated Akt/eNOS signaling in rats. J Cereb Blood Flow Metab 35:1790-1803.
Christakis DA, Ramirez JS, Ramirez JM (2012) Overstimulation of newborn mice leads to behavioral differences and deficits in cognitive performance. Sci Rep 2:546.
Dalpke A, Heeg K, Bartz H, Baetz A (2008) Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology 213:225-235.
Desikan A, Wills DN, Ehlers CL (2014) Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacol Biochem Behav 122:279-285.
Desoubeaux G, Pantin A, Peschke R, Joachim A, Cray C (2017) Application of Western blot analysis for the diagnosis of Encephalitozoon cuniculi infection in rabbits: example of a quantitative approach. Parasitol Res 116:743-750.
Dewhirst MW, Ashcraft KA (2016) Implications of increase in vascular permeability in tumors by VEGF: a commentary on the pioneering work of harold dvorak. Cancer Res 76:3118-3120.
Dhar K, Rakesh K, Pankajakshan D, Agrawal DK (2013) SOCS3 promotor hypermethylation and STAT3-NF-kappaB interaction downregulate SOCS3 expression in human coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 304:H776-785.
Dobrogowska DH, Lossinsky AS, Tarnawski M, Vorbrodt AW (1998) Increased blood–brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol 27:163-173.
Erdogdu O, Eriksson L, Xu H, Sjöholm A, Zhang Q, Nyström T (2013)Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI3K,eNOS, p38 MAPK, and JNK pathways. J Mol Endocrinol 50:229-241.
Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N,Saito Y, Kangawa K, Mochizuki N (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25:136-146.
Goeckeler ZM, Wysolmerski RB (2005) Myosin phosphatase and cofilin mediate cAMP/cAMP-dependent protein kinase-induced decline in endothelial cell isometric tension and myosin II regulatory light chain phosphorylation. J Biol Chem 280:33083-33095.
Gorentla BK, Moritz AE, Foster JD, Vaughan RA (2009) Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry 48:1067-1076.
Himadri P, Kumari SS, Chitharanjan M, Dhananjay S (2010) Role of oxidative stress and inflammation in hypoxia-induced cerebral edema: a molecular approach. High Alt Med Biol 11:231-244.
Holscher C (2014) Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 221:T31-41.
Hovsepian E, Penas F, Siffo S, Mirkin GA, Goren NB (2013) IL-10 inhibits the NF-kappaB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes. PLoS One 8:e79445.
Huang X, Zhou Y, Zhao T, Han X, Qiao M, Ding X, Li D, Wu L, Wu K,Zhu LL, Fan M (2015) A method for establishing the high-altitude cerebral edema (HACE) model by acute hypobaric hypoxia in adult mice. J Neurosci Methods 245:178-181.
Iacovetta C, Rudloff E, Kirby R (2012) The role of aquaporin 4 in the brain.Vet Clin Pathol 41:32-44.
Kang HM, Sohn I, Jung J, Jeong JW, Park C (2015) Exendin-4 protects hindlimb ischemic injury by inducing angiogenesis. Biochem Biophys Res Commun 465:758-763.
Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY (2009) Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 11:e19.
Lafuente JV, Bermudez G, Camargo-Arce L, Bulnes S (2016) Blood-brain barrier changes in high altitude. CNS Neurol Disord Drug Targets 15:1188-1197.
Li AQ, Zhao L, Zhou TF, Zhang MQ, Qin XM (2015) Exendin-4 promotes endothelial barrier enhancement via PKA- and Epac1-dependent Rac1 activation. Am J Physiol Cell Physiol 308:C164-175.
Li HT, Zhao XZ, Zhang XR, Li G, Jia ZQ, Sun P, Wang JQ, Fan ZK, Lv G(2016) Exendin-4 enhances motor function recovery via promotion of autophagy and inhibition of neuronal apoptosis after spinal cord injury in rats. Mol Neurobiol 53:4073-4082.
Liu W, Wang P, Shang C, Chen L, Cai H, Ma J, Yao Y, Shang X, Xue Y (2014)Endophilin-1 regulates blood-brain barrier permeability by controlling ZO-1 and occludin expression via the EGFR-ERK1/2 pathway. Brain Res 1573:17-26.
Liu X, Wang Z, Wang P, Yu B, Liu Y, Xue Y (2013) Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement Altern Med 13:187.
MacDonald PE, Wang X, Xia F, El-kholy W, Targonsky ED, Tsushima RG, Wheeler MB (2003) Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 278:52446-52453.
Mahony R, Ahmed S, Diskin C, Stevenson NJ (2016) SOCS3 revisited: a broad regulator of disease, now ready for therapeutic use? Cell Mol Life Sci 73:3323-3336.
Okiyama K, Smith D H, White W F, Richter K, McIntosh T K (1997) Effects of the novel NMDA antagonists CP-98, 113, CP-101,581 and CP-101,606 on cognitive function and regional cerebral edema following experimental brain injury in the rat. J Neurotrauma 14:211-222.
Park J, Yang WS, Kim SB, Park SK, Lee SK, Park JS, Chang JW (2009) Usefulness of segmental bioimpedance ratio to determine dry body weight in new hemodialysis patients: a pilot study. Am J Nephrol 29:25-30.
Patir H, Sarada SK, Singh S, Mathew T, Singh B, Bansal A (2012) Quercetin as a prophylactic measure against high altitude cerebral edema. Free Radic Biol Med 53:659-668.
Plant T, Aref-Adib G (2008) Travelling to new heights: practical high altitude medicine. Br J Hosp Med (Lond) 69:348-352.
Quigley I, Zafren K (2016) Subtle cognitive dysfunction in resolving high altitude cerebral edema revealed by a clock drawing test. Wilderness Environ Med 27:256-258.
Reischl S, Li L, Walkinshaw G, Flippin LA, Marti HH, Kunze R (2014) Inhibition of HIF prolyl-4-hydroxylases by FG-4497 reduces brain tissue injury and edema formation during ischemic stroke. PLoS One 9:e84767.
Rodrigues SF, Granger DN (2015) Blood cells and endothelial barrier function. Tissue Barriers 3:e978720.
Sandoval D, Sisley SR (2015) Brain GLP-1 and insulin sensitivity. Mol Cell Endocrinol 418 Pt 1:27-32.
Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM (2006)Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells.Mol Cell Biol 26:6333-6346.
Savourey G, Guinet A, Besnard Y, Garcia N, Hanniquet AM, Bittel J (1995)Evaluation of the Lake Louise acute mountain sickness scoring system in a hypobaric chamber. Aviat Space Environ Med 66:963-967.
Skolnick P, Kos T, Czekaj J, Popik P (2015) Effect of NMDAR antagonists in the tetrabenazine test for antidepressants: comparison with the tail suspension test. Acta Neuropsychiatr 27:228-234.
Song TT, Bi YH, Gao YQ, Huang R, Hao K, Xu G, Tang JW, Ma ZQ, Kong FP, Coote JH, Chen XQ, Du JZ (2016) Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflammation 13:63.
West JB (2002) Highest permanent human habitation. High Alt Med Biol 3:401-407.
West JB (2010) English translation of “Nomenclature, classification, and diagnostic criteria of high altitude disease in China”. High Alt Med Biol 11:169-172.
White GE, Cotterill A, Addley MR, Soilleux EJ, Greaves DR (2011) Suppressor of cytokine signalling protein SOCS3 expression is increased at sites of acute and chronic inflammation. Journal of molecular histology 42:137-151.
Woolson HD, Thomson VS, Rutherford C, Yarwood SJ, Palmer TM (2009)Selective inhibition of cytokine-activated extracellular signal-regulated kinase by cyclic AMP via Epac1-dependent induction of suppressor of cytokine signalling-3. Cell Signal 21:1706-1715.
Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA,Romberger DJ (2010) Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol 42:706-715.
Yarwood SJ, Borland G, Sands WA, Palmer TM (2008) Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem 283:6843-6853.
Zanotto C, Abib RT, Batassini C, Tortorelli LS, Biasibetti R, Rodrigues L, Nardin P, Hansen F, Gottfried C, Leite MC, Goncalves CA (2013)Non-specific inhibitors of aquaporin-4 stimulate S100B secretion in acute hippocampal slices of rats. Brain Res 1491:14-22.
Zhao L, Li AQ, Zhou TF, Zhang MQ, Qin XM (2014) Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol 307:C1130-1141.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration

