A DFT study on TNGU isomers and aluminized cis-TNGU composites
Lemi Türker
Middle East Technical University,Department of Chemistry,Üniversiteler,Eskis¸ehir Yolu No:1,06800,Çankaya,Ankara,Turkey
1.Introduction
Among the various high energy density materials(HEDMs),heterocyclic nitrogen compounds have attracted significant attention,such as the well-known explosives 1,3,4,6-tetranitroglycouril(TNGU,Sorguyl,see Fig.1)[1],hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) [2,3], and 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane(HMX)[4,5]and the newer compounds trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin(TNAD)[6],2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(CL-20)[7]and cis-2,4,6,8-tetranitro-1H,5H-2,4,6,8-tetraazabicyclo[3.3.0]octane
(Bicycle-HMX)[8,9].They are all explosives with high positive HOFs and excellent detonation properties.
The inspection of properties of nitrourea compounds suggests that they would make excellent candidates as both insensitive and highly energetic materials,as well as they serve as precursors of other energetic compounds:(1)the urea moiety inherently has a high molecular density,suggesting that the mono and dinitrourea derivatives of it should also have attractive molecular densities.Indeed,this conjecture was supported by the work[10]at Picatinny Arsenal where glycoluril was first nitrated using 100%HNO3and P2O5at 50°C to yield TNGU,which has one of the highest densities of known organic materials(2.04 g/cm3).1,4-Dinitroglycoluril(DNGU),the analog of TNGU,also has an attractive crystal density of 1.992 g/cm3[11].
Although there are many types of HEDMs,each one has different advantages and disadvantages with respect to its stability and detonation properties.For example,TNGU(Fig.1),whose density and detonation properties are superior to many popular explosives,such as RDX and HMX.This molecule has four nitro groups for improved density and detonation properties[12].
The synthesis of TNGU has been achieved by many researches via different routes[13-17].An improved synthetic method was presented for TNGU via the in situ decomposition of a nitrimino group with elimination of nitrogen without the use of dinitrogen pentoxide[14].
Some studies about morphology of TNGU have been published[11,18,19].Sherrill et al.,described a new method for the preparation of TNGUinwhichimidazo-[4,5-d]-imidazoles are nitratedwith the elimination of N2O to generate TNGU.This method of TNGU synthesis yields a material which is less sensitive than material produced with some alternative routes.Additionally,a new spherical morphology of TNGU was described.This morphology exhibits an even higher resistance to external insult even than material synthesized with the new method[19].Its usage in preparation of some eco-friendly propellant compositions was published by Lim and Byun[19].The stability of TNGU was investigated by many researchers[20-22].Relations betweenproperties and electronic structure of cyclic nitroureas including TNGU was studied by Xi et al.,[21].
TNGU was also the subject of many calculations[23-26].A simple correlation for predicting detonation velocity of ideal and non-ideal explosives including TNGU was given by Keshavarz[24].A brief thermodynamic calculation of the thermo chemical properties of monopropellants,composite propellants,and metalized solid composite propellants considering TNGU and others(binder,Al or Al/Mg and AP)was given[24].
In the present study, firstly cis and trans isomers of TNGU have been investigated quantum chemically.Then,the aluminized cis-TNGU composites having different spin states are subjected to quantum chemical treatment.
