Do large brains of long-living mammals prefer non-newly generated,immature neurons?
Brain plasticity is heterogeneous in mammals:Brain regeneration and repair are the dream of every neurobiologist as well as every common citizen in the world who knows that most neurological diseases, dementia and other age-related problems affecting the central nervous system(CNS) do represent a heavy health and social burden. Efficacious regenerative processes are not a natural property of the mammalian CNS,rather, due to evolutionary constraints they seem substantially reduced (if compared to those occurring in non-mammalian vertebrates) and hardly inducible by therapeutic approaches (reviewed in Martino et al., 2011).Paradoxically, different types of remarkable structural plastic processes have been shown to occur in the young and adult mammalian brain,spanning from widespread synaptic plasticity and gliogenesis to a more spatially-restricted, yet clearly demonstrable, genesis of new neurons(references in Martino et al., 2011). The failure in mammalian brain regeneration can be explained by a combination of several factors acquired through evolution (e.g., brain complexity, scarcity of stem cells, incapability of cell de-differentiation, role of immune system; see Bonfanti,2011 for review). Ultimately, all aspects involved converge into two main reasons: i) unlike non-mammalian vertebrates (e.g., fish or amphibians)in which neurogenic processes are far more extended and also provide repair, mammals have retained mostly the physiological role of plasticity,useful to cope with environmental changes but hardly helpful in regeneration; ii) adult mammalian neurogenesis appears to be highly reduced in humans with respect to rodents (Sanai et al., 2011; Paredes et al.,2015), recent work carried out in dolphins further confirming that it can be vestigial in large-brained, long-living mammals (Parolisi et al., 2017).Especially humans face further neurological risks in the future since they possess less potential for brain regeneration than other animal species in contrast with their extended (and progressively increasing) lifespan.
The “immature” neurons: non-newly generated, young cells in the adult brain:In parallel with research on brain stem cells and related neurogenic processes which have dominated the field during the last three decades, some studies identified cell populations in the adult brain which express markers usually found in newly generated neurons (Bonfanti et al., 1992; Bonfanti and Nacher, 2012), yet being born prenatally(Gómez-Climent et al., 2008). The best known example is a subpopulation of neurons located in the layer II of the piriform cortex, within the paleocortex, namely, the evolutionarily oldest part of the cerebral cortex(Figure 1A, B). These cells express markers of immaturity, such as doublecortin (DCX; a cytoskeletal protein involved in cell migration) and polysialic acid-neural cell adhesion molecule (PSA-NCAM) (a low-adhesive form of the neural cell adhesion molecule, involved in structural plasticity rather than in stabilization of cell-to-cell contacts). The cortical“immature” neurons display various morphologies, which were named differently by Authors who studied them across the years (see Bonfanti and Nacher, 2012). Most of them fall into two cell types as to their size and morphology: type 1 cells, small and mostly bipolar, and type 2 cells,larger and with longer, ramified dendrites (Figure 1A), the former being usually far more abundant than the latter (Piumatti et al., 2018).
