The corrosion behavior and mechanical property of the Mg-7Y-xNd ternary alloys
Quantong Jiang,Xianzi Lv,Dongzhu Lu,Jie Zhang,Baorong Hou
aCAS Key Laboratory of Marine Environmental Corrosion and Bio-fouling,Institute of Oceanology,Chinese Academy of Sciences,No.7 Nanhai Road,Qingdao 266071,China
bOpen Studio for Marine Corrosion and Protection,Pilot Qingdao National Laboratory for Marine Science and Technology,No.1 Wenhai Road,Qingdao,266235,China
Abstract The corrosion behavior and mechanical property of Mg-7Y-xNd(x=0.5,1.0,1.5wt%)alloys were investigated.The microstructure and precipitations of Mg-7Y-xNd alloys were studied by scanning electron microscopy,energy-dispersive spectrometry and X-ray Diffraction.The quantities of the Mg12(Y,Nd)phase increased,whereas that of the Mg24(Y,Nd)5phase decreased with increasing Nd-content.The weight loss rate decreased from 17.5020 mg cm-2d-1(36.7542 mm y-1)to 9.3744 mg cm-2d-1(19.6862 mm y-1).The electrochemical measurement also demonstrated the similar tendency.The loss in mechanical properties after corrosion reaction followed the order Mg-7Y-0.5Nd>Mg-7Y-1.0Nd>Mg-7Y-1.5Nd.The precipitations played dual roles in the corrosion resistance that depended on type and distribution.
Keywords:Mg-Y-Nd alloy;Microstructure;Corrosion;Mechanical property.
1.Introduction
Magnesium alloys contained rare earths have extensive applications due to their excellent performance[1-4].Yttrium element can re fine grain and improve the strength of magnesium alloys via changing the deformation mechanism[5,6].Suzuki et al.[7]researched the compressive creep behavior and deformation substructures of binary Mg-Y alloys(0.2-2.4mol%),and found that the addition of yttrium improves creep strength of magnesium more efficiently.Neodymium element can enhance the room and high temperature mechanical properties of magnesium alloys[8].Williams et al.[9]Localized corrosion of binary Mg-Nd(Nd=0.47-3.53wt%)alloys in chloride-containing electrolyte using a scanning vibrating electrode technique,and clarified the role of the Nd-rich intermetallic phase on the localized corrosion mechanism.
In the Mg-Y-Nd alloy system,WE43 and WE54 have been successfully developed as the commercial alloys[10-12].Rokhlin et al.[13]researched relationship between characteristic feature and precipitated phase of Mg-Nd-Y alloy,and found the volumetric transformation of solid solution decomposition.Xin et al.[14]researched the effect of aging precipitation on mechanical anisotropy of an extruded Mg-Y-Nd alloy,and demonstrated that aging precipitation largely reduced yield strength anisotropy.Zhu et al.[15]studied the serrated flow and tensile properties of a Mg-Y-Nd alloy,and found that the serrated flow is due to interactions between dislocations and solute atoms.Different kinds of precipitations(Mg3Nd,Mg12(YNd)and Mg3(YNd)et.al)may form in the Mg-Y-Nd system[16].Previous studies have been focusing on the relationship between precipitations and mechanical strength.However,the influence of precipitations with the increasing Nd-content on the corrosion behavior of Mg-YNd alloy remains unclear[17,18].Thus,investigation on this topic is interesting and necessary for scientific studies and practical applications.
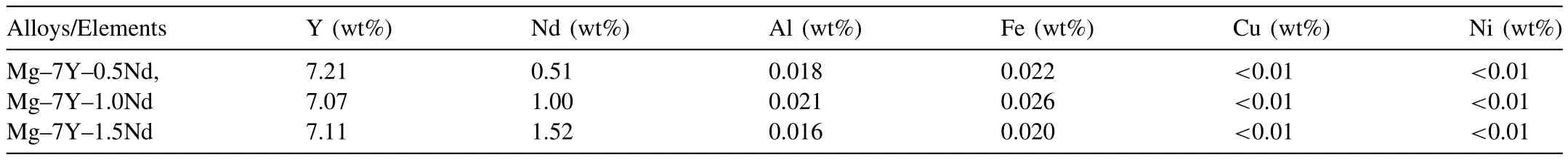
Table 1 Compositions of Mg-7Y-xNd alloys detected by ICP-AES.
In this work,Mg-7Y-xNd(x=0.5,1.0,1.5wt%)alloys were studied to demonstrate precipitations with the addition of Nd-content on corrosion behavior.The microstructure,precipitations and corrosion behavior of the different samples were studied.The effect mechanism was showed that the condition of the precipitations changed from Mg24(Y,Nd)5to continuous Mg12(Y,Nd)with the increasing Nd-content.The precipitation strengthening and barrier effect of Mg-7Y-1.5Nd alloys led to improved corrosion resistance and mechanical property.
2.Experimental
2.1.Material preparation
Mg-7Y-xNd(x=0.5,1.0,1.5wt%)alloys were prepared by melting in an electrical resistance furnace in a steel crucible under the protection of the Argon and tetra fl uoroethane gas.The commercial purity Mg(>99.95%),Y(99.9%),and Nd(99.9%)materials were used during the melting of the Mg-7Y-xNd alloys.The maximum smelting temperature was not above 850°C,whereas the cast temperature was between 720 °C and 750 °C.The nominal component of the experimental samples was Mg-7Y-0.5Nd,Mg-7Y-1.0Nd and Mg-7Y-1.5Nd,respectively.
2.2.Material characterization
The actual compositions of Mg-7Y-xNd alloys,along with the major impurity levels,were detected by inductively coupled plasma atomic emission spectrometry(ICP-AES;Table 1).
The microstructure was measured via scanning electron microscopy.Energy-dispersive spectrometry technology was used to analyze the components of Mg-7Y-xNd alloys.More than three replicate spots were tested in order to ensure the reliability of the experimental results.X-ray diffraction was employed to identify the types of different phases via CuKα radiation operated at 40kV and 40mA.
2.3.Corrosion and mechanical properties
The Mg-7Y-xNd alloy samples were polished by using the abrasive paper for metallograph.The original weight(W0)of the samples was tested via the analytical balance.And then the immersion test was completed in 3.5%sodium chloride solution for 72h.There were three replicate samples for each alloy in order to ensure the reliability of the experimental results.After the immersion test,the boiling chromic acid(20%chromium trioxide+1%silver nitrate)was used to remove the corrosion products on the surface of the Mg-7Y-xNd alloy samples[19].The samples without corrosion products were washed with deionized water and ethyl alcohol successively.When the samples were dry,the final weight(W1)was obtained via the analytical balance.The original weight(W0)minus final weight(W1)is the weight loss(△w)during the corrosion reaction.The surface morphologies of the samples without corrosion products were characterized via scanning electron microscopy.
Open circuit potential,potentiodynamic polarization and electrochemical impedance spectroscopy were tested using a Princeton P4000+Electrochemical workstation.The three electrode system was held at the open circuit potential for 400s,and then the potentiodynamic scanning was carried out at 0.5mV/s.Electrochemical impedance spectra was tested with the frequency ranged from 10,000Hz to 0.01Hz.The tensile samples were processed according to the ISO 6892-1:2009 standard.The tensile samples were exposed in 3.5%sodium chloride salt spray for 72h.A tensile test machine was employed to measure the degradation of the mechanical strength.
3.Results and discussion
3.1.Microstructure of Mg-7Y-xNd alloys
Fig.1(a),(c)and(e)shows that the microstructure of Mg-7Y-xNd alloys was mainly composed of the α-Mg matrix and precipitations.A large quantity of precipitations was distributed throughout the α-Mg matrix and slightly dispersed in morphology.The previous study demonstrated that the precipitations mainly served as cathode of the electrochemical reaction to accelerate corrosion[20].Precipitations showed a continuous uniform distribution gradually with the increasing Nd-content.
Fig.1(b),(d)and(f)shows the images of EDS analysis for different samples,and results are shown in Table 2.The gray matrix location(marked “A”)mainly contained magnesium element,and only very little yttrium and neodymium elements existed.The white contrast particle phase was marked as “B”,whereas the other phase was marked as “C”.It is obvious that there was a larger yttrium content in B phase than C phase,but the neodymium element showed the opposite trend.The EDS results of different alloys,showed that spot B was arranged to be Mg24(Y,Nd)5,whereas spot C was considered to be Mg12(Y,Nd).The contents of yttrium and neodymium in the locations “B”and “C”were large due to the beneficiation of solute atoms in the solid/liquid interface during non-equilibrium solidification[21-23].

Fig.1.SEM and EDS analysis of different samples:(a,b)Mg-7Y-0.5Nd;(c,d)Mg-7Y-1.0Nd;and(e,f)Mg-7Y-1.5Nd.
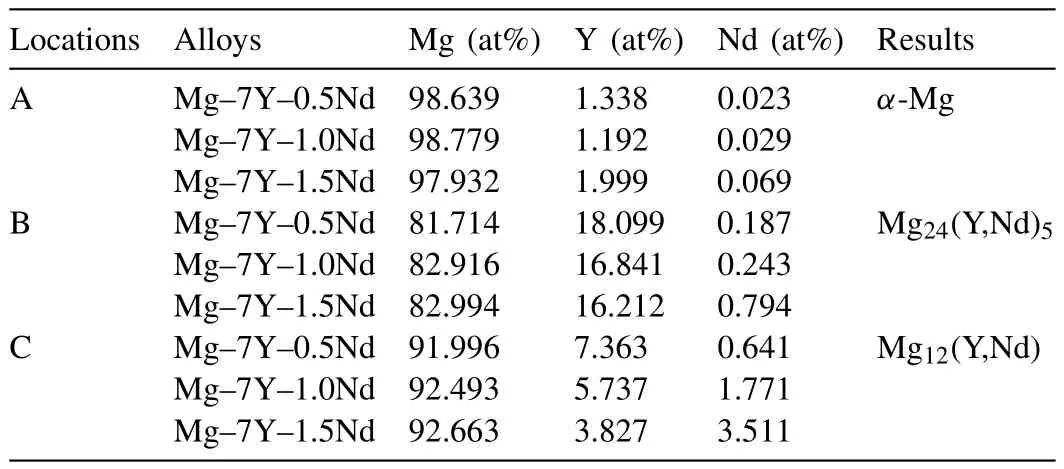
Table 2 EDS results of different samples Mg,Y and Nd(at%).
XRD analysis(Fig.2)revealed obvious Mg diffraction peaks.Moreover,both Mg24(Y,Nd)5and Mg12(Y,Nd)precipitations were detected,but the characteristic peak intensity of Mg24(Y,Nd)5decreased with the increasing Nd-content,whereas Mg12(Y,Nd)showed the opposite trend.Actually,both theMg24(Y,Nd)5and Mg12(Y,Nd)were consisted of α-Mg,Mg24Y5and Mg41Nd5.There are two balance reactions in the Mg-Y-Nd alloy system(α-Mg ↔Mg24(Y,Nd)5↔Mg24Y5;α-Mg↔ Mg12(Y,Nd)↔ Mg41Nd5).The spot “B”contained amounts of α-Mg and Mg24Y5,but less Mg41Nd5,and the components of precipitated phase were Mg24(Y,Nd)5.On the other hand,the spot“C”contained amounts of α-Mg and Mg41Nd5,but less Mg24Y5,and the components of precipitated phase were Mg24(Y,Nd)5.With the increase in Nd-content,more and more Mg41Nd5formed,so the amount of Mg24(Y,Nd)5decreased,whereas the Mg12(Y,Nd)increased.Previous studies showed,that Mg12(Y,Nd)phase was the common phase in Mg-Y-Nd system,whereas the Mg24(Y,Nd)5phase rarely existed in the grain boundary[24,25](Fig.3).

Fig.2.X-Ray diffraction analysis of Mg-7Y-xNd samples.

Fig.3.Weight loss rate of Mg-7Y-xNd:(a)△W(mg cm-2d-1),(b)PW(mm y-1).
