肠道微生态失衡与肝性脑病发病的研究进展
张 柳, 田德安
1.华中科技大学同济医学院,湖北 武汉 430000;2.华中科技大学同济医学院附属同济医院消化内科
肝性脑病(hepatic encephalopathy,HE)是由严重肝病引起的、以代谢紊乱为基础的中枢神经系统功能失调综合病征,表现为亚临床改变到昏迷的各种神经或精神异常,分为轻型肝性脑病(mild hepatic encephalopathy,MHE)和显性肝性脑病(overt hepatic encephalopathy,OHE)[1]。过去几十年有关HE的发病机理研究中主要有氨中毒学说、氨基酸失衡学说、假性神经递质学说、GABA-BZ复合体学说、毒物的协同作用学说。其中高血氨仍是HE发生的重要因素。最开始人们普遍认为,HE是由肝脏解毒功能下降和/或门静脉-体循环分流导致循环系统毒物入脑引起的脑功能障碍[2]。而近年来研究[3-4]发现,肠道微生物在导致HE发生的高血氨、炎症发生中扮演重要角色。肝硬化状态下,肠道黏膜屏障破坏、肠道菌群失调,导致肠道菌群易位、小肠细菌过度生长(small intestine bacterial overgrowth,SIBO)、代谢产物异常、肠道局部及全身免疫激活、炎症反应等,最终导致循环系统血氨及炎症因子水平升高,导致血脑屏障破坏、脑细胞水肿、代谢异常,引发HE[5]。因此,具有纠正肠道微生态失调功能的益生菌将有望成为治疗HE的新方法。
1 肠道正常微生物
正常人肠道内微生物数量达1010个,是人体细胞数量的10倍[6]。肠道微生物种类和数量受年龄、性别、种族,甚至分娩方式、怀孕年龄、抗生素应用、饮食、卫生条件的影响[7-8],由于其多样、多态且影响因素多、检测技术问题等,研究其对人类病理生理状态的影响十分复杂。近年随着新的微生物检测技术(焦磷酸测序技术)诞生和新的菌群研究为解决这一问题带来了突破。研究发现,微生物绝非随机组合,2011年ARUMUGAM等[9]发现人类肠道微生物大致分3种菌属,即拟杆属、普氏属、瘤胃球菌属,正常情况下这3种菌属在肠道中占优势,且处于稳态。一旦人体发育成熟,这样的肠道菌群组合不受年龄、种族、体质量指数、性别的影响,但机体消化代谢、功能状态会使微生物比例发生改变[10]。
2 微生态失衡与肝硬化、HE
菌群失调是指机体某部位正常菌群间各菌种比例发生较大幅度改变并超过正常幅度的状态,临床常用CDR(肠道有益菌与有害菌的比值)评估菌群失调的严重程度[11]。研究[12]发现,在肝硬化、HE患者的肠道中存在严重的菌群失调。门静脉高压引起的肠道淤血、水肿、缺氧,一方面影响肠道动力学,导致肠道自主清除能力下降,使过路菌接触、黏附黏膜的概率增加;另一方面破坏黏膜屏障,肠壁局部抵抗力下降,使得各种致病菌大量繁殖,导致SIBO,多种因素综合作用致使肠腔内微生态环境遭到严重破坏、菌群比例失调。研究[13]发现,CDR在不同生理病理状态下有显著性差异,HE组显著低于肝硬化组和正常对照组,且CDR与血清内毒素水平呈负相关。BAJAJ等[12-14]多项研究表明,MHE与正常对照组之间存在肠道菌群的差异,前者粪便标本拟杆菌、疣微菌科减少,肠杆菌、梭杆菌、韦荣球菌、链球菌、葡萄球菌、变形杆菌、梭状芽胞杆菌、普氏菌科增加,并且另有研究[15]显示,肠道内存在不同程度的SIBO,其发生率为35%~61%。进一步研究发现,细菌比例失衡与肝功能及认知受损无单纯的相关性,CHEN等[13]发现,Child-Turcotte-Pugh (CTP)评分与链球菌比例呈正相关,与毛螺旋菌呈负相关。终末期肝病模型(MELD)评分与肠杆菌科比例呈正相关,与疣微菌科比例呈负相关。此外,SIBO与HE严重程度相关,CTP评分越高的患者SIBO发生率越高[16-17]。由此说明肝硬化、HE患者肠道确实存在不同程度的菌群紊乱。目前调节肠道菌群的药物成功治疗HE更证实了该观点。MARLICZ等[18-19]采用益生菌、合生元、利福昔明治疗后发现,肠道菌群比例发生改变,肠杆菌科丰度减少,乳酸杆菌比例增加,同时伴随血氨水平的降低,MHE的逆转、OHE发生率显著降低。也有研究[20]表明,肝移植能通过调节肠道菌群改善大脑的认知功能。因此,我们猜测菌群失调与HE的发生之间可能存在关联性。
肠道菌群紊乱如何导致HE的发生、发展,涉及到肠道代谢产物的异常、黏膜屏障功能受损、SIBO的背景下引发高血氨症、炎症激活等多个环节。
3 肠道微生物、高血氨与HE