The piriform cortex is a three-layered cortex interposed in the olfactory pathways, from the olfactory bulb to the hippocampus. Since neurons in these three regions do express markers of immaturity and are involved in striking processes of structural plasticity (the olfactory bulb and hippocampus being also sites of adult neurogenesis), the DCX+/PSA-NCAM+cells of the piriform cortex were considered potentially newly generated. Nevertheless, most studies carried out in the last decades substantially excluded such hypothesis (reviewed in Nacher and Bonfanti, 2015), confirming that these cells are born prenatally then remaining in a prolonged state of immaturity for extended periods, theoretically throughout life (Gómez-Climent et al., 2008). Analyses conducted in other (non-rodent) species described similar cells to reach into the neocortex (reviewed in Bonfanti and Nacher, 2012), thus suggesting that immature neurons might differ as to their abundance and location in larger mammals. On this basis, we recently investigated the occurrence, origin, and distribution of immature neurons in sheep, namely,animals with far larger brain and longer lifespan than rodents (Piumatti et al., 2018). In addition to canonical locations within neurogenic sites,neurons expressing DCX and PSA-NCAM were found to be present in the whole cerebral cortex (including neocortex) and in several subcortical regions (Figures 1and2), involving both white (external capsule)and grey matter (amygdala and claustrum). By using a combination of the local cell proliferation marker Ki-67 antigen and pulse labelling of 5-bromo-2′-deoxyuridine (BrdU, injected both during pregnancy and through adulthood), the study by Piumatti et al. (2018) shows that all these immature neuronal populations were born prenatally and were not newly generated after birth. Hence, immature neurons are far more widespread in sheep with respect to laboratory rodents (Figure 1C). In addition to their extension into layer II of the whole neocortex (a brain region processing higher cognitive functions), they are also present in neuroanatomical domains involved in the coupling of emotions and conscious perception: the amygdala and the claustrum. These facts, independently of the possible fate and functional role of immature neurons(still unknown, as discussed below), underline the idea that they could have higher importance in some animal species in a general picture of heterogeneous brain structural plasticity which includes a sharp reduction of adult neurogenesis in some large mammals.
Which fate and function for the immature neurons?In spite of stimulating hypotheses about the possible fate of the immature neurons throughout life of individuals, a definitive proof for their functional integration into the pre-existing neural circuits is at present lacking. The progressive decrease of their number with increasing age of the animal, as well as some studies reporting changes as a consequence of experimental manipulations (reviewed in Bonfanti and Nacher, 2012), do suggest that immature cortical neurons might represent a reservoir of “young” cells to be possibly recruited through time, without the need to be generatedex novo. In their original study, Gómez-Climent et al. (2008) showed that some immature neurons in the cortical layer II are ensheathed by astroglial lamellae which would prevent them from receiving synapses. This observation, to be further confirmed with thorough ultrastructural analyses, rises the intriguing question: how these “naked” neurons can survive in the assembling cortical tissue without establishing contacts with other neuronal cells? By using a combination of neuronal markers for maturity/immaturity (Piumatti et al., 2018) the DCX+“immature” neurons of the sheep brain were found to show an intermediate degree of immaturity with respect to the cells generated in the main neurogenic sites (subventricular zone and hippocampus). Among the DCX+cells, most also express PSA-NCAM, and type 2 cells start to express some molecules linked to neuronal differentiation(e.g., HuC/D, NeuN) which are hardly detectable in type 1 cells. These observations strongly support the hypothesis that a maturational process progressively occurs in immature neuron populations shifting from the small, highly immature cells (type 1), to the large, ramified type 2 cells(principal neurons), the latter being the candidates for further maturation and possible integration. In perspective, additional information is required concerning the pattern of expression of molecules determining the immature/mature state (or, better, gradient) of these neurons in the different brain regions and at different ages. The issue of the complete maturation and integration of immature neurons will be solved only by future experiments able to show their fate after they have lost the markers of immaturity as a consequence of their maturational process. Such goal can be reached either by using inducible transgenic animal models in which the DCX-expressing cells can be permanently labelled and/or by developing viral tracing techniques aimed at following single cells/cell populations through long periods of the animal’s life. The observation that the number of immature neurons undergoes progressive reduction in the cortex but not elsewhere (in sheep, their number in subcortical regions is changeless from neonatal to adult ages, as shown by Piumatti et al., 2018) does suggest that the final fate and functional role of immature neurons might be different depending on the brain region. Indeed, brain plasticity, in terms of structural remodeling of neuronal circuits, is a common feature across developmental ages and brain regions, the key difference between the developing and adult brain being only that of scale (Berry and Nedivi, 2016).For instance, the visual system retains a high degree of plasticity in terms of reorganization of the adult visual cortex in experimental conditions (e.g.,monocular deprivation or environmental enrichment; references in Berry and Nedivi, 2016). The existence of immature neurons could be among the factors explaining why cortical plasticity is still present during adulthood well beyond the critical periods of the developing/young brain.
