Neuromodulator interactions and spinal cord injury in lamprey
Neuromodulation is mediated by neurotransmitters that typically act on G-protein-coupled receptors. It can confer behavioural flexibility by modifying the functional properties of anatomically hard-wired neural circuits.Single neuromodulators generally have divergent cellular and synaptic effects (Harris-Warrick and Johnson, 2010), and different modulators, of which there are many in even simpler systems, can interact by converging onto the same effectors. These interactions can generate synergistic, antagonistic, or novel effects (see Harris-Warrick and Johnson, 2010). Further modulator complexity is offered by the potential for concentration, time,and state-dependent influences (see Parker, 2015 and references therein).All of these effects can make neuromodulation highly flexible, making it difficult to predict or explain the functional effects resulting from the targeting of even a single modulatory system. As many clinically used drugs target G-protein-coupled receptors, being able to explain and predict these effects is important.
Serotonin (5-HT) is one of the best studied neuromodulators, and one that is also targeted clinically. Its effects have been examined in detail in the spinal cord, where it modulates various sensory and motor processes. 5-HT has also been studied in the context of spinal cord injury (SCI;Ghosh and Pearse, 2015). The major focus of research into SCI has been on promoting the regeneration of axons across lesion sites. While regeneration can be evoked, the functional improvements are modest (Steward et al., 2012). In addition to disconnecting the spinal cord, lesions also evoke functional changes above and below lesion sites (e.g., changes in cellular excitability, changes in excitatory and inhibitory synaptic inputs, and changes in sensory feedback) (ElBasiouny et al., 2010; Moraud et al., 2016;Parker, 2017). The relevance of these changes is often unclear. They could be “homeostatic” attempts to restore function by compensating for lesion-induced reductions in descending excitatory drive, or injury-induced epiphenomena that lack any direct functional relevance. Whatever their basis, they have to be considered as they mean that any intervention after SCI (e.g., regeneration) will occur in a functionally different spinal cord.
Locomotor, sensory, and autonomic networks persist below lesion sites.This offers a complementary approach to regeneration strategies after SCI.If these networks can be utilised they could help to improve locomotor and other functions. Motor outputs are facilitated by a locomotor central pattern generator that can generate basic locomotor activity in isolation(Stuart and Hultborn, 2008), although normal activity requires sensory and descending inputs (e.g., Akay et al., 2014). Given that sensory and motor networks in the spinal cord are subject to modulation by various transmitter systems, an obvious approach is to pharmacologically modulate the functional state of the spinal cord to improve locomotor and other outputs. This has been attempted experimentally and clinically for locomotion, primarily by targeting monoamine and glutamatergic systems (see Rossignol and Frigon, 2011). However, despite a vast literature on drug effects, there is still little insight into what would constitute an optimal pharmacological approach after SCI.
There is evidence that constitutive activation of 5-HT2receptors or 5-HT receptor agonists can improve locomotor function after SCI, greater effects being evoked by simultaneously targeting multiple 5-HT receptors or by combining 5-HT modulation with electrical stimulation of the spinal cord (Ghosh and Pearse, 2015). However, while various cellular effects of 5-HT are known in the unlesioned spinal cord, how these influence global aspects of normal locomotor function and improvements after SCI remains unclear. This could reflect the complexities of neuromodulator effects outlined above, including state-dependent changes in modulation caused by SCI-induced changes in cellular and synaptic properties; direct changes in modulatory systems; or because exogenously-evoked modulation fails to match the effects of endogenous release. Insight into all of these aspects would allow beneficial effects to be targeted to improve functional recovery. This insight requires defined networks that allow the divergent cellular and synaptic effects of modulators that alter system outputs to be identified (this is currently lacking for even the simplest spinal cord; Parker, 2010), a detailed analysis of the factors that influence modulatory effects (Harris-Warrick and Johnson, 2010), and how these aspects are altered after SCI.
The lamprey has been used since the 1970’s as a model system for studying the recovery of locomotor function after spinal cord lesions. This work has focused almost exclusively on characterising the spontaneous regeneration that occurs across the lesion site. However, the lamprey spinal cord has been used as a model system to examine spinal cord locomotor networks, sensory feedback, and neuromodulation. Thus, despite receiving very little attention, changes in the functional properties of locomotor and sensory networks above and below lesion sites and their modulation can also be examined in lamprey. We have started to perform these analyses(see Parker, 2017 for review). The focus on neuromodulation has shown differences in GABAergic and neuropeptide-mediated modulation of sensory inputs after SCI compared to the unlesioned spinal cord (reviewed in Parker, 2017). There are also differences in the effects of 5-HT on locomotor networks in unlesioned and lesioned animals that depend on the location examined relative to the lesion site and the degree of recovery.These include changes in the 5-HT-mediated modulation of synaptic transmission, changes in 5-HT effects on the resting membrane potential,and changes in the modulation of action potential properties (Parker,2017). Differences in modulatory effects also occur in mammals (see Rossignol and Frigon, 2011). These differences have obvious implications for pharmacological approaches to SCI, as they mean that drug effects cannot confidently be predicted from effects in the unlesioned spinal cord. There is thus a need to understand the changes in modulatory effects.
In McClelland and Parker (2017), we aimed to examine the mechanisms underlying the differences in 5-HT modulation after spinal cord lesions in lamprey using a simple experimental assay, monitoring ventral root activity in response to spinal cord stimulation in unlesioned animals and comparing this to the effects after lesioning. This approach was preferred to analyses using fictive locomotion as it reduces the number of variables that have to be considered, many of which remain unknown(see Parker, 2010), and avoids concerns over the physiological relevance of fictive activity (see Parker, 2017). In common with most modulators,5-HT has a range of cellular (excitability, resting potential) and synaptic effects (changes in inhibitory and excitatory inputs) in lamprey (see references in McClelland and Parker, 2017). These generally match effects in mammals, with 5-HT1A and 5-HT2receptors being implicated in the modulation of cellular and synaptic properties, respectively (see Parker,2017). The reduction of glutamatergic synaptic inputs and the reduction of a slow afterhyperpolarisation (sAHP) following an action potential are the best characterised effects of 5-HT in lamprey, and can occur in various cell types, including motor neurons. In mammals, 5-HT also reduces glutamatergic synaptic inputs, but activates persistent inward sodium and calcium conductances that increase motor neuron excitability (ElBasiouny et al.,2010). The latter effect is absent in lamprey, but the reduction of the sAHP and the associated spike frequency adaptation does increase the excitability of lamprey motor neurons.
In unlesioned animals, we expected that the significant reduction of excitatory synaptic inputs by 5-HT would reduce the ventral root response.However, 5-HT did not significantly affect ventral root responses (McClelland and Parker, 2017). While initially surprising, this could have reflected a parallel reduction of either the motor neuron sAHP or of inhibitory synaptic inputs to the motor neuron. We examined this experimentally and in a computer simulation. We found no evidence for the involvement of an effect on inhibitory synaptic transmission. However, blocking the sAHP resulted in a 5-HT-mediated reduction of the ventral root response. This suggested that the failure to affect ventral root responses under normal conditions reflected the parallel reduction of glutamatergic synaptic inputs and the reduction of the sAHP. These two effects have opposite influences on motor neuron excitability. The sAHP is functionally comparable to inhibition that will act in opposition to an excitatory synaptic input (this could in principle be a simple linear subtraction from the depolarisation caused by an excitatory synaptic input). A reduction of the sAHP could maintain motor neuron excitability at the same level despite the reduced excitatory synaptic input if there was an appropriate balance between the two effects. Computer simulations suggested that a relatively large range of values of the glutamatergic synaptic input and the sAHP can allow this(McClelland and Parker, 2017; seeFigure 1A, C).