2.Method of calculation
Geometry optimizations of all the structures leading to energy minima were initially achieved by using MM2 method followed by semi-empirical PM3 self-consistent fields molecular orbital(SCF MO)method[27,28]at the restricted level[29,30].Subsequent optimizations were achieved at Hartree-Fock level using various basis sets.Then,geometry optimizations were managed within the framework of density functional theory(DFT)using B3LYP functional[31,32]at the level of 6-31G(d,p)and cc-PVDZ for cis-and trans-TINGU.Note that cc-PVDZ is a correlation consisted and refers to the fact that the basis set is designed so that functions which contribute similar amounts of correlation energy are included at the same stage,independently of the function type[33]and the set includes polarization functions[34].Whereas for the aluminized cis-TINGU composites(cis-TNGU+nAl,n:1,2)B3LYP/6-31+G(d)(unrestricted)[29]level of calculations were adopted.The exchange term of B3LYP consists of hybrid Hartree-Fock and local spin density(LSD)exchange functions with Becke's gradient correlation to LSD exchange[32,35].Note that the correlation term of B3LYP consists of the Vosko,Wilk,Nusair(VWN3)local correlation functional[36]and Lee,Yang,Parr(LYP)correlation correction functional[37].The vibrational analyses were also done at the same level of calculations which had been performed for the optimizations.The total electronic energies(E)are corrected for the zero point vibrational energy(ZPE)to yield Ecvalues.The normal mode analysis for each structure yielded no imaginary frequencies for the 3N-6 vibrational degrees of freedom,where N is the number of atoms in the system.This indicates that the structure of each molecule corresponds to at least a local minimum on the potential energy surface.All these calculations were done by using the Spartan 06 package program[38].

Table 1Various energies of cis-and trans-TINGU.
3.Results and discussion

Table 2Some properties of cis-and trans-TINGU.

Table 3HOMO,LUMO energies,FMO energy gaps(Δε)and μ and η values of cis-and trans-TINGU.
TNGU having four nitramine groups and two carbonyls does not have much possibility for constitutional isomerization.However,the bridgehead hydrogens can have different orientations which lead to the cis-and trans-TNGU structures.
3.1.Cis-and trans-TNGU
Cis-and trans-TNGU differ from each other only with respect to the orientation of hydrogen atoms linked to bridgehead positions(see Fig.2).In the figure,optimized structures of these isomeric compounds,obtained by employing two different basis sets(6-31G(d,p)and cc-PVDZ)have been displayed.Fig.3 shows the IR and UV-VIS(Time dependent,TDDFT)spectra of the isomers.Depending on basis set variation,some orientation changes of the nitro groups in space occurs,thus some energy and bond lengths changes take place(see Table 1 and Figs.2 and 4).

Table 4Mulliken charges on nitro groups linked to Niand the respective nitramine bond lengths in cis-TNGU.

Table 5Mulliken charges on nitro groups linked to Niand the respective nitramine bond lengths in trans-TNGU.
In the calculated IR spectra(in vacuum,unstandardized),the peaks about 1910 cm-1stand for the carbonyl stretchings.The nitro stretchings occur in the range of 1764-1717 cm-1.The bendings of hydrogens happen at 1294 cm-1(cis-TNGU)and 1255 cm-1(trans-TNGU).Both of the isomers absorb in the same UV region,but the trans isomer has somewhat flattened spectrum.Due to the competing actions of nitro and lactam carbonyl groups to attract the lone-pair of nitrogen of the lactam moiety,a rather small chromophore group exists in the structure(limited conjugation).Therefore,the light absorption occurs only in the UV-region.
Table 1 tabulates the total electronic energy(E),zero point vibrational energy(ZPE)and the corrected total energy(Ec).The calculations(performed using two different basis sets)have revealed that in each case,cis-TNGU is more stable than its transisomer(at 298 K).The stability is probably due to existence of bettercharge-charge,charge-dipole and dipole-dipole interactions of the nitro groups or nitramine moieties present in the cis-TNGU.
Fig.4 exhibits bond length data which reveals that cis and trans isomers have rather comparable bond lengths.Table 2 displays some properties of TNGU isomers.Cis-isomer is characterized with a higher dipole moment than the trans.The B3LYP/cc-PVDZ level of calculations yield appreciably high dipole moment for the cis-isomer.
The heats of formation values(at the standard conditions)obtained by using T1 method of calculation[39,40](The T1 method is a little bit less accurate than the expensive G3(MP2)method)are 91.33 kJ/mol and 186.26 kJ/mol,respectively for the cis-and transforms.However,Smirnov et al.,reported the value ofΔHf°for TNGU(without mentioning its cisness or transness)as 75.3 kJ/kg(density:2.03 g/cm3)which corresponds to 24.25 kJ/mol[41].
According to the following formula[42],
Ispvalues of cis and trans TNGU are obtained as 69.01 s and 98.56 s,respectively for the cis-and trans-isomers.
Table 3 includes the HOMO,LUMO energies and the interfrontier molecular orbital energy gaps(FMO energy gap,Δε)of the TNGU isomers of present concern.At each level of calculations the HOMO energy of cis-TNGU is higher than the trans-isomer.Whereas,the LUMO energy of cis-isomer is lower than the trans at each level of calculations.Consequently,Δε values for cis-TNGU is less than the trans-TNGU.It is known that the impact sensitivity of explosives increases as the HOMO-LUMO energy gap decreases[43].Hence,cis-TNGU is expected to be more sensitive to impact than its trans-isomer should be.Table 3 also includesμ(electronegativity)andη(hardness)values of these isomers which are de fined as[44-46],
According to the both level of calculations,trans-TINGU is more electronegative and harder than its cis isomer.
Zhang et al.,proposed a method of correlation to predict the impact sensitivities of nitro compounds based on nitro group charges(Mulliken)[47].The more negative charge the nitro group possesses it is less likely to split from the backbone(R-NO2).In systems conjugated with the nitro group,some electron population can betransferred to NO2moietyviamesomerism andconsequently the bond order between the NO2and its neighbor atom in the backbone increases.Meanwhile the bond length is expected to shorten.However,that relationship is not so explicitly occurring all the time.Fig.5 shows the numbering of atoms in cis and trans TNGU.Tables 4 and 5 show the Mulliken charges on NO2atoms and the respective N-NO2bond lengths in cis-and trans-TNGU,respectively.

Table 6Various energies of aluminized cis-TINGU.
According to the data in Table 4,the nitro group linked to N5 atom is more likely to be split off from cis-TNGU structure(both level of calculations predict the same).Note that the corresponding nitramine bonds is the longest among all.As for the trans-TNGU case,the nitro groups linked to N1 and N5 atoms are more likely candidates to split off than the others.The corresponding bond lengths are comparatively longer too.
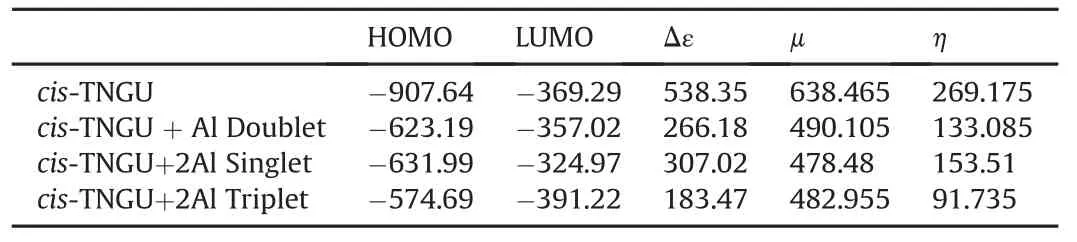
Table 7HOMO,LUMO energies,FMO energy gaps(Δε)andμ and ηvalues of aluminized cis-TINGU composites.
Relying on higher stability of the cis-isomer,the aluminized composites of it has been further focus of investigation presently.
3.2.Aluminized cis-TNGU
Being aluminum powder a combustible high energy material,it is widely employed as a component of explosive and propellant formulations to increase the explosive/propellant performance.
In the present study,cis-TNGU,which is the more stable isomer compared to the trans form,has been considered to be investigated for the interaction with aluminum.The composite systems having one and two atoms of aluminum per molecule of cis-TNGU have been subjected to density functional treatment at the level of B3LYP/6-31+G(d).Hence,cis-TNGU+Al and cis-TNGU+2Al type composite systems are investigated.Note that aluminum has 1s22s22p63s23p1electronic con figuration.Thus,cis-TNGU+Al system is a doublet,having an unpaired electron.Whereas,cis-TNGU+2Al composite might have a singlet state(a closed shell system)or a triplet state having two unpaired electrons with parallel spins(open shell system).Fig.6 shows the optimized structures of the aluminized cis-TNGU composites of the present concern.Fig.7 exhibits the bond lengths/distances of those systems.The doublet system(cis-TNGU+Al)has very reasonable bond lengths.Its aluminum content corresponds to 7.73%Al by weight.On the other hand,the composite system(cis-TNGU+2Al)having 14.35%Al are structurally unstable that is cis-TNGU moiety undergoes some bond cleavages(see Fig.6).
Interestingly enough,the singlet system with two aluminum atoms undergoes bond rupture of the ring(see Figs.6 and 7).There C-N distances are 3.17 Å and 3.33 Å.Whereas the triplet system having two aluminum atoms emanates an NO2moiety.Hence,cis-TNGU seems to be incompatible with the presence of a second Al atom.Fig.8 shows the electrostatic charges(ESP)on the atoms of the aluminized cis-TNGU composites.Note that ESP charges are obtained by the program based on a numerical method that generates charges that reproduce the electrostatic field from the entire wavefunction[38].In the triplet system,the overall charge of the dispelled NO2moiety is-0.566 esu.The result indicates that Al atom supplies some electron population to cis-TNGU which causes the elimination of the NO2group(quasi nitrite ion).Since the calculations reveal that some electronpopulation has been transferred to the organic component from Al atom(s),the anions of cis-TNGU are considered for comparison purpose to have a better understanding of the process.Fig.7 also contains the bond lengths of mono and dianions of cis-TNGU.Note that they are not aluminized.The aluminized triplet(TNGU+2Al)system highly resembles the mono anion of cis-TNGU(doublet)both of which expel single NO2moiety but no resemblance exists to dianions of cis-TNGU.So,the aluminum atom should have transferred some electron population,nearly a single electron,to cis-TNGU molecule.In the case of cis-TNGU+2Al(singlet)composite system,the structure is completely different from the structures of anions of cis-TNGU.Hence,it implies that a more complex electron transfer process should have occurred from aluminum atom to the organic system resulting in a ring opening reaction.In organic chemistry there exist many examples of metal reduction processes and also many examples of nitro compounds acting as oxidizers(eg.,Skraup synthesis)[48-50].All these data reveal that cis-TNGU can be incorporated with approximately 7-8%Al safely,but 14%Al content causes bond cleavages in the singlet or triplet states.Of course,in between those limitssomemuch betterweightlimitscan beobtained experimentally.
Fig.9 shows the spin densities for the open shell composites cis-TNGU+Al(doublet)and cis-TNGU+2Al(triplet).The aluminum atom in the doublet and one of the aluminum atoms in the triplet composites are characterized with very low spin population,which is an other evidence that those aluminum atoms have transferred some electron population to the organic moiety.
Table 6 shows various energiesof the aluminized cis-TNGU.They are all stable in terms of overall energy.The corrected total electronic energy of the triplet system(cis-TNGU+2Al)stands for a much more stable composite than the singlet(cis-TNGU+2Al).Note that both the triplet and the singlet composites are structurally decomposed.
Fig.10 displays the calculated(in vacuum)IR spectra of the cis-TNGU and its aluminized composites.In the figure the peaks about 1887-1896 cm-1stand for the carbonyl stretchings.In the case of cis-TNGU+Al composite,the carbonyl nearby the Al atom vibrates at 1756 cm-1.The carbonyl of the broken ring in the case of singlet cis-TNGU+2Al composite occurs at 2175 cm-1.Whereas,in the triplet case the carbonyl stretch is at 1740 cm-1.The nitro stretchings are lowered by the effect of presence of Al atom(s).
Table 7 shows the HOMO,LUMO energies and the interfrontier molecular orbital energy gaps as well as theμandηvalues for cis-TNGU and its aluminized forms.The HOMO energy order is cis-TNGU<cis-TNGU+2Al(singlet)<cis-TNGU+Al(doublet)<cis-TNGU+2Al(triplet).Whereas the LUMO energy order is TNGU+2Al(triplet)<cis-TNGU<cis-TNGU+Al(doublet)<cis-TNGU+2Al(singlet).Consequently,the Δε values have the sequence of TNGU+2Al(triplet)<cis-TNGU+Al(doublet)<cis-TNGU+2Al(singlet)<cis-TNGU.The sequence of HOMO energies indicates that the presence of Al atom raises up the HOMO energy level as compared to cis-TNGU.This is also true for the LUMO levels with the exception of triplet cis-TNGU+2Al case.Such kind of situation arises whenever electron donating effect exists[51].Although,some composite systems considered presently are decomposed,cis-TNGU+Al(doublet)is an intact system.Therefore,arose of its frontier molecular orbital energy levels compared to cis-TNGU can be mainlyattributed toelectron donation fromthe aluminum atom.Note that cis-TNGU+Al(doublet)is a stable system which is less electronegative and softer as compared to cis-TNGU at the same level of calculation.

Table 8Mulliken charges on nitro groups linked to Ni and the respective nitramine bond lengths in cis-TNGU+Al composite.
Fig.11 shows the time-dependent UV-VIS spectra of the systems of present concern.One observes that the presence of Al atom(s)causes different extents of bathochromic shift in the spectra of these composites.This effect is more pronounced in the triplet cis-TNGU+2Al composite.The conformational changes of nitramine groups in the doublet case and both the structural and conformational changes in the cases of singlet and triplet should have created chromophores responsible for the observed bathochromic shifts in the calculated spectra.
Fig.12 shows the electrostatic potential maps of cis-TNGU and its aluminized forms(B3LYP/6-31+G(d)).In the figure,the electron de ficient regions(blue)occur at the central region of the rings.
Of the presently concerned composite structures,cis-TNGU+Al is the only stable one.Fig.13 shows the numbering of cis-TNGU+Al composite system and Table 8 lists the Mulliken charges on nitro groups as well as the respective nitramine bond lengths in cis-TNGU+Al composite.
Inspection of the data inTable 8 reveals that NO2group linked to N1 atom has the least negative charge and the longest nitramine bond length hence it is more susceptible to cleavage.
4.Conclusion
The present DFT treatment,within the limitation of the method,has revealed that cis-TNGU is more stable than trans-TNGU.As for the aluminized cis-TNGU composites(cis-TNGU+Al)doublet has been found to be structurally stable.Whereas,the singlet and triplet of cis-TNGU+2Al systems are unstable.The former one undergoes a ring cleavage whereas the triplet system expels a NO2moiety.Structurally comparing with cis-TNGU mono and dianions and referring to some quantum chemical analyses one concludes that the bond cleavages occur with transfer of some electron population from the aluminum atoms in the TNGU+2Al composites.The present study put some light not only from the quantum chemical aspects at the molecular level but also to the stability of the aluminized cis-TNGU composites.
[1]Pagoria PF,Lee GS,Mitchell AR,Schmidt RD.A review of energetic materials synthesis.Thermochim Acta 2002;384:187-204.
[2]Hudson RJ,Zioupos P,Gill PP.Investigating the mechanical properties of RDX crystals using nano-indentation.Propellants Explos Pyrotech 2012;37:191-7.
[3]Hunter S,Sutinen T,Parker SF,Morrison CA,Williamson DM,Thompson S,et al.Experimental and DFT-D studies of the molecular organic energetic material RDX.J Phys Chem C 2013;117:8062-71.
[4]Zhi HZ,Luo J,Feng GA,Lu CX.An efficient method to synthesize HMX by nitrolysis of DPT with N2O5and a novel ionic liquid.Chin Chem Lett 2009;20:379-82.
[5]Landenberger HB,Matzger AJ.Cocrystals of 1,3,5,7-Tetranitro-1,3,5,7-tetrazacyclooctane(HMX).Cryst Growth Des 2012;12:3603-9.
[6]Yan QL,Li XJ,Chen ZQ,Ren XN,Nie LH.Thermal behavior and thermolysis kinetics ofthe explosive trans-1,4,5,8-Tetranitro-1,4,5,8-Tetraazadecalin(TNAD).Propellants Explos Pyrotech 2009;34:357-62.
