Effect of different intensities of physical activity on cardiometabolic markers and vascular and cardiac function in adult rats fed with a high-fat high-carbohydrate diet
Romo B.Bataan Jr*,Mith J.Dunan,Vinnt J.Dalbo,Graldin L.Buitrago,Andrw S.Fnning
aSchool of Medical and Applied Sciences,Central Queensland University,Rockhampton,QLD 4702,Australia
bCentre for Physical Activity Studies,Central Queensland University,Rockhampton,QLD 4702,Australia
cSchool of Medicine&Public Health,Priority Research Centre for Physical Activity and Nutrition,Faculty of Health and Medicine,The University of Newcastle,University Drive,Callaghan,NSW 2308,Australia
dClinical Biochemistry Laboratory,Central Queensland University,Rockhampton,QLD 4702,Australia
eCentre for Biodiscovery and Molecular Development of Therapeutics,Australian Institute of Tropical Health and Medicine,James Cook University,Cairns,QLD 4870,Australia
1.Introduction
Globally,an increasing proportion of people are consuming a diet characterized by a high intake of fat and sugar, also known as the Western diet.1Diets such as these increase the risk of developing the cardiometabolic syndrome1-3by inducing sustained increases in triglycerides,very low-density lipoprotein,and plasma glucose.4Cardiometabolic syndrome is the clustering of the following risk factors:central obesity,hypertension,insulin resistance, dyslipidemia, microalbuminuria, and hypercoagulability.5,6Collectively,these risk factors also increase the risk of developing cardiovascular disease and diabetes.5
The key risk factors for the development of cardiometabolic syndrome are low physical activity(PA)and a poor-quality diet predominantly high in saturated fat,protein,and simple sugars.7-9The intensity of PA occurs on a continuum from sedentary to light,moderate,and vigorous.Substantial research has documented the positive effects of moderate-to-vigorous PA(MVPA)on traditional cardiometabolic risk factors such as obesity,dyslipidemia,diabetes mellitus,and hypertension.10,11Recently,owing to evidence implicating inflammation and oxidative stress in the pathogenesis of cardiometabolic disease,12several studies have evaluated the effects of MVPA on novel risk factors and found decreases in plasma biomarkers of endothelial dysfunction(P-selectin)and inflammation(tumor necrosis factor α)following MVPA.13-16
Currently,there is growing interest in how time spent in activities at the lower end of the PA intensity continuum affect health relative to MVPA,because a greater proportion of the waking day is spent in these activities.17There is mixed evidence regarding the impact of sedentary behavior18and lightintensity activity19on some cardiovascular disease(CVD)risk factors(i.e.,obesity,hypertension,and dyslipidemia).However,despite the knowledge that greater total activity is better for health,there has been a trend toward overall declines in total activity20,21and increased sedentary behavior.17,22To this end,efforts to optimize health by increasing PA have led to numerous studies focused on identifying alternative approaches to MVPA.23,24Light-intensity training(LIT)25,26and high-intensity interval training(HIIT)23have been suggested as an alternate means of promoting PA to improve health.LIT refers to PA prescriptions that call for an expenditure of less than 40%of maximal oxygen uptake;it has been suggested that this is a more attainable target compared with continuous MVPA.10In contrast,HIIT involves alternating short bursts of highintensity exercise with less-intense recovery periods requiring less time to perform than continuous MVPA.23Recent published data have demonstrated that both LIT26-29and HIIT30-32induce positive adaptations that may reduce cardiometabolic risks.Specifically,LIT was found to improve blood pressure in physically inactive populations with a medical condition,19whereas HIIT was found to improve glucose control33in healthy individuals and to improve insulin sensitivity and lipoproteins34in individuals with metabolic syndrome.In rats fed standard chow,LIT and HIIT were found to improve body weight,fat accumulation,glucose control,high-density lipoprotein(HDL)and total cholesterol(TC)levels,and mesenteric vessel contractile response.35
Several studies have examined the impact of low-calorie low-fat diet and PA on health and found a significant reduction in the risk of developing CVD and diabetes compared with a placebo oran educational support group.36-39However,although healthy diet and increased PA improve health,40many people in developed countries and increasingly in developing countries are becoming sedentary and are consuming significant elements of the Western diet.41,42Thus,it is valuable to understand how sedentary behavior and alternative activity modes such as LIT and HIIT affect cardiometabolic risk in populations consuming the Western diet.Therefore,this study aims to determine the effects of sedentary behavior,LIT,and HIIT on metabolic markers and cardiac and vascular function in male adult rats fed with a high-fat high-carbohydrate(HFHC)diet.
2.Methods
2.1.Study design and animals
All research procedures were granted prior approval by Central Queensland University(CQU)Animal Ethics Research Committee(A13/08-303)in accordance with the guidelines of the National Health and Medical Research Council of Australia.Male Wistar rats(n=48)were bred and housed at CQU in an environmentally controlled room(temperature 22°C ± 1°C,relative humidity 50%±2%)with a 12 h light-dark cycle.Male rats were used to avoid variability caused by hormonal cycles in females as well as because of their more similar homeostatic adaptation(negative energy balance)to exercise in humans compared with female rats.43,44Water and standard rat chow(Riverina Stockfeeds,South Brisbane,Australia)were providedad libitumfor 12 weeks.After 12 weeks of aging,rats(437.8±3.9 g)were randomly divided into 4 groups,each comprising 12 animals:control(CTL),sedentary(SED),LIT,and HIIT.Rats were subjected to 12 weeks of PA intervention and HFHC diet feeding in all groups.All rats at 24 weeks of age were euthanized at the end of the intervention period.
2.2.PA protocol
During the 12-week intervention period,animals were subjected to each of the following PA protocols.The CTL group was housed 3 or 4 rats per standard cage with 2400 cm2floor area(400 cm2/400 g rat)and was not subjected to any exercise training beyond normal cage activity.The SED group was not subjected to any exercise training and was housed 3 rats per small cage with 1080 cm2floor area(240 cm2/400 g rat)to initiate a significant reduction in PA.45The LIT group was housed 3 or 4 rats per standard cage with 2400 cm2floor area(400 cm2/400 g rat)and ran at a treadmill speed of 8 m/min,0%incline,for 125 min/day divided into 4 bouts(30-30-30-35 min)separated by a 2 h rest period,5 days/week for 12 weeks.46The HIIT group was housed 3 or 4 rats per standard cage with 2400 cm2floor area(400 cm2/400 g rat)and was trained starting at a treadmill speed of 10 m/min,0% incline,for 10-15 min/day,progressively increased at a speed of 50 m/min,10%incline,divided into four 2.5 min work bouts separated by a 3 min rest period,5 days/week for 12 weeks(training protocol followed as described by Matsunaga et al.47).
2.3.Diet protocol
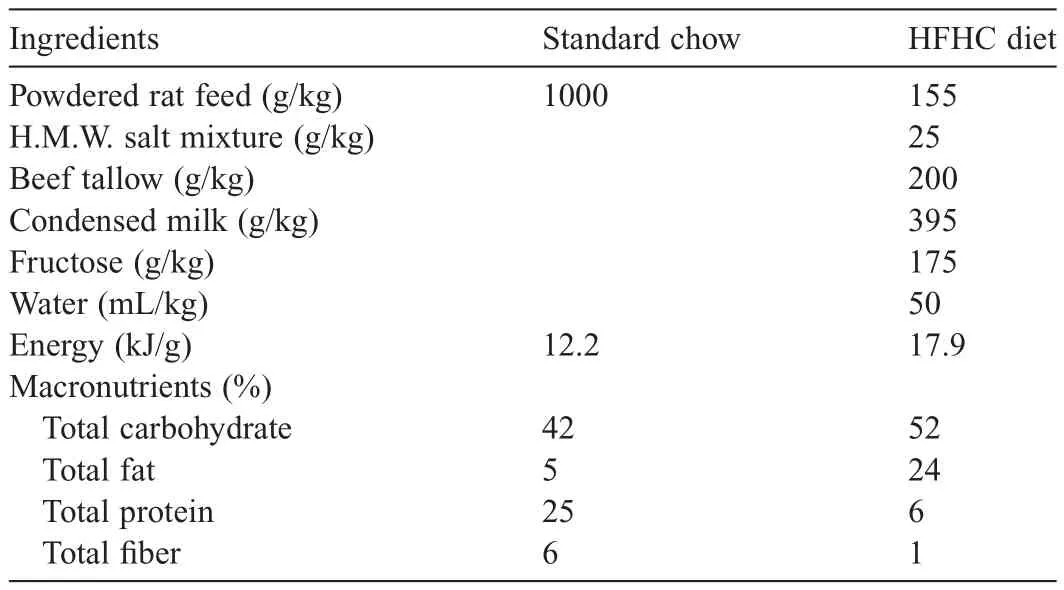
Table 1Animal diet composition.