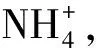
4 肠道微生物、炎症与HE
虽然高血氨症在HE发病机制中占有重要地位,但近年有研究[11,22,30-31]发现,由肠道菌群失调所致的炎症在HE中发挥了作用。在部分认知功能严重受损的HE患者中无明显高氨血症,但炎症因子水平显著升高,包括IL-10、IL-6、IL-2和TNF-α[30],且发现炎症能协同氨加重大脑认知功能受损[21,27,31-32]。研究发现,肝硬化时存在严重的菌群失调、SIBO,导致毒性物质(脂多糖、肽糖和微生物核酸[14])产生增多、排泄减少,大量积聚在肠腔,加之肝硬化时肠道黏膜屏障受到严重破坏,这些毒性物质能与肠道上皮细胞、肝细胞上多种模式识别受体(如TLR2)相互作用[33-34]。这样的相互作用通过MyD88-NF-κB-依赖通路激活下游的信号通路,促炎因子增加,同时下调TGF-β使抑炎反应减弱,引起炎症瀑布级联反应[18],最终导致HE的发生。炎症因子导致大脑认知功能受损的机制尚不明确,可能与炎症因子导致脑血流量改变、血脑屏障的通透性增加、脑细胞水肿、谷氨酰胺合成减少有关[5]。
动物模型研究证实,微生物失调是肝硬化发生大脑炎症的关键,研究发现,与GF(肠道无微生物)肝硬化组比较,在肠道菌群失调肝硬化小鼠中有明显的小胶质细胞、星形胶质细胞的激活,小脑IL-1b、MCP-1和皮层IL-1b的mRNA表达显著增加,而抗炎因子IL-10的水平下降[35]。多项临床研究[14,19]也发现了肠道微生物紊乱与炎症的发生存在关联性。肠杆菌科、韦荣球菌和梭杆菌科细菌、肠球菌、产碱杆菌、紫单胞菌比例增加和疣微菌科比例减少的患者血液炎症水平更高,且认知功能更差。由此可见,肠道菌群异常是导致HE炎症因子升高的关键。此外,炎症的存在确实能导致大脑认知功能的损伤,小鼠模型研究发现,腹腔注射脂多糖所导致的炎症反应会诱导肝硬化前驱昏迷的出现,进一步研究发现,肝硬化小鼠存在小胶质细胞的激活和原位促炎细胞因子TNF-α、IL-1β和IL-6的合成增加[21]。WRIGHT等[36]发现,促炎因子主要是通过导致脑细胞水肿从而诱发昏迷。临床研究[21,37]也发现,炎症的存在会加重HE认知功能的受损。LOREN AGUSTI等[38-39]的多项动物研究采用西地那非、布洛芬等抗炎治疗,发现其通过抑制小胶质细胞和星形胶质细胞的活化、降低炎症因子水平后能降低小鼠门静脉的压力、改善认知功能。同样BAJAJ等[11,17,40]临床试验发现,采用益生菌治疗能降低内毒素血症和TNF的水平从而改善HE认知功能。这些都提示肠道微生物所诱导的炎症在HE发病中发挥作用。
5 益生菌治疗HE
肠道微生物失衡在HE发病中占据重要地位,因此调节肠道菌群,从而增强黏膜屏障、减少细菌易位、SIBO、炎症等是治疗HE的新方案。近年益生菌在改善微生态失衡从而改善HE认知功能上备受关注。DHIMAN等[40]的多项临床研究表明,与安慰剂对照组相比,益生菌能改善肝功能和大脑认知功能,发现治疗后CTP评分、末期肝病评分和数字测试试验显著好转,在逆转MHE、减少OHE的发生、降低HE的住院风险上疗效显著[29,40-41]。SAAB等[41-44]比较了益生菌与目前治疗HE的推荐用药如利福昔明、乳果糖的疗效,结果发现其在降低血氨及炎症因子水平、改善大脑认知功能上疗效相仿,并且在增加有益菌群、减少致病性细菌和长期耐受性上更胜一筹[26,42],但益生菌使用并不能降低HE的死亡率[41]。益生菌在治疗HE上疗效确切,目前尚无报道严重不良反应,安全性得到认可。
HE严重威胁人类健康,它是由高血氨、炎症、代谢毒性物质等多种因素综合作用所导致的大脑认知功能损伤。近年发现肠道微生态失衡在其发病中扮演重要角色,肠道菌群失调、SIBO、肠黏膜受损等诱导高血氨、系统神经炎症,最终引发HE。而益生菌因其调节肠道菌群比例、改善肠道微生态,对HE有确切的治疗效果,且不良反应小,长期耐受性好,有望成为治疗HE的一线药物。但其剂量剂型、适应证等尚无统一推荐标准,未来需更多大规模、多中心临床试验研究其疗效及用法。此外多项研究发现,炎症在HE发病中发挥作用,但目前尚无直接抗炎制剂治疗HE的临床试验,未来可在抗炎制剂治疗HE上进一步研究,有望发现更多的HE治疗方案,使HE患者获益。