3.2.Surface morphologies and weight loss rate
The corrosion rate is set as△W(mg cm-2d-1),which is calculated via the formula:△W=(W0-W1)/ST.In the formula above,W0(mg)represents the original weight,whereas W1(mg)is the final weight without the corrosion product.S(cm2)represents the surface area of the samples,and T(d)represents the period of immersion tests.The corrosion rate in the formula represents an average measurement over the entire sample area during the whole immersion tests.Moreover,the corrosion rate PW(mm y-1)was calculated via the formula[26-28]:

The corrosion rate of Mg-7Y-0.5Nd samples showed a maximum value at 17.5020 mg cm-2d-1(36.7542 mm y-1),whereas that of Mg-7Y-1.5Nd samples showed a minimum value.The corrosion rate of Mg 7Y 1.0Nd samples was intermediate.This phenomemon was mainly due to the corrosion barrier of continuous Mg12(YNd)phases.All the samples had a larger corrosion rate than high-purity Mg(1.38 mm y-1)[29].This phenomemon was mainly because that the precipitations accelerated the degradation of α-Mg matrix.Alloying in magneisum cannot impart appreciable corrosion resistance and the corrosion rate is proportional to increase.Elements Y and Nd in Mg-Y-Nd system has a very large solubility and can be exploited to retard anodic reaction kinetics rather well.On the other hand,elements Y and Nd given the low exchange current density of Mg,essentially all alloying additions will enhance cathodic activity and as is the case here,such increases outpaced anodic retardation such that the Mg-Y-Nd system was under cathodic control.
The corrosion morphology was characterized by scanning electron microscope.The sample surfaces covered with corrosion products are shown in Fig.4(a),(c)and(e).Difference in surface appearances was distinct obvious.Fig.4(a)shows that Mg-7Y-0.5Nd alloy was covered with a large amount of corrosion products,which was like a dry riverbed,whereas a few corrosion products were deposited on the immersed surface of Mg-7Y-1.0Nd alloy.However,Mg-7Y-1.5Nd alloy was corroded the slightest,as shown in Fig.4(e),which had a more compact corrosion products formed on the surface.The sample surfaces were not covered with corrosion products,shown in Fig.4(b),(d)and(f).Fig.4(b)shows that the attack of Mg-7Y-0.5Nd sample occurred obviously,largely and deeply.The corrosion attacks and shallow pits of Mg-7Y-1.0Nd sample surface were irregularly distributed in Fig.4(e),whereas the Mg-7Y-1.5Nd sample surface showed the slightest micro-crack damage in Fig.4(f).
To research the influence of precipitations with increasing Nd-content on corrosion property,the high-resolution surface morphology and energy dispersive spectrum analysis of Mg-7Y-0.5Nd alloy were studied in Fig.5.Both the uncorroded α-Mg matrix and the precipitations were dispersively distributed in the surface.This corrosion morphology was due to the fact that the micro-galvanic influence led to a prior degradation of α-Mg matrix.On one hand,the granular Mg24(Y,Nd)5phases distributed uniformly to accelerate the corrosion.On the other hand,continuous Mg12(Y,Nd)phases formed a barrier to protect the α-Mg matrix.Thus,this phenomenon showed that corrosion rate of the Mg-7Y-xNd samples was attributed to the type and distribution of the precipitations.
3.3.Electrochemical characterization

Fig.4.Corrosion morphology of the different samples:(a)Mg-7Y-0.5Nd alloy with corrosion products,(b)Mg-7Y-0.5Nd alloy without corrosion products,(c)Mg-7Y-1.0Nd alloy with corrosion products,(d)Mg-7Y-1.0Nd alloy without corrosion products,(e)Mg-7Y-1.5Nd alloy with corrosion products and(f)Mg-7Y-1.5Nd alloy without corrosion products.

Fig.5.High resolution surface morphology and energy dispersive spectrum analysis of Mg-7Y-0.5Nd alloy without corrosion products.