2.4.Body weight,systolic blood pressure(SBP),and heart rate(HR)
Body weights,SBP,and HR were recorded every 4 weeks.Rats were anesthetized with an intraperitoneal injection of Zoletil®(zolazepam/tiletamine,15 mg/kg)prior to SBP readings.SBP and HR measurements were taken via tail-cuff plethysmography using an MLT1010 Pulse Transducer(ADInstruments,Bella Vista,Australia)and inflatable tail-cuff connected to an MLT844 Physiological Pressure Transducer(ADInstruments),and PowerLab data acquisition unit(ADInstruments).50
2.5.Oral glucose tolerance test(OGTT)
OGTT was performed every 4 weeks after an overnight fast(10-12 h).Rats were administered 40%glucose(2 g/kg)via oral gavage.Tail-vein blood samples were taken at 0,30,60,90,and 120 min,and blood glucose concentrations were measured using a Medisense Precision QID glucose meter(Abbott Laboratories,Princeton,NJ,USA).Area under the curve was calculated using the blood glucose levels measured over the 2 h period.
2.6.Terminal assessments
At completion of the 12-week intervention period,all rats were euthanized after a 24 h rest period via intraperitoneal injection of Lethabarb®(sodium pentobarbitone,1.5 mg/kg)prepared at a concentration of 375 mg/mL.Following euthanasia,the chest cavity was opened and heart,kidney,liver,spleen,and fats pads(visceral and epididymal)were removed and weighed(weight normalized to tibial length).Blood serum was collected and stored at-80°C for subsequent analysis.Electrophysiological studies and isolated thoracic aortic and mesenteric ring organ baths were then performed to assess changes in cardiovascular function.
2.7.Biochemical assays
Biochemical measurements were performed using blood collected from the abdominal vena cava of each rat.Blood samples were allowed to clot,then centrifuged for 10 min at 4000 rpm.Serum was aliquoted to microtubes and stored at-80°C until further analysis.The Sorte and Basak51modified copper-cadmium reduction method was used to quantify total nitric oxide concentrations.Abcam assay kits(Abcam,Cambridge,UK)prepared according to manufacturer-provided standards and protocols were used to measure serum triglycerides,low-density lipoprotein(LDL),and HDL concentrations.TC was calculated using a modified Fried lander formula.52Mercodia Rat Insulin ELISA kit(Mercodia,Uppsala,Sweden)was used to determine serum levels of insulin,and R&D Systems Rat IL-6 DuoSet ELISA(Catalogue Number DY506)(R&D Systems,Minneapolis,MN,USA)was used to quantify interleukin-6(IL-6)concentration.The Briskey et al.53optimized gas chromatography-tandem mass spectrometry protocol was used to measure total F2-isoprostane levels.
2.8.Cardiac function
The left ventricular papillary muscle was pinned between 2 earth-isolated platinum electrodes in a 1.0 mL experimental chamber filled with Tyrode’s physiological salt solution(37°C,aerated with 95%O2-5%CO2).The papillary muscle was slowly stretched to a maximum preload(5-10 m·N)and was then stimulated using the Grass SD9 stimulator(West Warwick,RI,USA)at a frequency of 1 Hz,pulse width of 0.5 ms,and stimulus strength of 20%above threshold.After a 5 min equilibration period,the papillary muscle was then impaled by glass microelectrodes filled with 3M potassium chloride((World Precision Instruments;Sarasota,FL,USA) filamented borosilicate glass,outer diameter 1.5 mm,tip resistance of 5-15 mΩ)using a silver/silver chloride reference electrode.The electrical activity(mV)of a cell was recorded with a Cyto 721 electrometer(World Precision Instruments)connected to a PowerLab data recording system(ADInstruments).
2.9.Vascular function
Thoracic aortic rings isolated from each rat were suspended in individual 25 mL organ baths filled with Tyrode’s buffer at 37°C aerated with 95%O2-5%CO2.Mesenteric arteries dissected from the intestine vasculature were threaded with 40 μm stainless steel wire while bathed in Tyrode’s solution,then transferred to 10 mL myograph chambers containing Tyrode’s buffer at 37°C aerated with 95%O2-5%CO2.Aortic and mesenteric rings were allowed to equilibrate for 30 min at a resting tension of ~10 m·N.After equilibration,mesenteric arteries were normalized using an automated normalization function,54were contracted with 10 mmol/L potassium chloride,and were relaxed with 0.01 mmol/L acetylcholine.Cumulative concentration-contraction curves were measured for noradrenaline(Sigma-Aldrich,Castle Hill,Australia)on both aortic and mesenteric vessels.Cumulative concentration-relaxation curves were measured for acetylcholine(Sigma-Aldrich)and sodium nitroprusside(Sigma-Aldrich)following submaximal(70%)contraction to noradrenaline.50
2.10.Statistical analysis
Data are presented as mean±SEM.Food and water intake,body weight,SBP,HR,and vascular functional parameters were analyzed using separate two-way(group×time)analysis of variance with Bonferronipost hocanalysis for multiple comparisons.Cardiometabolic and one-way analyses of variance with Bonferronipost hoccomparisons.Analyses were performed using GraphPad Prism Version 6(GraphPad Software Inc.,La Jolla,CA,USA).Statistical significance was set atp<0.05.
3.Results
3.1.Food,water,and energy intake
Table 2 presents the mean 24 h food,energy,and water intake of the experimental groups at Weeks 0,4,8,and 12 of intervention.There were no significant between-group differences in food,water,and energy intake throughout the intervention period.No group×time interaction was observed for food(p=0.5435),water(p=0.1555),and energy(p=0.2034)intake.No main effect for group was observed for food(p=0.1335),water(p=0.0891),and energy(p=0.1660)intake,but there was a significant main effect for time(allp<0.0001).Follow-up analyses showed all groups had significantly lower food and water intake,and significantly higher energy intake at Weeks 4,8,and 12 compared with Week 0(allp<0.0001).No significant difference in food,water,and energy intake in all groups was observed at Week 12 compared with Weeks 4 and 8.
3.2.Body and organ weights
Table 3 presents the body weights of the experimental groups at Weeks 0,4,8,and 12 and organ weights of experimental groups after 12 weeks of the intervention.Initial body weights ranged from 420.7±12.2 g to 449.2±8.2 g.No significant main effects or interactions were observed for body weight throughout the intervention period.At Week 12,the LIT(p=0.0057)and HIIT(p=0.0120)groups’visceral fat weight was significantly lower compared with the CTL group.The LIT group’s epididymal fat weight was significantly lower compared with the CTL(p<0.0001)and SED(p=0.0447)groups,and the HIIT group’s epididymal fat weight was significantly lower compared with the CTL group(p=0.0002).No significant between-group differences were observed in the left and right ventricle,kidney,spleen,and liver weights at Week 12 of the intervention.
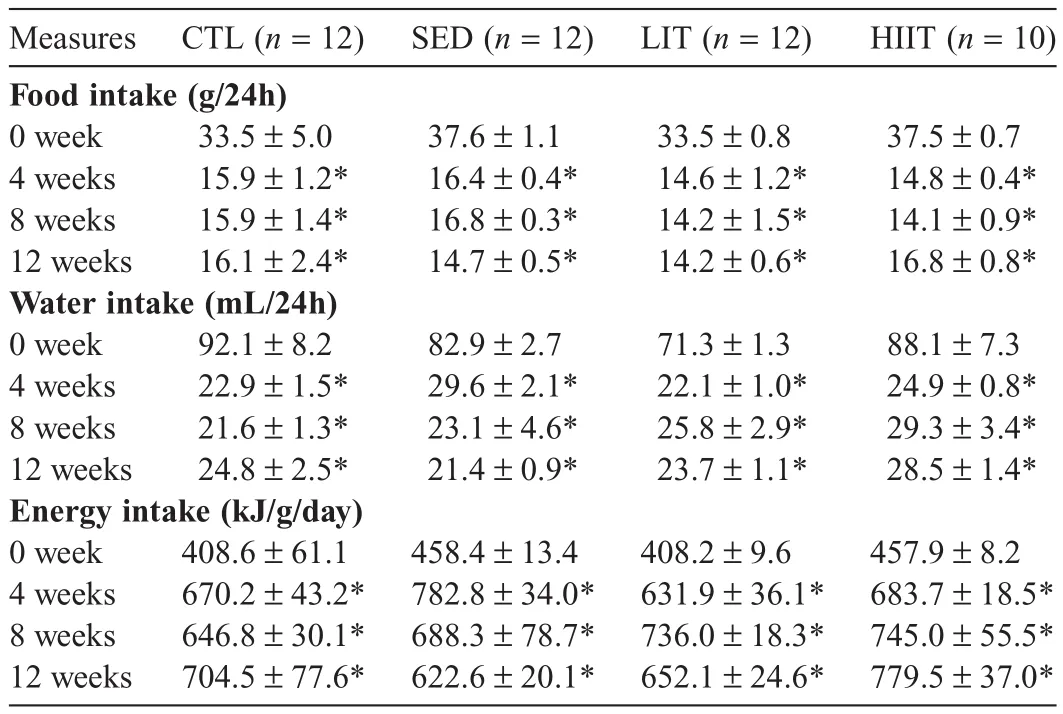
Table 2Mean 24 h food and water intake of experimental groups at 0,4,8,and 12 weeks of intervention(mean±SEM).