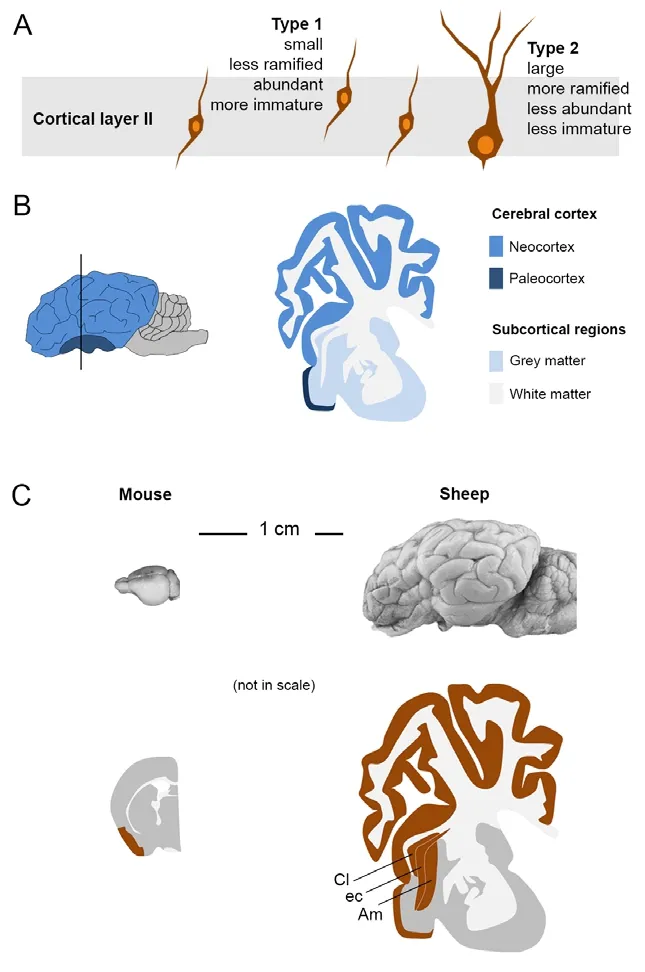
Figure 1 Occurrence of immature neurons in different mammals.
The results obtained in sheep strongly support the view that such kind of “non-neurogenic” plasticity might have been an evolutionary choice in large-brained, long-living mammals whose brains are less efficient to perform adult neurogenesis with respect to rodents. Adult neurogenesis is an expensive biological process, likely less suitable for large brains able to perform high cognitive functions in long-living mammals (Bonfanti,2011; Paredes et al., 2015; Parolisi et al., 2017). Yet, convincing results about the real rate of adult neurogenesis are available only in a few mammalian species, mostly depicting the extremes: high neurogenic capacity in laboratory rodents and strong reduction in humans and dolphins.Thus, we lack systematic quantitative analyses in mammals. In parallel, we need quantitative, really comparable data on the distribution of immature neurons in widely different mammalian species. In perspective, at least two types of analyses are required: i) systematic studies of the rate of adult neurogenesis in different mammals (current literature on this aspect is fragmentary and hardly comparable, still preventing the attainment of a“decrease slope” beyond the extremes); ii) comparative analyses on the occurrence and relative amount of immature neurons to be performed on a wide range of mammalian species and orders (including humans).
In the absence of deeper insights on the fate of immature neurons, we still do not know if such putative reservoir of young cells can have a role in regeneration and/or in the prevention of neurological disorders linked to aging. A role in regeneration is at present unlikely, since most scientific literature supports the incapability of these cells to be generatedex novo, at least in physiological conditions (Nacher and Bonfanti, 2015).Only a few studies addressed the behavior of immature neurons in the brain affected by lesions or neurodegenerative diseases. A recent report suggested that substantial structural plasticity can occur in DCX+neurons of the rat piriform cortex after induction of status epilepticus (Sakurai et al., 2017), thus opening the possibility that immature neurons can be responsive in pathological states. Furthermore, the increase in number of DCX+neurons in experimental conditions does suggest an even challenging idea: that mature cells of the adult brain might re-express juvenile molecules and re-acquire features of immaturity. Most likely,a reserve of “stand by” neurons not fully integrated into neural circuits might be an implement for large brains of long-living mammals to cope with structural/cognitive impairing linked to aging.