[1] VILSTRUP H, AMODIO P, BAJAJ J. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the american association for the study of liver diseases and the european association for the study of the liver [J]. Hepatology, 2014, 60(2): 715-735. DOI: 10.1002/hep.27210.
[2] BUTTERWORTH R F. Pathophysiology of hepatic encephalopathy: a new look at ammonia [J]. Metab Brain Dis, 2002, 17(4): 221-227.
[3] KARAKAN T. Gut microbiota modulation in cirrhosis: a new frontier in hepatology [J]. Turk J Gastroenterol, 2014, 25(1): 126. DOI: 10.5152/tjg.2014.0007.
[4] BETRAPALLY N S, GILLEVET P M, BAJAJ J S. Gut microbiome and liver disease [J]. Transl Res, 2017, 179: 49-59. DOU: 10.1016/j.trsl.2016.07.005.
[5] DHIMAN R K. Gut microbiota, inflammation and hepatic encephalopathy: a puzzle with a solution in sight [J]. J Clin Exp Hepatol, 2012, 2(3): 207-210. DOI: 10.1016/j.jceh.2012.08.004.
[6] SENDER R, FUCHS S, MILO R. Revised estimates for the number of human and bacteria cells in the body [J]. PLoS Biol, 2016, 14(8): e1002533. DOI: 10.1371/journal.pbio.1002533.
[7] MARQUES T M, WALL R, ROSS R P, et al. Programming infant gut microbiota: influence of dietary and environmental factors [J]. Curr Opin Biotechnol, 2010, 21(2): 149-156. DOI: 10.1016/j. copbio. 2010.03.020.
[8] FOUHY F, ROSS R P, FITZGERALD G F, et al. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps [J]. Gut Microbes, 2012, 3(3): 203-220. DOI: 10.4161/gmic.20169.
[9] ARUMUGAM M, RAES J, PELLETIER E. Enterotypes of the human gut microbiome [J]. Nature, 2011, 473(7346): 174-180. DOI: 10.1038/nature09944.
[10] GHOSHAL U C, SHUKLA R, GHOSHAL U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy [J]. Gut Liver, 2017, 11(2): 196-208. DOI: 10.5009/gnl16126.
[11] BAJAJ J S, HEUMAN D M, PHILLIP B. Altered profile of human gut microbiome is associated with cirrhosis and its complications [J]. J Hepatol, 2014, 60(5): 940-947. DOI: 10.1016/j.jhep.2013.12.019.
[12] BAJAJ J S, RIDLON J M, HYLEMON P B. Linkage of gut microbiome with cognition in hepatic encephalopathy [J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(1): G168-G175. DOI: 10.1152/ajpgi.00190.2011.
[13] CHEN Y, YANG F, LU H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis [J]. Hepatology, 2011, 54(2): 562-572. DOI: 10.1002/hep.24423.
[14] QIN N, YANG F, LI A, et al. Alterations of the human gut microbiome in liver cirrhosis [J]. Nature, 2014, 513(7516): 59-64. DOI: 10.1038/nature13568.
[15] GUPTA A, DHIMAN R K, KUMARI S. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy [J]. J Hepatol, 2010, 53(5): 849-855. DOI: 10.1016/j.jhep.2010.05.017.
[17] LUNIA M K, SHARMA B C, SACHDEVA S. Small intestinal bacterial overgrowth and delayed orocecal transit time in patients with cirrhosis and low-grade hepatic encephalopathy [J]. Hepatol Int, 2013, 7(1): 268-273. DOI: 10.1007/s12072-012-9360-9.
[18] MARLICZ W, WUNSCH E, MYDLOWSKA M, et al. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis[J].J Physiol Pharmacol, 2016, 67(6): 867-877.
[19] 王瑶芬.利福昔民预防肝性脑病复发的Meta分析[J].胃肠病学和肝病学杂志,2015,24(9):1133-1136. DOI:10.3969/j.issn. 1006-5709. 2015.09.027.
WANG Y F. The Meta-analysis of rifaximin prevent recurrence of hepatic encephalopathy [J]. Chin J Gastroenterol Hepatol, 2015, 24(9): 1133-1136. DOI: 10.3969/j.issn.1006-5709.2015.09.027.
[20] BAJAJ J S, FAGAN A, SIKAROODI M, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis [J]. Liver Transpl, 2017, 23(7): 907-914. DOI: 10.1002/lt.24754.
[21] ALDRIDGEA D R, TRANAHA E J, SHAWCROSS D L. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation [J]. J Clin Exp Hepatol, 2015, 5(Suppl 1): S7-S20. DOI: 10.1016/j.jceh.2014.06.004.
[22] RAHUL R, VIVEK A, RADHA K, et al. Gut microbiota: its role in hepatic encephalopathy [J]. J Clin Exp Hepatol, 2015, 5(Suppl 1): S29-S36. DOI: 10.1016/j.jceh. 2014.12.003.
[23] MARCHESE A, SALERNO A, PESCE A, et al. In vitro activity of rifaximin, metronidazole and vancomycin against Clostridium difficile and the rate of selection of spontaneously resistant mutants against representative anaerobic and aerobic bacteria, including ammonia-producing species [J].Chemotherapy, 2000, 46(4): 253-266. DOI: 10.1159/000007297.
[24] SHEN T C, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia [J]. J CLIN INVEST, 2015, 125(7): 2841-2850. DOI: 10.1172/JCI79214.
[25] BAJAJ J S, HEUMAN D M, HYLEMON P B, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis [J]. Aliment Pharmacol Ther, 2014, 39(10): 1113-1125. DOI: 10.1111/apt.12695.
[26] VIRAMONTES HÖRNER D, AVERY A, STOW R. The effects of probiotics and symbiotics on risk factors for hepatic encephalopathy: a systematic review [J]. J Clin Gastroenterol, 2017, 51(4): 312-323. DOI: 10.1097/MCG.0000000000000789.
[27] LUNIA M K, SHARMA B C, SHARMA P, et al. Probiotics prevent hepatic encephalopathy in patients with cirrhosis:a randomized controlled trial [J]. Clin Gastroenterol Hepatol, 2014, 12(6): 1003-1008.e1. DOI: 10.1016/j.cgh.2013.11.006.
[28] XU J, MA R, CHEN L F, et al. Effects of probiotic therapy on hepatic encephalopathy in patients with liver cirrhosis: an updated meta-analysis of six randomized controlled trials [J]. Hepatobiliary Pancreat Dis Int, 2014,13(4): 354-360. DOI.org/10.1016/S1499-3872(14)60280-0.
[29] ZHAO L N, YU T, LAN S Y, et al. Probiotics can improve the clinical outcomes of hepatic encephalopathy: an update meta-analysis [J]. Clin Res Hepatol Gastroenterol, 2015,39(6): 674-682. DOI: 10.1016/j.clinre.2015.03.008.
[30] SHAWCROSS D L, SHARIFI Y, CANAVAN J B. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis [J]. J Hepatol, 2011, 54(4): 640-649. DOI: 10.1016/j.jhep.2010.07.045.
[31] SHAWCROSS D L. Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy [J]. Expert Rev Gastroenterol Hepatol, 2015, 9(5): 539-542. DOI: 10.1586/17474124.2015.1035257.
[32] BOSOI C R, TREMBLAY M, ROSE C F. Induction of systemic oxidative stress leads to brain oedema in portacaval shunted rats [J].Liver Int, 2014, 34(9): 1322-1329. DOI: 10.1111/liv.12414.
[33] 张静雯,王玉平,周永宁.肠道菌群在非酒精性脂肪性肝病中的作用[J].胃肠病学和肝病学杂志, 2017, 26(4): 468-471. DOI:10.3969/j.issn.1006-5709.2017.04.027.
ZHANG J W, WANG Y P, ZHOU Y N. The role of intestinal microflora in nonalcoholic fatty liver disease [J]. Chin J Gastroenterol Hepatol, 2017, 26(4): 468-471. DOI: 10.3969/j.issn.1006-5709.2017.04.027.
[34] 陈小林, 任宏宇.肠道微生物群组与肠道免疫的关系[J].胃肠病学和肝病学杂志, 2014, 23(11): 1245-1248. DOI: 10.3969/j.issn.1006-5709.2014.11.001.
CHEN X L, REN H Y. The relationship between intestinal microorganism group and intestinal immunity [J]. Chin J Gastroenterol Hepatol, 2014, 23(11): 1245-1248. DOI: 10.3969/j.issn.1006-5709.2014.11.001.
[35] KANG D J, BETRAPALLY N S, GHOSH S A, et al. Gut microbiota drive the development of neuroin flammatory response in cirrhosis in mice [J]. Hepatology, 2016, 64(4): 1232-1248. DOI: 10.1002/hep.28696.
[36] WRIGHT G, DAVIES N A. SHAWCROSS D L. Endotoxemia produces coma and brain swelling in bile duct ligated rats [J]. Hepatology, 2007, 45(6): 1517-1526. DOI: 10.1002/hep. 21599.
[37] SEYAN A S, HUGHES R D, SHAWCROSS D L. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress [J]. World J Gastroenterol, 2010, 16(27): 3347-3357. DOI: 10.3748/wjg.v16.i27.3347.
[38] AGUSTI A. Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone, and improves motor in-coordination in rats with hepatic encephalopathy [J]. CNS Neurosci Ther, 2017, 23(5): 386-394. DOI: 10.1111/cns.12688.
[39] CAULI O, RODRIGO R, PIEDRAFITA B, et al. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with porto-caval shunts [J]. Hepatology, 2007, 46(2): 514-519. DOI: 10.1002/hep.21734
[40] DHIMAN R K, RANA B, AGRAWAL S, et al.Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial [J]. Gastroenterology, 2014, 147(6): 1327-1337, e3. DOI: 10.1053/j.gastro.2014.08.031.
[41] SAAB S, SURAWEERA D, AU J, et al. Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials [J]. Liver Int. 2016, 36(7): 986-993. DOI: 10.1111/liv.13005.
[42] SHAVAKHI A, HASHEMI H, EABESH E, et al. Multistrain probiotic and lactulose in the treatment of minimal hepatic encephalopathy [J]. J Res Med Sci, 2014,19(8):703-8
[43] SHARMA K, PATN S, MISRA S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial [J]. Saudi J Gastroenterol, 2014, 20(4): 225-232. DOI: 10.4103/1319-3767.136975.
[44] DING X, ZHANG G F, WANG Y. Letter: probiotics VS. Lactulose for minimal hepatic encephalopathy therapy [J]. Aliment pharmacol Ther, 2014, 39(9): 1000. DOI: 10.1111/apt.122661.