3.3.SBP and HR
Table 4 presents SBP and HR of the experimental groups at 0,4,8,and 12 weeks of intervention.No significant main effects or interactions were observed for SBP or HR throughout the intervention period.
3.4.Cardiometabolic parameters
Table 5 presents the cardiometabolic parameter outcomes of the experimental groups after 12 weeks of the intervention.There were no significant between-group differences observed in OGTT area under the curve at Weeks 0,4,8,and 12 of the intervention.No significant between-group differences were observed in LDL cholesterol(LDL-C),HDL cholesterol(HDLC),triglycerides,TC,insulin,nitric oxide,IL-6,or F2-isoprostane.
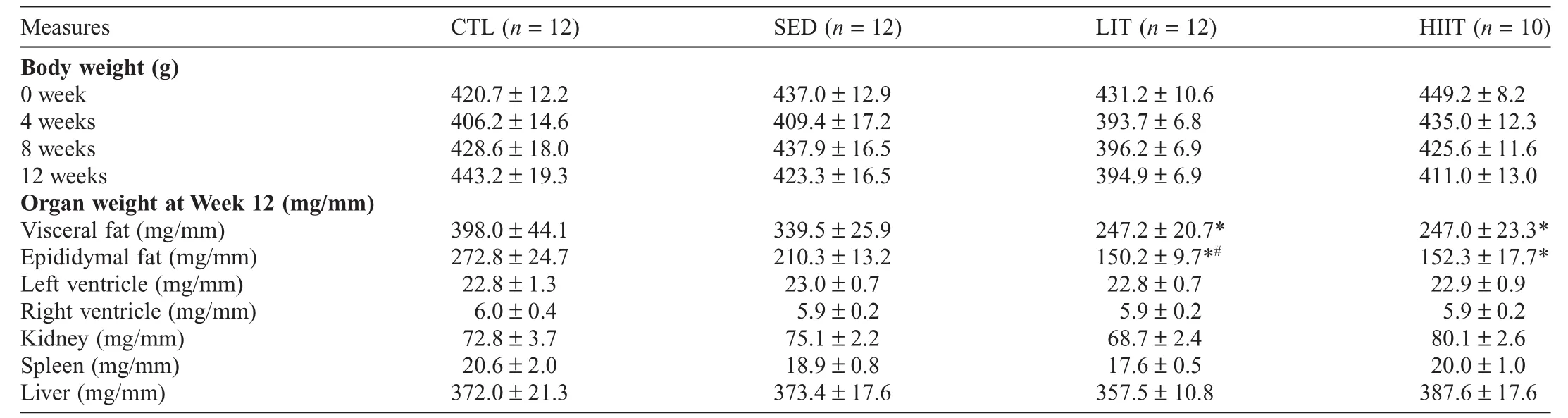
Table 3Body weights at Weeks 0,4,8,and 12 and organ weights of experimental groups after 12 weeks of intervention(mean±SEM).
3.5.Cardiac function
Table 6 presents cardiac function of the experimental groups after 12 weeks of the intervention.The SED group’s action potential amplitude(APA)was significantly lower compared with the CTL group(p=0.0426),and the LIT group’s APA was significantly higher compared with the SED group(p=0.0003).No significant between-group differences were observed in action potential duration(APD)at 20%,50%,or 90%of the repolarization,in resting membrane potential,or in force of contraction.
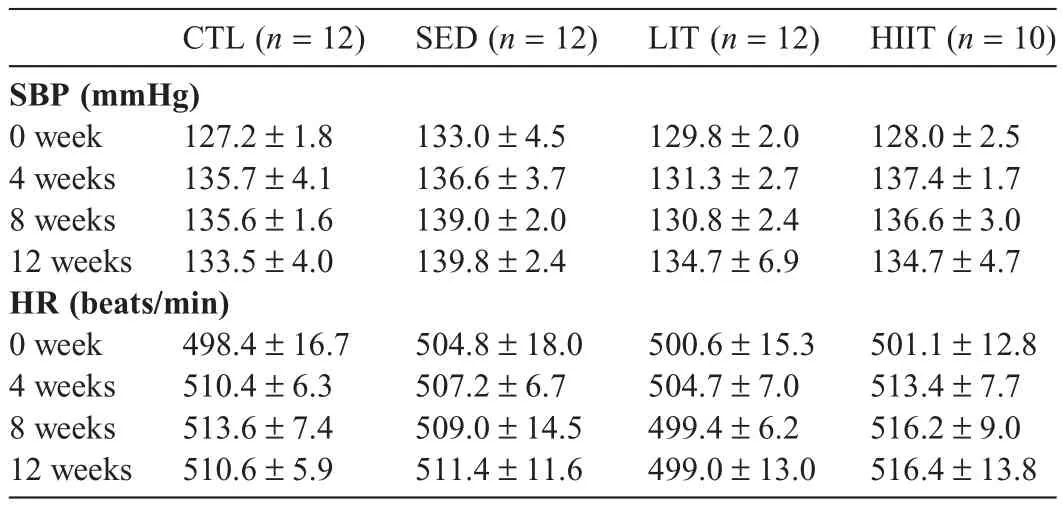
Table 4SBP and HR of experimental groups at 0,4,8,and 12 weeks of intervention(mean±SEM).
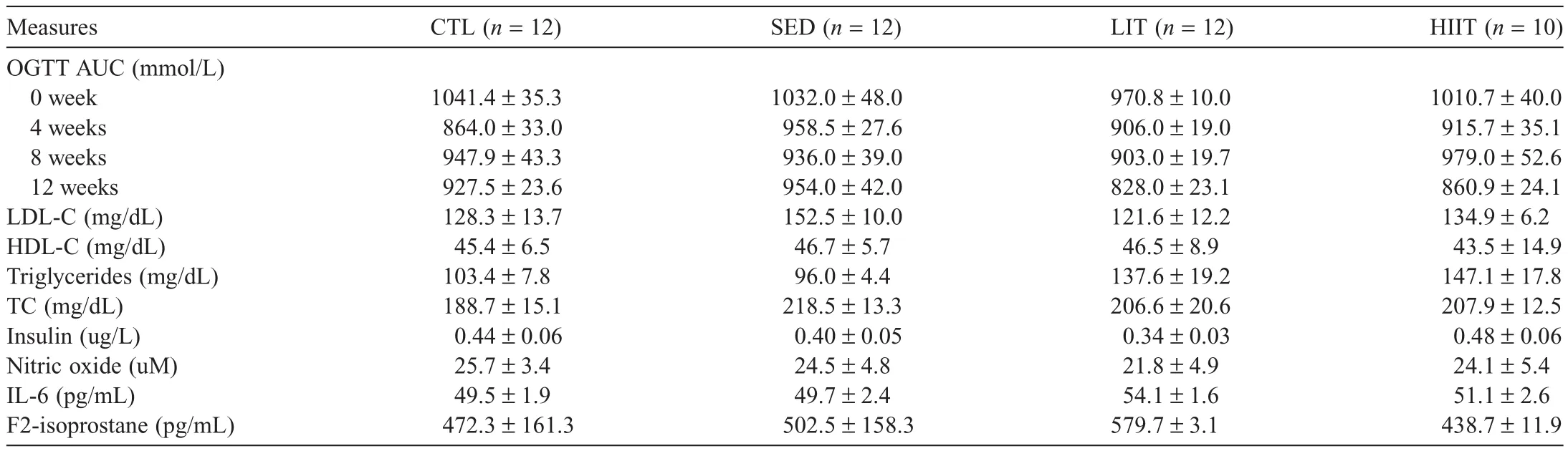
Table 5Cardiometabolic parameter outcomes of experimental groups after 12 weeks of intervention(mean±SEM).