In conclusion, in the awareness of a substantial reduction of adult neurogenesis in humans, the non-newly generated immature neurons could be an alternative source of brain plasticity with unknown potential, on which it might be worthwhile to invest further efforts in future research.
This work was supported by MIUR-PRIN2015 (grant 2015Y5W9YP)and University of Turin (Ricerca locale 2016).
Ottavia Palazzo, Chiara La Rosa, Matteo Piumatti, Luca Bonfanti*Neuroscience Institute Cavalieri Ottolenghi (NICO), Orbassano, Italy(Palazzo O, La Rosa C, Piumatti M, Bonfanti L)Institute of Zoology-Neurogenetics, Universität Regensburg,Regensburg, Germany (Palazzo O)Department of Veterinary Sciences, University of Turin, Grugliasco(TO), Italy (La Rosa C, Bonfanti L)Université Libre de Bruxelles (ULB), Institute for Interdisciplinary Research in Human Biology (IRIBHM), Brussels, Belgium (Piumatti M)

Figure 2 Immature neurons in the sheep brain.
orcid:0000-0002-1469-8898 (Luca Bonfanti)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Jose A. Garcia-Sanz, Centro de Investigaciones Biologicas (CIB-CSIC),Spain.
Comments to authors:The manuscript is interesting, describes large differences in neuroregeneration potential between rodent and mammals with larger brains (including humans), describing the possible development of a relatively large number of immature cells that are of pre-natal origin and can remain undifferentiated during most of the individual’s life, playing possible regenerative roles. The concept is highly interesting and worth discussing.
Reviewer 2:Yousef Hannawi, Ohio State University College of Medicine, USA.
Berry KP, Nedivi E (2016) Experience-dependent structural plasticity in the visual system. Annu Rev Vis Sci 2:17-35.
Bonfanti L (2011) From hydra regeneration to human brain structural plasticity: a long trip through narrowing roads. Scienti fi cWorldJournal 11:1270-1299.
Bonfanti L, Olive S, Poulain DA, Theodosis DT (1992) Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience 49:419-436.
Bonfanti L, Nacher J (2012) New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: the case of cortical layer II immature neurons.Prog Neurobiol 98:1-15.
Gómez-Climent MA, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Martínez-Guijarro FJ, Nácher J (2008) A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex 18:2229-2240.
Martino G, Pluchino S, Bonfanti L, Schwartz M (2011) Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 91:1281-1304.
Nacher J, Bonfanti L (2015) New neurons from old beliefs in the adult piriform cortex? A Commentary on: “Occurrence of new neurons in the piriform cortex”. Front Neuroanat 9:62.
Paredes MF, Sorrells SF, Garcia-Verdugo JM, Alvarez-Buylla A (2016) Brain size and limits to adult neurogenesis. J Comp Neurol 524:646-664.
Parolisi R, Cozzi B, Bonfanti L (2017) Non-neurogenic SVZ-like niche in dolphins,mammals devoid of olfaction. Brain Struct Funct 222:2625-2639.
Piumatti M, Palazzo O, La Rosa C, Crociara P, Parolisi R, Luzzati F, Lévy F, Bonfanti L (2018) Non-newly generated, “immature” neurons in the sheep brain are not restricted to cerebral cortex. J Neurosci 38:826-842.
Sakurai M, Suzuki H, Tomita N, Sunden Y, Shimada A, Miyata H, Morita T (2017)Enhanced neurogenesis and possible synaptic reorganization in the piriform cortex of adult rat following kainic acid-induced status epilepticus. Neuropathology doi:10.1111/neup.12445.
Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS,Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A (2011) Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478:382-386.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration