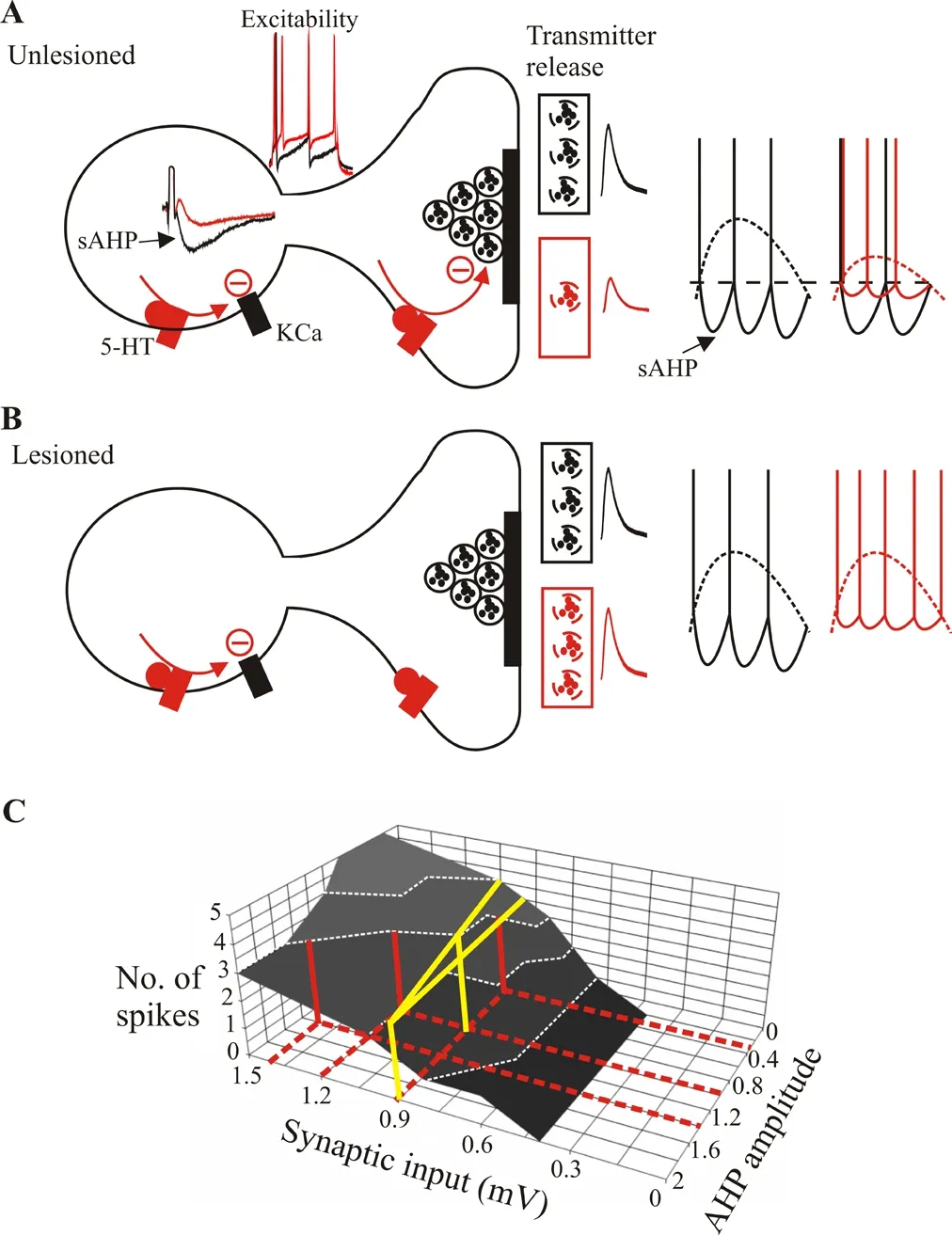
Figure 1 Summary of serotonin (5-HT) effects on lamprey motor neurons.
We also examined the effects of 5-HT on ventral root responses in animals after complete spinal cord lesions (McClelland and Parker, 2017).Here effects differed: in animals that failed to recover the effect was the same as in unlesioned animals, no effect of 5-HT on the ventral root response. However, in animals that recovered well 5-HT now increased the ventral root response. This could be explained by the failure of 5-HT to reduce glutamatergic synaptic transmission in lesioned animals, an effect that will allow the 5-HT-mediated reduction of the sAHP that occurs only in animals that recover well to dominate, and thus increase motor neuron excitability (see Parker, 2017;Figure 1B). This effect is functionally, but not mechanistically, comparable to the increased excitability caused by it could provide a mechanism to constrain single effects and prevent“over-modulation” by ensuring that the system does not go too far in one direction (Harris-Warrick and Johnson, 2010). Thus, the sAHP-mediated increase in excitability could ensure that a reduction of the synaptic input does not cause the motor output to fail. Parallel effects may also be designed to be energetically favourable. Maximization of information transmission for energy used is an important functional principle in nervous systems. In the cerebral cortex and cerebellum the highest percentage of ATP use is required for synaptic transmission (see Mcclelland and Parker,2017). A reduction of excitatory synaptic transmission with a reduction of the sAHP would offer an energetically favourable shift to cellular-driven motor neuron activation that allows the same motor output to be generated from an approximately 50% smaller postsynaptic input. This may be useful after spinal cord injury where energy supplies may be compromised
The changes in 5-HT effects after lesioning, and similar changes in mammalian systems (Rossignol and Frigon, 2011), may also influence pharmacological approaches after SCI. A rational pharmacological therapy requires knowing what manipulation is needed and how it can be achieved. To do this we need to know how the functional state of the spinal cord has changed after lesioning, the adjustments needed to restore locomotor or other functions, and how these can be evoked using pharmacological (or other) approaches. Appreciating that the spinal cord either side of the lesion site is altered and not just disconnected will facilitate this understanding. We also need to recognise the nuances of neuromodulation that make explaining the mechanisms underlying system or behavioural effects difficult, and that these can be altered by injury.This is less widely appreciated, but awareness of this aspect should help to improve the effectiveness of pharmacological approaches after spinal and other injuries.
David Parker*, Thomas J. McClelland
Department of Physiology, Neuroscience and Development, University of Cambridge, Cambridge, UK
orcid:0000-0002-5345-348X (David Parker)
This study thus adds to the evidence for the divergence and interaction of neuromodulator effects (see Harris-Warrick and Johnson, 2010). It also adds to the evidence for changes in neuromodulation after spinal cord lesions (Parker, 2017). Modulators typically affect multiple cellular and synaptic properties. In the example discussed here, this allows the same motor output to be generated from a varying combination of cellular and synaptic mechanisms. If this was a common effect it would make assumptions of the mechanisms underlying modulation difficult when just the system or behavioural output was monitored. Even though 5-HT did not affect the motor output using our experimental paradigm, it could of course still affect outputs under other conditions. It could also be argued that the approach we used does not reflect the normal effects of 5-HT. Endogenously released 5-HT could lead to local differences in 5-HT concentration or the location at which the 5-HT receptors are activated, which could separate the parallel cellular or synaptic effects necessarily evoked by exogenous application. However, parallel modulation of cellular and synaptic properties to maintain the same motor output may also occur by design. Firstly,
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-Share-Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Akay T, Tourtellotte WG, Arber S, Jessell TM (2014) Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci U S A 111:16877-16882.
ElBasiouny SM, Schuster JE, Heckman CJ (2010) Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121:1669-1679.
Ghosh M, Pearse DD (2015) The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circuits 8:151.
Harris-Warrick RM, Johnson BR (2010) Checks and balances in neuromodulation.Front Behav Neurosci 4: 47.
McClelland TJ, Parker D (2017) Inverse modulation of motor neuron cellular and synaptic properties can maintain the same motor output. Neuroscience 360:28-38.
Moraud EM, Capogrosso M, Formento E, Wenger N, DiGiovanna J, Courtine G,Micera S (2016) Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron 89:814-828.
Parker D (2010) Neuronal network analyses: premises, promises and uncertainties.Philos Trans R Soc Lond B Biol Sci 365:2315-2328.
Parker D (2015) Synaptic variability introduces state-dependent modulation of excitatory spinal cord synapses. Neural Plast 2015:512156.
Parker D (2017) The lesioned spinal cord is a “new” spinal cord: evidence from functional changes after spinal injury in lamprey. Front Neural Circuits 11:84.
Rossignol S, Frigon A (2011) Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34:413-440.
Steward O, Popovich PG, Dietrich WD, Kleitman N (2012) Replication and reproducibility in spinal cord injury research. Exp Neurol 233:597-605.
Stuart DG, Hultborn H (2008) Thomas Graham Brown (1882--1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Rev 59:74-95.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration