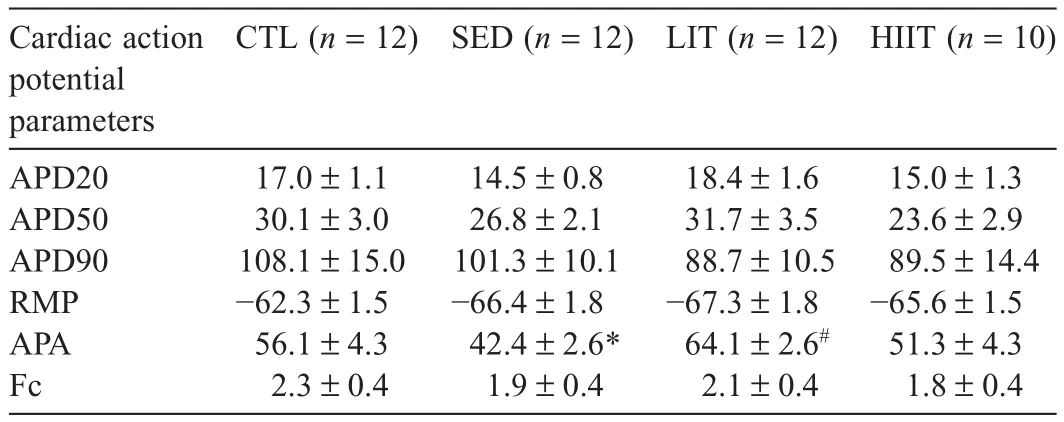
Table 6Cardiac electrophysiology outcomes of experimental groups after 12 weeks of intervention(mean±SEM).
3.6.Vascular function
Fig.1 presents vascular reactivity of the experimental groups at Week 12 of the intervention.The SED group demonstrated increased thoracic aortic contractile responses to noradrenaline compared with the CTL group(p<0.05)(Fig.1A),whereas the HIIT group demonstrated decreased mesenteric artery contractile responses to noradrenaline compared with all other groups(p<0.05)(Fig.1D).Results regarding vasorelaxation of noradrenaline-precontracted vessels at Week 12 of the intervention demonstrated that HIIT improved endothelium dependent mesenteric artery relaxation responses to acetylcholine compared with the LIT group(p<0.05)(Fig.1E).No significant differences in endothelium-dependent aortic and endotheliumindependent aortic and mesenteric relaxation were observed between groups after 12 weeks of the intervention.
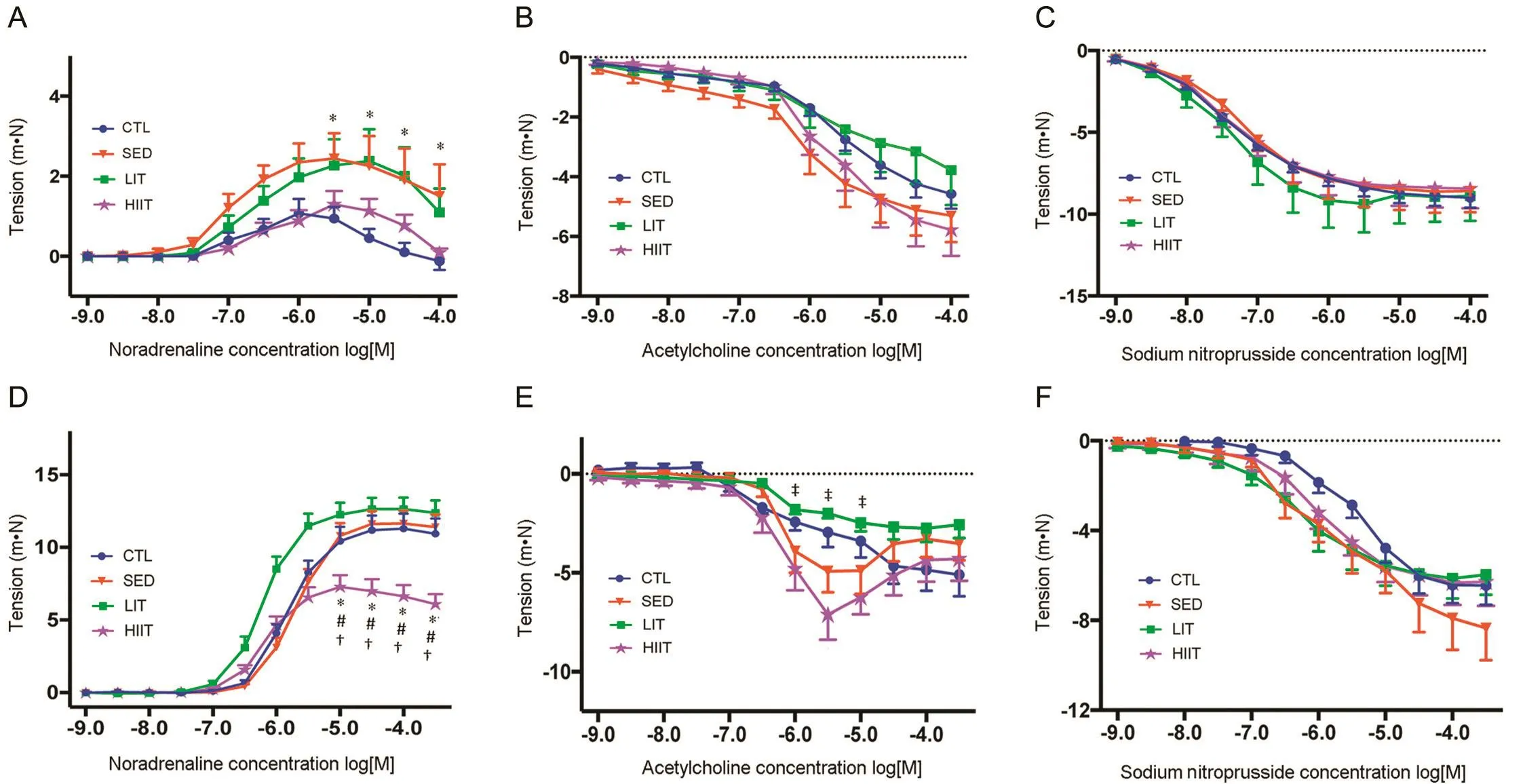
Fig.1.Vascular functional changes in high-fat high-carbohydrate-fed rats after 12 weeks of intervention.(A)Noradrenaline-mediated contraction of the thoracic aorta;(B)Endothelium-dependent relaxation to acetylcholine of noradrenaline-precontracted thoracic aorta;(C)Endothelium-independent relaxation to sodium nitroprusside of noradrenaline-precontracted thoracic aorta;(D)Noradrenaline-mediated contraction of the mesenteric artery;(E)Endothelium-dependent relaxation to acetylcholine of noradrenaline-precontracted mesenteric artery;(F)Endothelium-independent relaxation to sodium nitroprusside of noradrenaline-precontracted mesenteric artery.Data expressed as mean±SEM.Log[M]=negative logarithm to the base 10 of the molar concentration.*p<0.05,compared with CTL;#p<0.05,compared with SED;†p<0.05,compared with LIT;‡p<0.05,compared with HIIT.CTL=control group;HIIT=high-intensity interval trained group;LIT=light-intensity trained group;SED=sedentary group.
4.Discussion
Studies have established that dietary factors and activity levels largely contribute to the development of the cardiometabolic syndrome.55,56The present study demonstrated that in rats fed with an HFHC diet,the SED group had lower APA and greater thoracic aortic contractile responses compared with the CTL group.The LIT group had a higher APA compared with the SED group,whereas the HIIT group showed a decreased mesenteric artery contractile response compared with all other groups and improved endothelium-dependent mesenteric artery relaxation compared with the LIT group.Additionally,the LIT and HIIT groups had decreased visceral and epididymal fat weights compared with the CTL group.These findings differ from those we recently reported showing that in animals fed standard chow and trained using the same LIT and HIIT protocols,LIT and HIIT groups demonstrated beneficial changes in body weight,fat accumulation,glucose control,HDL and TC levels,and mesenteric vessel contractile response to noradrenaline compared with the CTL group.35Furthermore,the LIT group demonstrated significant improvements in insulin sensitivity and cardiac conduction,whereas the HIIT group demonstrated significant improvements in SBP and enhanced sensitivity to endothelium-independent relaxation in the aorta and mesenteric artery compared with the CTL group.35These results suggest that limited beneficial effects are observed following LIT and HIIT prescriptions in rats consuming an HFHC diet.
Results of this study demonstrated significant decreases in food and water(fructose)intake in all groups at Weeks 4, 8, and 12 compared with Week 0 of intervention.This finding is in agreement57-59with previous studies in rodents suggesting that the hypophagic effect of an HFHC diet is a way to maintain a stable body weight or fat.In contrast,other animal studies60-62have shown increased food intake following an HFHC diet.Several factors are hypothesized to have contributed to this discrepancy.These include differences in experimental design,strain susceptibility to fructose manipulations,and palatability of the diet,which depends on the concentration and the ratio of fructose to fat.63However,it should be noted that although several hypotheses63have been proposed,it is still unclear what exactly influences an HFHC diet to stimulate or inhibit food intake in rats.
Although a significantly lower food and water intake was observed at Weeks 4,8,and 12 compared with Week 0,energy intake was significantly higher in all groups at Weeks 4,8,and 12 compared with Week 0.This finding was due to the higher energy content of the HFHC diet,leading to a higher energy intake.As observed in this study,rats given sugar solutions(fructose)in addition to solid food consumed more energy than rats given solid food alone,promoting changes in adipose deposition even without excessive weight gain.64Thus,a sustained increase in intake was not essential to promote an increased energy intake in this model.
Several studies have demonstrated that MVPA can mitigate high-fat diet-induced weight gain.65-67In the current study,no significant main effects or interactions were observed for body weight throughout the intervention period,suggesting that body weight was not altered in either sedentary,LIT,or HIIT rats fed with an HFHC diet.This is perhaps due to the hypophagic effect of an HFHC diet,57-59which suppresses the effect of PA on body weights.Additionally,although feeding an HFHC diet has been linked to obesity and the metabolic syndrome in several animal models,68data from this study failed to induce excessive weight gain in all groups,as evidenced by the nonsignificant difference in weight in all groups throughout the intervention period.Several rat studies have reported the failure of an HFHC diet to induce obesity,57,69-71and various reasons have been suggested to explain this occurrence.Investigators have suggested that following 6-8 weeks of HFHC feeding,increases in leptin(satiety hormone)production and central leptin sensitivity are observed in the animals,decreasing food intake and body weight.72After 12 weeks,animals start to develop leptin resistance and obesity.72Another suggestion is that Wistar rats are less responsive to weight gain following an HFHC diet compared with Sprague-Dawley rats owing to their higher basal metabolic rate.73-75Lastly,it is hypothesized that induction of obesity is more effective when a high-fat diet is started at an early age(9 weeks of age)and administered for 8 weeks or more.76These factors may have contributed to the failure of HFHC to induce obesity in this study.
It is established that body fat distribution is a better indicator of disease risk than body weight itself.77In this study,although no changes in body weights were observed,the LIT and HIIT groups’visceral and epididymal fat weights were significantly lower compared with the CTL group.This finding suggests that LIT and HIIT can prevent fat accumulation (even without excessive weight gain),potentially decreasing cardiometabolic risk.78Possible mechanisms underlying this LIT-and HIIT-induced fat prevention are an increase in the muscle enzymes that stimulate increased fat oxidation,79,80an increase in neurotransmitters contributing to post-training inhibition of food intake,81and an increase in release of anorectic peptide(corticotropin-releasing factor)by the hypothalamus.82Additionally,although not statistically significant,the SED group’s visceral and epididymal fat weights were lower compared with the CTL group.In rodents,food intake is coupled with energy expenditure,such that decreased PA acutely reduces energy expenditure and food intake and consequently lowers fat accumulation.83
No significant main effects or interactions were observed for SBP or HR throughout the intervention period.The autonomic nervous system and its sympathetic arm play important roles in regulating blood pressure and HR.84,85Previous studies have confirmed that MVPA lessens sympathetic modulation of the heart,86,87whereas HFHC feeding enhances sympathetic activity.88One study further demonstrated that MVPA attenuated high-fat diet-induced sympathetic activation.86Results of this study showed that sedentary behavior,LIT,and HIIT had no effect on SBP and HR.Rats used were relatively young with normal vascular function,so it is possible that 12 weeks of restricted activity may not be enough to cause adverse cardiometabolic changes.The effect of LIT and HIIT on sympathetic activity has not been widely examined,and it is assumed that the HFHC feeding reduced the effect of LIT and HIIT on the sympathetic system;however,this remains to be clarified.Notably,blood pressure readings were showing an upward trend in the HFHC-fed groups,which may have become more apparent if the feeding trial had been extended beyond 12 weeks.
The role of PA and diet on glucose and lipid metabolism was also examined in the current study.No significant between group differences were observed in OGTT area under the curve at 0,4,8,and 12 weeks and no between-group differences in insulin,LDL-C,HDL-C,triglycerides,and TC at Week 12 of intervention.In rats fed with a standard diet,previous studies have demonstrated that LIT and HIIT89,90stimulate the rate of muscle glucose transport,reducing plasma glucose,whereas physical inactivity decreases the number of muscle glucose transporters,resulting in increased plasma glucose.91Similarly,several animal studies using standard chow diets have shown that LIT and HIIT improve the lipid profile,31,92-96whereas physical inactivity reduces plasma triglyceride uptake into muscle and decreases HDL-C levels29in rats fed with a standard diet.Results of this study showed that sedentary behavior,LIT,and HIIT had no effect on glucose and lipid metabolism in rats consuming an HFHC diet.The underlying mechanism is not fully understood,but it appears that the HFHC diet is hindering the effect of PA in glucose and lipid metabolism.This is an important finding in the context of this project,where the short duration of HFHC feeding(12 weeks)compared with other studies97,98did alter glucose responses to exercise training despite limited evidence of classic metabolic syndrome changes.
Recently,it has become apparent that the complex interaction of inflammation,endothelial dysfunction,and oxidative stress are contributors to the pathogenesis of several diseases(e.g.,hypertension,diabetes,and coronary artery disease).99-101In this study,no significant between-group differences were observed in nitric oxide,IL-6,and F2-isoprostane concentrations following the various PA prescriptions.Similar animal studies have demonstrated different findings,where moderateintensity exercise was found to reduce circulating inflammatory cytokines(IL-6)69and oxidative stress markers(superoxide anion,thiobarbituric acid reactive substances,and carbonyl)102in rats fed with 8 weeks of a high-fat diet.However,these studies employed different training intensity and outcome measures69,102as well as rat models(adolescent rats).69On the other hand,no significant differences in serum nitric oxide concentrations were observed between groups,which is in agreement with a previous study that demonstrated no change in the level of plasma nitric oxide after 4 weeks of moderate intensity exercise in Zucker obese rats.103Results of this study showed that sedentary behavior,LIT,and HIIT had no effect on inflammatory and oxidative stress markers.However,because this is the first study to examine the influence of an HFHC diet with different intensities of PA on markers of inflammation and oxidative stress,more research is required to fully understand these results.
In this study,no significant between-group differences were observed for APD20,APD50,APD90,resting membrane potential,or force of contraction.After12 weeks of intervention,the LIT group demonstrated a higher APA compared with the SED group despite the HFHC diet,whereas the SED group showed decreased APA compared with the CTL group.This finding suggests that LIT improved cardiac conduction,resulting in greater efficacy of cardiac impulse propagation.104,105In contrast,the SED group demonstrated slower cardiac conduction,potentially increasing CVD risk by impairing ventricular pressure development and increasing myocardial oxygen demand.104-106These cardiac effects are mediated by the activation of β1-adrenergic receptors,which are important in regulating heart function.107However,to date no study has examined the effect of these different intensities of PA on β1-adrenergic receptors.This is a worthwhile future area of investigation to gain insight into how exercise intensity may affect cardiac function.
Vascular dysfunction,a hallmark of several cardiovascular diseases,is frequently associated with increased vasoconstriction and decreased vasodilation.108,109The current study demonstrated increased thoracic aortic contractile responses to noradrenaline in the SED group compared with the CTL group.This increased vascular reactivity to noradrenaline observed in the SED group supports previous observation that increased sedentary behavior promotes vascular deconditioning by increasing arterial tone.110Studies have demonstrated that sedentary behavior increases plasma triglycerides and decreases HDL-C,29whereas a high-fat diet is known to induce elevated levels of the triglyceride-rich very low-density lipoprotein and the cholesterol-rich LDL.111Thus,this combined effect of sedentary behavior and diet is perhaps the reason for the increased α-adrenergic contractile responses (vascular constriction)observed in the SED group.112
In the mesenteric vessels,where vascular responsiveness is faster and greater compared with large vessels,113the HIIT group(compared with CTL,SED,and LIT groups)had significantly lower contractile response to noradrenaline.These results are encouraging because they indicate that HIIT can mitigate mesenteric α-adrenergic contractile responses even with concurrent intake of an HFHC diet and can possibly decrease vessel pressure.Possible mechanism is the stimulation of an α-adrenergic receptor on the vascular endothelial cells,leading to release of endothelium-derived relaxing factor through the nitric oxide synthase pathway.114This assumption is supported by the finding in this study of increased sensitivity of HIIT to endothelium-dependent mesenteric artery relaxation compared with the LIT group.
The present data also showed no between-group differences in endothelium-dependent aortic and endothelium-independent aortic and mesenteric relaxation after 12 weeks of intervention.This finding is consistent with previous studies,which demonstrated that an HFHC diet impairs endothelium-dependent and endothelium-independent dilation,even in the absence of increase in fat mass.115It has been suggested that the presence of insulin resistance and fatty acids released during fat oxidation,which activate the nuclear factor-κB resulting in nitric oxide inhibition,contribute to the impairment of the endothelium.116Thus,it is possible that this HFHC diet is suppressing the effects of PA,if there are any.
5.Conclusion
The present study examined the influence of an HFHC diet on cardiometabolic,cardiac,and vascular function following 12 weeks of different intensities of PA in male adult rats.This study demonstrated negative effects on cardiac conduction and thoracic aortic contractile response in the SED group compared with the CTL group.LIT induced positive adaptations in fat accumulation and cardiac conduction compared with the CTL and SED groups,respectively.The HIIT group induced a positive effect on fat accumulation compared with the CTL group,a positive effect on mesenteric artery contraction compared with all other groups,and a positive effect on endotheliumdependent mesenteric artery relaxation compared with the LIT group.Our data provide evidence that few positive health effects are achieved through LIT and HIIT when consuming an HFHC diet.It is possible that the duration of the exercise interventions in this study is insufficient to prevent and/or reverse the changes occurring following HFHC feeding.Evidently,diet and PA play significant roles in influencing the cardiometabolic effect of LIT and HIIT and thus should both be targeted when developing interventions for improving cardiometabolic health.
The authors would like to thank Kylie Connolly,Candice Pullen,and Douglas Jackson for their assistance during the terminal experiments and Dr.Jeff Coombes for allowing RBB to work in his laboratory for the F2-isoprostane analysis.RBB is supported by the Strategic Research Scholarship grant from Central Queensland University(CQU).This manuscript is in part supported by CQU Health CRN.MJD is supported by a Future Leader Fellowship(ID 100029)from the National Heart Foundation of Australia.
Authors’contributions
RBB designed and conducted the study,analyzed the data,and drafted the manuscript;MJD contributed to the study design,data analysis,and manuscript drafting;VJD helped in data analysis and critically reviewed the manuscript;GLB contributed in data collection and data analysis;ASF helped with the study design and data analysis,edited and revised the manuscript.All authors have read and approved the final version of the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
1.Hu FB.Globalization of food patterns and cardiovascular disease risk.Circulation2008;118:1913-4.
2.Kanoski SE,Davidson TL.Western diet consumption and cognitive impairment:links to hippocampal dysfunction and obesity.Physiol Behav2011;103:59-68.
3.Dissard R,Klein J,Caubet C,Breuil B,Siwy J,Hoffman J,et al.Long term metabolic syndrome induced by a high fat high fructose diet leads to minimal renal injury in C57BL/6 mice.PLoS One2013;8:e76703.doi:10.1371/journal.pone.0076703
4.Lê KA,Faeh D,Stettler R,Ith M,Kreis R,Vermathen P,et al.A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans.Am J Clin Nutr2006;84:1374-9.
5.Ashen MD.Management of cardiometabolic syndrome in the primary and secondary prevention of cardiovascular disease.J Nurse Pract2008;4:673-80.
6.Rangaraj VR,Knutson KL.Association between sleep deficiency and cardiometabolic disease:implications for health disparities.Sleep Med2016;18:19-35.
7.Reddigan JI,Ardern CI,Riddell MC,Kuk JL.Relation of physical activity to cardiovascular disease mortality and the influence of cardiometabolic risk factors.Am J Cardiol2011;108:1426-31.
8.Ekelund U,Franks PW,Sharp S,Brage S,Wareham NJ.Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness.Diabetes Care2007;30:2101-6.
9.Saunders TJ,Larouche R,Colley RC,Tremblay MS.Acute sedentary behaviour and markers of cardiometabolic risk:a systematic review of intervention studies.J Nutr Metab2012;2012:712435.doi:10.1155/2012/712435
10.Norton K,Norton L,Sadgrove D.Position statement on physical activity and exercise intensity terminology.J Sci Med Sport2010;13:496-502.
11.Peterson MD,Al Snih S,Stoddard J,McClain J,Lee IM.Adiposity and insufficient MVPA predict cardiometabolic abnormalities in adults.Med Sci Sports Exerc2014;46:1133-9.
12.Fernández-García JC,Cardona F,Tinahones FJ.Inflammation,oxidative stress and metabolic syndrome:dietary modulation.CurrVasc Pharmacol2013;11:906-19.
13.Lwow F,Dunajska K,Milewicz A,Jedrzejuk D,Kik K,Szmigiero L.Effect of moderate-intensity exercise on oxidative stress indices in metabolically healthy obese and metabolically unhealthy obese phenotypes in post menopausal women:a pilot study.Menopause2011;18:646-53.
14.Zoppini G,Targher G,Zamboni C,Venturi C,Cacciatori V,Moghetti P,et al.Effects of moderate-intensity exercise training on plasma biomarkers of in flammation and endothelial dysfunction in older patients with type 2 diabetes.Nutr Metab Cardiovasc Dis2006;16:543-9.
15.Ranadive SM,Kappus RM,Cook MD,Yan H,Lane AD,Woods JA,et al.Effect of acute moderate exercise on induced inflammation and arterial function in older adults.Exp Physiol2014;99:729-39.
16.Starkie R,Ostrowski SR,Jauffred S,Febbraio M,Pedersen BK.Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans.FASEB J2003;17:884-6.
17.Matthews CE,Chen KY,Freedson PS,Buchowski MS,Beech BM,Pate RR,et al.Amount of time spent in sedentary behaviors in the United States,2003-2004.Am J Epidemiol2008;167:875-81.
18.Tremblay MS,Colley RC,Saunders TJ,Healy GN,Owen N.Physiological and health implications of a sedentary lifestyle.Appl Physiol Nutr Metab2010;35:725-40.
19.Batacan RB,Duncan MJ,Dalbo VJ,Tucker PS,Fenning AS.Effects of light intensity activity on CVD risk factors:a systematic review of intervention studies.Biomed Res Int2015;2015:596367.doi:10.1155/2015/596367
20.Church TS,Thomas DM,Tudor-Locke C,Katzmarzyk PT,Earnest CP,Rodarte RQ,et al.Trends over 5 decades in U.S.occupation-related physical activity and their associations with obesity.PLoS One2011;6:e19657.doi:10.1371/journal.pone.0019657
21.Brownson RC,Boehmer TK,Luke DA.Declining rates of physical activity in the United States:what are the contributors?Annu Rev Public Health2005;26:421-43.
22.Rhodes RE,Mark RS,Temmel CP.Adult sedentary behavior:a systematic review.Am J Prev Med2012;42:e3-28.
23.Gibala MJ,Little JP,MacDonald MJ,Hawley JA.Physiological adaptations to low-volume,high-intensity interval training in health and disease.J Physiol2012;590:1077-84.
24.Smith KL,Carr K,Wiseman A,Calhoun K,McNevin NH,Weir PL.Barriers are not the limiting factor to participation in physical activity in Canadian seniors.J Aging Res2012;2012:890679.doi:10.1155/2012/890679
25.Loprinzi PD,Lee H,Cardinal BJ.Evidence to support including lifestyle light-intensity recommendations in physical activity guidelines for older adults.Am J Health Promot2015;29:277-84.
26.Buman MP,Hekler EB,Haskell WL,Pruitt L,Conway TL,Cain KL,et al.Objective light-intensity physical activity associations with rated health in older adults.Am J Epidemiol2010;172:1155-65.
27.Healy GN,Dunstan DW,Salmon J,Cerin E,Shaw JE,Zimmet PZ,et al.Objectively measured light-intensity physicalactivity is independently associated with 2-h plasma glucose.Diabetes Care2007;30:1384-9.
28.Healy GN,Dunstan DW,Shaw JE,Zimmet PZ,Owen N.Objectively measured sedentary time and light-intensity physical activity are independently associated with components of the metabolic syndrome:the AusDiab study.Diabetologia2008;50(Suppl.1):S67-8.
29.Bey L,Hamilton MT.Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity:a molecular reason to maintain daily low-intensity activity.J Physiol2003;551:673-82.
30.Kessler HS,Sisson SB,Short KR.The potential for high-intensity interval training to reduce cardiometabolic disease risk.Sports Med2012;42:489-509.
31.Rocha RE,Coelho I,Pequito DC,Yamagushi A,Borghetti G,Yamazaki RK,et al.Interval training attenuates the metabolic disturbances in type 1 diabetes rat model.Arq Bras Endocrinol Metabol2013;57:594-602.
32.Hoshino D,Yoshida Y,Kitaoka Y,Hatta H,Bonen A.High-intensity interval training increases intrinsic rates of mitochondrial fatty acid oxidation in rat red and white skeletal muscle.Appl Physiol Nutr Metab2013;38:326-33.
33.Nybo L,Sundstrup E,Jakobsen MD,Mohr M,Hornstrup T,Simonsen L,et al.High-intensity training versus traditional exercise interventions for promoting health.Med Sci Sports Exerc2010;42:1951-8.
34.Tjonna AE,Lee SJ,Rognmo O,Stolen TO,Bye A,Haram PM,et al.Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome:a pilot study.Circulation2008;118:346-54.
35.Batacan RB,Duncan MJ,Dalbo VJ,Connolly KJ,Fenning AS.Light intensity and high intensity interval training improve cardiometabolic health in rats.Appl Physiol Nutr Metab2016;41:945-52.
36.Diabetes Prevention Program Research Group.The Diabetes Prevention Program(DPP):description of lifestyle intervention.Diabetes Care2002;25:2165-71.
37.The Look Ahead Research Group,Wadden TA,West DS,Delahanty L,Jakicic J,Rejeski J,et al.The Look AHEAD Study:a description of the lifestyle intervention and the evidence supporting it.Obesity(Silver Spring)2006;14:737-52.
38.Wadden TA,Webb VL,Moran CH,Bailer BA.Lifestyle modification for obesity:new developments in diet,physical activity,and behavior therapy.Circulation2012;125:1157-70.
39.The Look Ahead Research Group,Wing RR,Bolin P,Brancati FL,Bray GA,Clark JM,et al.Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes.N Engl J Med2013;369:145-54.
40.Centers for Disease Control,National Center for Chronic Disease Prevention and Health Promotion.Physical activity and good nutrition:essential elements to prevent chronic diseases and obesity 2003.Nutr Clin Care2003;6:135-8.
41.Popkin BM.Global nutrition dynamics:the world is shifting rapidly toward a diet linked with noncommunicable diseases.Am J Clin Nutr2006;84:289-98.
42.Nazni P.Association of western diet&lifestyle with decreased fertility.Indian J Med Res2014;140(Suppl.1):S78-81.
43.Pi-Sunyer FX,Woo R.Effect of exercise on food intake in human subjects.Am J Clin Nutr1985;42:983-90.
44.Titchenal CA.Exercise and food intake.What is the relationship?Sports Med1988;6:135-45.
45.Sharp J,Azar T,Lawson D.Does cage size affect heart rate and blood pressure of male rats at rest or after procedures that induce stress-like responses?Contemp Top Lab Anim Sci2003;42:8-12.
46.Lee JS,Bruce CR,Spriet LL,Hawley JA.Interaction of diet and training on endurance performance in rats.Exp Physiol2001;86:499-508.
47.Matsunaga S,Yamada T,Mishima T,Sakamoto M,Sugiyama M,Wada M.Effects of high-intensity training and acute exercise onin vitrofunction of rat sarcoplasmic reticulum.Eur J Appl Physiol2007;99:641-9.
48.Poudyal H,Panchal S,Brown L.Comparison of purple carrot juice and β-carotene in a high-carbohydrate,high-fat diet-fed rat model of the metabolic syndrome.Br J Nutr2010;104:1322-32.
49.Panchal SK,Brown L.Rodent models for metabolic syndrome research.J Biomed Biotechnol2011;2011:351982.doi:10.1155/2011/351982
50.Fenning A,Harrison G,Rose’meyer R,Hoey A,Brown L.I-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats.Am J Physiol Heart Circ Physiol2005;289:H1408-16.
51.Sorte K,Basak A.Development of a modified copper-cadmium reduction method for rapid assay of total nitric oxide.Anal Methods2010;2:944-7.
52.Friedlander Y,Kark JD,Eisenberg S,Stein Y.Calculation of LDL-cholesterol from total cholesterol,triglyceride and HDL-cholesterol:a comparison of methods in the Jerusalem Lipid Research Clinic Prevalence Study.Isr J Med Sci1982;18:1242-52.
53.Briskey DR,Wilson GR,Fassett RG,Coombes JS.Optimized method for quantification of total F(2)-isoprostanes using gas chromatographytandem mass spectrometry.J Pharm Biomed Anal2014;90:161-6.
54.Bridges LE,Williams CL,Pointer MA,Awumey EM.Mesenteric artery contraction and relaxation studies using automated wire myography.JVis Exp2011;22:3119.doi:10.3791/3119
55.Barnard RJ,Wen SJ.Exercise and diet in the prevention and control of the metabolic syndrome.Sports Med1994;18:218-28.
56.Roberts CK,Barnard RJ.Effects of exercise and diet on chronic disease.J Appl Physiol2005;98:3-30.
57.Picchi MG,Mattos AM,Barbosa MR,Duarte CP,Gandini Mde A,Portari GV,et al.A high-fat diet as a model of fatty liver disease in rats.Acta Cir Bras2011;26:25-30.
58.Tillman EJ,Morgan DA,Rahmouni K,Swoap SJ.Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice.PLoS One2014;9:e107206.doi:10.1371/journal.pone.0107206
59.Crescenzo R,Bianco F,Coppola P,Mazzoli A,Cigliano L,Liverini G,et al.The effect of high-fat-high-fructose diet on skeletal muscle mitochondrial energetics in adult rats.Eur J Nutr2015;54:183-92.
60.Charlton M,Krishnan A,Viker K,Sanderson S,Cazanave S,McConico A,et al.Fast food diet mouse:novel small animal model of NASH with ballooning,progressive fibrosis,and high physiological fidelity to the human condition.Am J Physiol Gastrointest Liver Physiol2011;301:G825-34.
61.Panchal SK,Poudyal H,Iyer A,Nazer R,Alam MA,Diwan V,et al.High-carbohydrate,high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats.J Cardiovasc Pharmacol2011;57:611-24.
62.Poudyal H,Campbell F,Brown L.Olive leaf extract attenuates cardiac,hepatic,and metabolic changes in high carbohydrate-,high fat-fed rats.J Nutr2010;140:946-53.
63.Vasselli JR,Scarpace PJ,Harris RB,Banks WA.Dietary components in the development of leptin resistance.Adv Nutr2013;4:164-75.
64.Ramirez I.Feeding a liquid diet increases energy intake,weight gain and body fat in rats.J Nutr1987;117:2127-34.
65.Touati S,Meziri F,Devaux S,Berthelot A,Touyz RM,Laurant P.Exercise reverses metabolic syndrome in high-fat diet-induced obese rats.Med Sci Sports Exerc2011;43:398-407.
66.Pinheiro-Mulder A,Aguila MB,Bregman R,Mandarim-de-Lacerda CA.Exercise counters diet-induced obesity,proteinuria,and structural kidney alterations in rat.Pathol Res Pract2010;206:168-73.
67.EL-Gohary OA,Hussien NI.Effect of exercise and quercetin on obesity induced metabolic and renal impairments in albino rats.J Phys Pharm Adv2015;5:589-602.
68.Angelova P,Boyadjiev N.A review on the models of obesity and metabolic syndrome in rats.Trak J Sci2013;11:5-12.
69.van Waveren A,Duncan M,Coulson FR,Fenning A.Moderate intensity physical activity prevents increased blood glucose concentrations,fat pad deposition and cardiac action potential prolongation following diet-induced obesity in a juvenile-adolescent rat model.BMC Obes2014;1:11.doi:10.1186/2052-9538-1-11
70.Moghadasian MH,Abdullah M,Xu ZY,Othman R,Riediger N,Le K.Metabolic effects of long-term consumption of a high fat,high fructose diet in rats.FASEB J2007;21:A1091.
71.Medeiros R,Gaique T,Bernardes T,Motta N,Brito F,Bertoldi J,et al.Aerobic training prevents the impaired vascular reactivity of fructose-treated rats.FASEB J2014;28:1106-22.
72.Lin S,Thomas TC,Storlien LH,Huang XF.Development of high fat diet-induced obesity and leptin resistance in C57B1/6J mice.Int J Obes Relat Metab Disord2000;24:639-46.
73.Kanarek RB,Orthen-Gambill N.Differential effects of sucrose,fructose and glucose on carbohydrate induced obesity in rats.J Nutr1982;112:1546-54.
74.de Moura RF,Ribeiro C,de Oliveira JA,Stevanato E,de Mello MA.Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols.Br J Nutr2009;101:1178-84.
75.Mamikutty N,Thent ZC,Sapri SR,Sahruddin NN,MohdYusof MR,Haji Suhaimi F.The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats.Biomed Res Int2014;2014:263897.doi:10.1155/2014/263897
76.Peckham SC,Entenman C.The influence of a hypercaloric diet on gross body and adipose tissue composition in the rat.J Nutr1962;77:187-97.
77.Herling AW,Kilp S,Juretschke HP,Neumann-Haefelin C,Gerl M,Kramer W.Reversal of visceral adiposity in candy-diet fed female Wistar rats by the CB1 receptor antagonist rimonabant.Int J Obes2008;32:1363-72.
78.Demerath EW,Reed D,Rogers N,Sun SS,Lee M,Choh AC,et al.Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels.Am J Clin Nutr2008;88:1263-71.
79.Boutcher SH.High-intensity intermittent exercise and fat loss.J Obes2011;2011:868305.doi:10.1155/2011/868305
80.Achten J,Jeukendrup AE.Optimizing fat oxidation through exercise and diet.Nutrition2004;20:716-27.
81.Guilland JC,Moreau D,Genet JM,Klepping J.Role of catecholamines in regulation by feeding of energy balance following chronic exercise in rats.Physiol Behav1988;42:365-9.
82.Rivest S,Richard D.Involvement of corticotropin-releasing factor in the anorexia induced by exercise.Brain Res Bull1990;25:169-72.
83.Kaiyala KJ,Morton GJ,Thaler JP,Meek TH,Tylee T,Ogimoto K,et al.Acutely decreased thermoregulatory energy expenditure or decreased activity energy expenditure both acutely reduce food intake in mice.PLoS One2012;7:e41473.doi:10.1371/journal.pone.0041473
84.Triposkiadis F,Karayannis G,Giamouzis G,Skoularigis J,Louridas G,Butler J.The sympathetic nervous system in heart failure:physiology,pathophysiology,and clinical implications.J Am Coll Cardiol2009;54:1747-62.
85.Joyner MJ,Charkoudian N,Wallin BG.Sympathetic nervous system and blood pressure in humans:individualized patterns of regulation and their implications.Hypertension2010;56:10-6.
86.Li G,Liu JY,Zhang HX,Li Q,Zhang SW.Exercise training attenuates sympathetic activation and oxidative stress in diet-induced obesity.Physiol Res2015;64:355-67.
87.Bertagnolli M,Schenkel PC,Campos C,Mostarda CT,Casarini DE,Belló-Klein A,et al.Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats.Am J Hypertens2008;21:1188-93.
88.Schwartz JH,Young JB,Landsberg L.Effect of dietary fat on sympathetic nervous system activity in the rat.J Clin Invest1983;72:361-70.
89.Koshinaka K,Kawasaki E,Hokari F,Kawanaka K.Effect of acute high-intensity intermittent swimming on post-exercise insulin responsiveness in epitrochlearis muscle of fed rats.Metab Clin Exp2009;58:246-53.
90.Cortez MY,Torgan CE,Brozinick JT,Ivy JL.Insulin resistance of obese Zucker rats exercise trained at two different intensities.Am J Physiol1991;261:E613-9.
91.Fushiki T,Kano T,Inoue K,Sugimoto E.Decrease in muscle glucose transporter number in chronic physical inactivity in rats.Am J Physiol Endocrinol Metab1991;260:E403-10.
92.Ravi Kiran T,Subramanyam MV,Asha Devi S.Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats:relationship to swim intensity and duration.Comp Biochem Physiol B Biochem Mol Biol2004;137:187-96.
93.Sun MW,Qian FL,Wang J,Tao T,Guo J,Wang L,et al.Low-intensity voluntary running lowers blood pressure with simultaneous improvement in endothelium-dependent vasodilatation and insulin sensitivity in aged spontaneously hypertensive rats.Hypertens Res2008;31:543-52.
94.Tucker PS,Scanlan AT,Dalbo VJ.High intensity interval training favourably affects angiotensinogen mRNA expression and markers of cardiorenal health in a rat model of early-stage chronic kidney disease.Biomed Res Int2015;2015:156584.doi:10.1155/2015/156584
95.Cunha TS,Moura MJ,Bernardes CF,Tanno AP,Marcondes FK.Vascular sensitivity to phenylephrine in rats submitted to anaerobic training and nandrolone treatment.Hypertension2005;46:1010-5.
96.Haram PM,Kemi OJ,Lee SJ,Bendheim MØ,Al-Share QY,Waldum HL,et al.Aerobic interval training vs.continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity.Cardiovasc Res2009;81:723-32.
97.Williams LM,Campbell FM,Drew JE,Koch C,Hoggard N,Rees WD,et al.The development of diet-induced obesity and glucose intolerance in C57Bl/6 mice on a high-fat diet consists of distinct phases.PLoS One2014;9:e106159.doi:10.1371/journal.pone.0106159
98.Angelova P,Boyadjiev N,Georgieva K,Hrischev P.Effects of the application of a high-fat-carbohydrate diet without additional cholesterol for the inducement of metabolic syndrome in rats.Am Int J Contemp Res2013;3:42-50.
99.Dinh QN,Drummond GR,Sobey CG,Chrissobolis S.Roles of inflammation,oxidative stress,and vascular dysfunction in hypertension.Biomed Res Int2014;2014:406960.doi:10.1155/2014/406960
100.Heitzer T,Schlinzig T,Krohn K,Meinertz T,Münzel T.Endothelial dysfunction,oxidative stress,and risk of cardiovascular events in patients with coronary artery disease.Circulation2001;104:2673-8.
101.van den Oever IA,Raterman HG,Nurmohamed MT,Simsek S.Endothelial dysfunction,inflammation,and apoptosisin diabetes mellitus.Mediators Inflamm2010;2010:792393.doi:10.1155/2010/792393
102.Barbosa VA,Luciano TF,Vitto MF,Cesconetto PA,Marques SO,Souza DR,et al.Exercise training plays cardioprotection through the oxidative stress reduction in obese rats submitted to myocardial infarction.Int J Cardiol2012;157:422-4.
103.Nakanishi R,Ohwaki J,Emoto S,Mori T,Mizuno K,Tsuda T,et al.Nitric oxide concentrations in gas emanating from the tails of obese rats.Redox Rep2013;18:233-7.
104.Yan D,Cheng LF,Song HY,Turdi S,Kerram P.Electrophysiological effects of haloperidol on isolated rabbit Purkinje fibers and guinea pigs papillary muscles under normal and simulated ischemia.Acta Pharmacol Sin2007;28:1155-60.
105.Clayton RH,Bernus O,Cherry EM,Dierckx H,Fenton FH,Mirabella L,et al.Models of cardiac tissue electrophysiology:progress,challenges and open questions.Prog Biophys Mol Biol2011;104:22-48.
106.Wieneke H,Sattler K,von Birgelen C,Böse D,Haude M,Rechenberg W,et al.Impact of intraventricular conduction delay on coronary haemodynamics:a study with intracoronary Doppler in patients with bundle branch blocks and normal coronary arteries.Europace2006;8:151-6.
107.Wallukat G.The β-adrenergic receptors.Herz2002;27:683-90.
108.Zhao Z,Luo Y,Li G,Zhu L,Wang Y,Zhang X.Thoracic aorta vasoreactivity in rats under exhaustive exercise:effects ofLycium barbarumpolysaccharides supplementation.J Int Soc Sports Nutr2013;10:47.doi:10.1186/1550-2783-10-47
109.Lundberg JO,Gladwin MT,Weitzberg E.Strategies to increase nitric oxide signalling in cardiovascular disease.Nat Rev Drug Discov2015;14:623-41.
110.Nosova EV,Yen P,Chong KC,Alley HF,Stock EO,Quinn A,et al.Short-term physical inactivity impairs vascular function.J Surg Res2014;190:672-82.
111.Wang L,Xu F,Zhang XJ,Jin RM,Li X.Effect of high-fat diet on cholesterol metabolism in rats and its association with Na+/K+-ATPase/Src/pERK signaling pathway.J Huazhong Univ Sci Technolog Med Sci2015;35:490-4.
112.West SG.Effect of diet on vascular reactivity:an emerging marker for vascular risk.Curr Atheroscler Rep2001;3:446-55.
113.Davis MJ.Myogenic response gradient in an arteriolar network.Am J Physiol1993;264:H2168-79.
114.Minami K,Kashimura O.Exercise training inhibits vasoconstriction of the rat pulmonary artery.Adv Exerc Sports Physiol2004;10:71-5.
115.Naderali EK,Williams G.Prolonged endothelial-dependentand-independent arterial dysfunction induced in the rat by short-term feeding with a high-fat,high-sucrose diet.Atherosclerosis2003;166:253-9.
116.Song GY,Gao Y,Di YW,Pan LL,Zhou Y,Ye JM.High-fat feeding reduces endothelium-dependent vasodilation in rats:differential mechanisms for saturated and unsaturated fatty acids?Clin Exp Pharmacol Physiol2006;33:708-13.
 Journal of Sport and Health Science2018年1期
Journal of Sport and Health Science2018年1期
- Journal of Sport and Health Science的其它文章
- Research highlights from the Status report for step it up!The surgeon general’s call to action to promote walking and walkable communities
- Environments favorable to healthy lifestyles:A systematic review of initiatives in Canada
- The built environment correlates of objectively measured physical activity in Norwegian adults:A cross-sectional study
- The association of various social capital indicators and physical activity participation among Turkish adolescents
- Feasibility of using pedometers in a state-based surveillance system:2014 Arizona Behavioral Risk Factor Surveillance System
- Matched or nonmatched interventions based on the transtheoretical model to promote physical activity.A meta-analysis of randomized controlled trials