[7]Bayat Y,Zeynali V.Preparation and characterization of nano-CL-20 explosive.J Energ Mater 2011;29:281-91.
[8]Qiu L,Xiao HM.Molecular dynamics study of binding energies,mechanical properties,and detonation performancesofbicyclo-HMX-based PBXs.J Hazard Mater 2009;164:329-36.
[9]Koppes WM,Chaykovsky MH,Adolph G,Gilardi R,George C.Synthesis and structure of some peri-substituted 2,4,6,8-tetraazabicyclo[3.3.0]octanes.J Org Chem 1987;52:1113-9.
[10]Federoff BT,et al.Encyclopedia of explosives and related items,vol.1.Dover,NJ:Picatinny Arsenal;1960A65.
[11]Pagoria PF,Mitchell AR,Jessop ES.Nitroureas 11.Synthesis of bicyclic monoand dinitrourea compounds.Propellants Explos Pyrotech 1996;21:14-8.
[12]Jin X,Hu B,Jia H,Liu Z,Lu C.DFT theoretical study of energetic nitrogen-rich C4N6H8-n(NO2)n Derıvatives.Quim Nova 2014;37(1):74-80.
[13]Ha H.Method for synthesis of tetranitroamine tetrasodium salt.Faming Zhuanli Shenqing.2016.CN 105777575 A 20160720.
[14]Sherrill WM,Johnson EC.Novel preparation of tetranitroglycoluril.U.S.Pat.Appl.Publ.;2016.US 20160176878 A1 20160623.
[15]Sherrill WM,Johnson EC,Banning JE.A method for the synthesis of tetranitroglycoluril from imidazo-[4,5-d]-imidazoles with loss of dinitrogen oxide.Propellants Explos Pyrotech 2014;39(5):670-6.
[16]Yi W,An Q,Cai C.Method for preparing 1,3,4,6-tetranitroglycoluril.Faming Zhuanli Shenqing.2013.CN 103242319 A 20130814.
[17]Zhang S,Zhu C,Wang H.Method for synthesizing tetranitroglycoluril.Faming Zhuanli Shenqing.2013.CN 103204854 A 20130717.
[18]Sherrill WM,Banning JE.Process for the production of spherical tetranitroglycouril.U.S.Pat.Appl.Publ;2016.US 20160090388 A1 20160331.
[19]Lim JH,Byun GM.Eco-friendly propellant composition with excellent reliability,and method for manufacturing propellant.Repub Korean Kongkae Taeho Kongbo.2012.KR 2012137643 A 20121224.
[20]Dong S,Zhang G.Hydrolysis of tetranitrohemiglycoluril and stability of the product.Huozhayao Xuebao 1997;19(2):60-1.
[21]Xi Y,Cai Z,Wang N,Xiao H,Tang Z,Yu M.Relations between properties and electronic structure of cyclic nitroureas.IV.Thermal decomposition.Fenxi Huaxue 1991;19(12):1387-91.
[22]Oyumi Y,Brill TB.Thermal decomposition of energetic materials.XXVIII.Predictions and results for nitramines of bis-imidazolidinedione:DINGU,TNGU and TDCD.Propellants Explos Pyrotech 1988;13(3):69-73.
[23]Keshavarz MH.Simple correlation for predicting detonation velocity of ideal and non-ideal explosives.J Hazard Mater 2009;166(2-3):762-9.
[24]Bogdan F,Lipinska K.Thermochemical properties of composite propellants combustion products.In:Zeman,editor.Svatopluk,new trends in research of energetic materials proceedings of the seminar;2001.p.86-98.4th,Pardubice,Czech Republic,Apr.11-12.
[25]Kozyrev NV.Reparametrization of the BKW equation of state for CHNO explosives which release no condensed carbon upon detonation.Central Eur J Energetic Mater 2015;12(4):651-69.
[26]Jin XH,Hu BC,Jia HQ,Liu ZL,Lu CX.DFT studies on two novel explosives based on the guanidine-fused bicyclic structure.Bull Korean Chem Soc 2014;35(4):1043.http://dx.doi.org/10.5012/bkcs.2014.35.4.1043.
[27]Stewart JJP.Optimization of parameters for semiempirical methods I.Method J Comput Chem 1989;10:209-20.
[28]Stewart JJP.Optimization of parameters for semi empirical methods II.Application.J Comput Chem 1989;10:221-64.
[29]Leach AR.Molecular modeling.Essex:Longman;1997.
[30]Fletcher P.Practical methods of optimization.New York:Wiley;1990.
[31]Kohn W,Sham L.Self-consistent equations including exchange and correlation effects.J Phys Rev 1965;140:1133-8.
[32]Parr RG,Yang W.Density functional theory of atoms and molecules.London:Oxford University Press;1989.
[33]Jensen F.Introduction to computational chemistry.Chichester:Wiley;1999.
[34]Young DC.Computational chemistry.New York:Wilet-Interscience;2001.
[35]Becke AD.Density-functional exchange-energy approximation with correct asymptotic behavior.Phys Rev A 1988;38:3098-100.
[36]Vosko SH,Vilk L,Nusair M.Accurate spin-dependent electron liquid correlation energies for local spin density calculations:a critical analysis.Can J Phys 1980;58:1200-11.
[37]Lee C,Yang W,Parr RG.Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density.Phys Rev B 1988;37:785-9.
[38]SPARTAN 06.Irvine CA,USA:Wavefunction Inc;2006.
[39]Ohlinger WS,Klunzinger PE,Deppmeier BJ,Hehre WJ.Ef ficient calculation of heats of formation.ACS Publications J Phys Chem A 2009;113:2165-75.
[40]Curtiss L,Raghavachari K,Redfern PC,Rassolov V,Pople JA.Gaussian-3(G3)theory for molecules containing first and second-row atoms.J Chem Phys 1998;109:7764-76.
[41]Smirnov A,Lempert D,Pivina T,Khakimov D.Basic characteristics for estimation polynitrogen compounds effciency.Central Eur J Energetic Mater(CEJEM)2011;8(4):233-47.
[42]Wilson KJ,Perera SA,Bartlett RJ,Watts JD.Stabilization of pseudo-benzene N6ring with oxygen.J Phys Chem A 2001;105:7693-9.
[43]Ovens EJ.Relationship between impact induced reactivity of trinitroaromatic molecules and their molecular structure.J Mol Struct(Theochem)1984;121:213-20.
[44]Pearson RG.Absolute electronegativity and hardness:applications to organic Chemistry.J Org Chem 1989;54:1423-30.
[45]Pearson RG.Chemical hardness.Weinheim:Wiley-VCH;1997.
[46]Zhou Z,Parr RG.Activation hardness:new ındex for describing the orientation of electrophilic aromatic substitution.J Am Chem Soc 1990;112:5720-4.
[47]Zhang C,Shu Y,Huang Y,Zhao X,Dong H.Investigation of correlation between ımpact sensitivities and nitro group charges in nitro compounds.J Phys Chem B 2005;109:8978-82.
[48]March J.Advanced organic chemistry,reactions,mechanisms and structure.NY:McGraw-Hill;1968.
[49]Fuson RC.Reactions of organic compounds.NY:Wiley;1962.
[50]Ono N.The nitro group in organic synthesis.NY:Wiley;2001.
[51]Fleming I.Frontier orbitals and organic chemical reactions.London:Wiley;1976.
- Defence Technology的其它文章
- Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction
- Process parameters-weld bead geometry interactions and their in fluence on mechanical properties:A case of dissimilar aluminium alloy electron beam welds
- Crystal lattice free volume in a study of initiation reactivity of nitramines:Friction sensitivity
- The kinetic of mass loss of grades A and B of melted TNT by isothermal and non-isothermal gravimetric methods
- Numerical simulation and optimized design of cased telescoped ammunition interior ballistic
- The effects of compressibility and strength on penetration of long rod and jet