The open circuit potential of Mg-7Y-xNd samples was comparatively stable before measurement.The open circuit potential and Tafel slopes of Mg-7Y-xNd samples measured in 3.5%NaCl solution are shown in Fig.6.The Mg-7Y-1.5Nd sample showed a most positive open circuit potential,indicated that the Mg-7Y-1.5Nd alloy had a lowest electrochemically activity.The Mg-7Y-0.5Nd sample showed a most negative open circuit potential,whereas the Mg-7Y-1.0Nd sample was the intermediate.A very interesting phenomenon was found that the cathodic polarization curves were almost exactly alike.However,the anode polarization curves of Mg-7Y-xNd samples relatively differed.The corrosion current density icorr(mA/cm2)of Mg-7Y-xNd alloys was calculated via the Tafel extrapolation of the cathodic polarization curve,and the average corrosion rate Piwas calculated via the formula[30,31]:

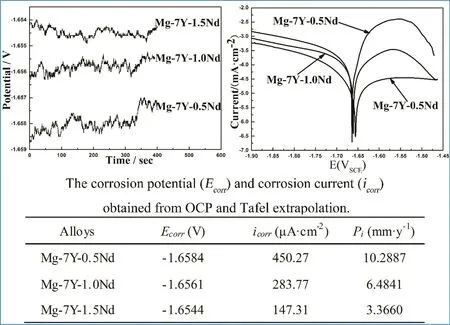
Fig.6.Open circuit potential and Tafel slopes of different samples measured in 3.5%NaCl solution.
The corrosion potential(Ecorr)and corrosion current(icorr)obtained from open circuit potential and Tafel extrapolation are also shown in Fig.6,which suggested that degradation rate of α-Mg matrix the followed the order:Mg-7Y-0.5Nd>Mg-7Y-1.0Nd>Mg-7Y-1.5Nd.The Mg-7Y-0.5Nd samples had the highest corrosion current density,because the amount of particle-phase Mg24(Y,Nd)5as the cathode of the electrochemical reaction accelerated the corrosion.The icorrof Mg-7Y-xNd samples decreased with the increasing Nd-content.The continuous distribution of precipitations formed the corrosion barrier to protect the Mg matrix,consistent with literature[32].
The EIS data of Mg-7Y-xNd alloys exhibited different characterizations of electrochemical reaction,as illustrated in Fig.7.The spectra of different alloys consisted of one capacitive reactance at high frequency after immersion in 3.5%NaCl solution,whereas one inductive reactance existed at low frequency.The equivalent circuit was used to fit the electrochemical impedance spectra plots.In the circuit,Rsis the solution resistance,Rtis the charge transfer resistance,and Qdlis the constant phase element of double layer.RLand L represent the pseudo resistance and inductance derived from the breakdown of the corrosion product film[33].
The different EIS spectra implied different electrochemical dynamic processes.The corrosion products formed due to the immersion of samples in sodium chloride solution;thus,the capacitive reactance was due to the double layer on the surface of Mg-7Y-xNd alloys.The corrosion rate evaluated from the capacitive dimension in the EIS spectra followed the order Mg-7Y-0.5Nd>Mg-7Y-1.0Nd>Mg-7Y-1.5Nd,which represented different charge-transfer resistances.With prolonged immersion time,the desorption of corrosion product film and movement of Mg+led to inductive reactance[34,35].
The polarization resistance Rpis an important parameter,becauseis believed to be proportional to the corrosion rate.Polarization resistance Rpcan be calculated via the formula on the basis of the equivalent circuits:

The icorr/EIS(mA cm-2)was calculated from the polarization resistance RP(Ω cm-2)via the formula[36]:

In the formula above,βaand βcrepresents the anodic Tafel slope and the cathodic Tafel slope,respectively.And B is a constant involved in the Tafel slope.The average corrosion rate Pi,EISwas calculated via the formula[37]:


Fig.7.Electrochemical impedance spectra of different samples.
The corrosion current density (icorr,EISand Pi,EIS)of Mg-7Y-xNd alloys obtained from electrochemical impedance spectra was shown in Fig.7.The Mg-7Y-1.5Nd sample showed a minimum corrosion current density(3.2959 mm y-1).This result demonstrated that the degradation of the α-Mg matrix was most difficult for the Mg-7Y-1.5Nd sample.Mg24(Y,Nd)5and Mg12(Y,Nd)precipitations were the common phases in Mg-Y-Nd based alloy.The amount of Nd element increased from 0.5 to 1.5(wt%),indicating that corrosion rate decreased with increased Mg12(Y,Nd)phase,whereas Mg24(Y,Nd)5phase decreased.The above conclusion clearly indicated that the type and distribution of precipitations played an important role in the corrosion resistance of the Mg-7Y-xNd alloys[37].Moreover,the influence mechanisms of Mg12(Y,Nd)and Mg24(Y,Nd)5phases on the corrosion properties were not expounded on clearly in this study.Thus,the relationship between precipitated phases and corrosion resistance requires futher research.
3.4.Mechanical property
As shown in Fig.8,the loss of the mechanical property of Mg-7Y-xNd alloys after corrosion salt spray was tested.Apparently,the Mg-7Y-1.5Nd alloy presented a minimum loss of mechanical property.After the corrosion salt spray test,the tensile strength of the Mg-7Y-1.5Nd sample decreased from 285 to 265 Mpa,resulting that the loss ratio was merely 7.02%.Conversely,the tensile strength of the Mg-7Y-0.5Nd sample decreased from 257 to 228 Mpa,and the loss ratio reached up to 11.28%.The loss of the mechanical property of Mg-7Y-1.0Nd alloy was in the intermediate level.Exfoliation corrosion led to the decreasing of the mechanical property for magnesium alloy after the salt spray test[38].
To research the corrosion crack-initiation mechanism,fracture appearance of the tensile sample was analyzed.For the uncorroded samples in Fig.9(a),(c)and(e)show that the fracture appearances mainly consisted of many voids,indicating the alloys had ductile fracture[39,40].However,cracks can be observed on the fracture surface in Fig.9(b),(d)and(f).Generally,cracks preferentially initiated at corrosion pits on the surface of the samples during the tensile test.
The corrosion of magnesium alloy in an aqueous solution includes both the Mg anodic dissolution and the cathodic hydrogen evolution.Thus,hydrogen evolution reaction plays an important role in the environment-assisted cracking of magnesium alloys[41].During the corrosion reaction,hydrogen diffuses into the magnesium matrix through the passage of the corrosion pits,which led to the hydrogen embrittlement and decrease the mechanical property.Based on theoretical extrapolation,Atrens et al.suggested that the hydrogen diffusion coefficient in magnesium at ambient temperature can sufficiently allow for significant hydrogen transport[42].Therefore,the poor corrosion resistance of Mg alloys caused corrosion pits to form on the surface of the tensile sample,which resulted in large size cracks in the fracture.
3.5.Corrosion mechanism
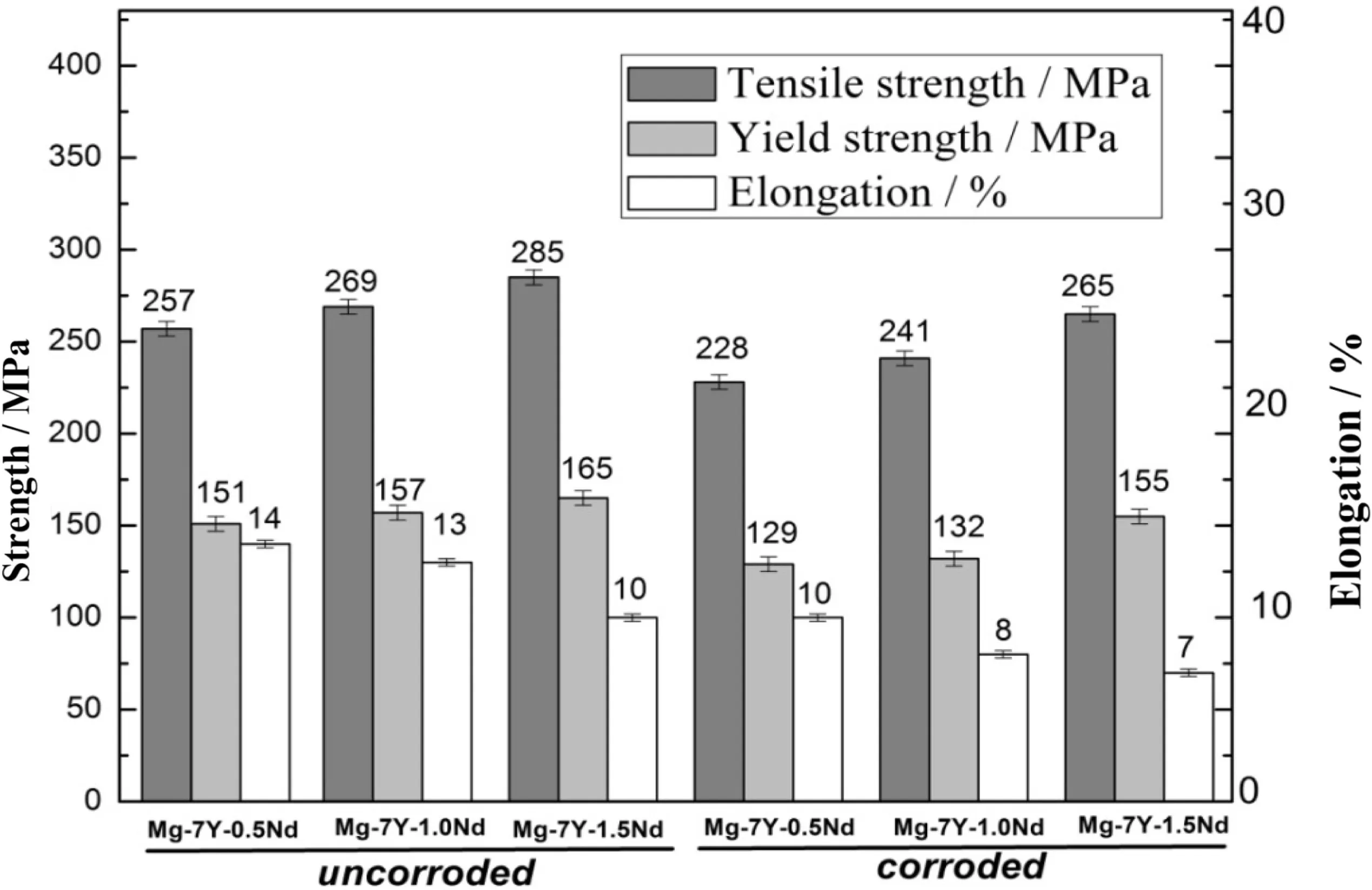
Fig.8.Loss of the mechanical property of the Mg-7Y-xNd alloys after corrosion salt spray test.

Fig.9.Fracture appearance of Mg-7Y-xNd alloys after exposure in the corrosion environment.
The schematic of microstructure related to the corrosion mechanism is shown in Fig.10.Actually,a difference exists in the volume fraction and distribution of Mg12(Y,Nd)and Mg24(Y,Nd)5phases among the three alloys.The volume fraction of Mg12(Y,Nd)phases in the Mg-7Y-0.5Nd sample showed the lowest value,whereas that of the Mg24(Y,Nd)5phases showed the opposite trend.However,the distinct location indicated that the Mg12(Y,Nd)phases of the Mg-7Y-1.5Nd sample nearly connected into nets in many areas.Compared with grain size,the precipitations played a dual role in the corrosion resistance for magnesium alloys according to the classical theory[43].The type and distribution determine that precipitations play dual roles in the corrosion of magnesium alloys.The precipitations acted as an effective galvanic cathode can accelerate corrosion process,whereas a fine and homogeneous phase can form an anti-corrosion barrier to protect the magnesium matrix.Then,the interconnected precipitations improve the corrosion properties.
The crystal structure of Mg24(Y,Nd)5is similar to that of Mg24Y5,which is a body-centered cubic(BCC)cell,whereas Mg12(Y,Nd)phase is orthorhombic according to the previous study[16].Fig.11 shows schematics of precipitated phase unit cells.The density of a BCC cell is larger than an orthorhombic cell.A larger density cell means that bodycentered cubic(BCC)is more stable than orthorhombic cell,which results in a more positive standard electrode potential.Thus,in the present work,the Mg24(Y,Nd)5and Mg12(Y,Nd)phases acted as the cathode to accelerate the corrosion of α-Mg.However,the accelerating force of the Mg24(Y,Nd)5phase was larger because of the larger potential difference between the precipitated phase and α-Mg.Therefore,the amount of Nd element increased from 0.5wt%to 1.5wt%,indicating that corrosion rate decreased with increased Mg12(Y,Nd)phase,whereas Mg24(Y,Nd)5phase decreased.Accordingly,the microstructure of Mg-7Y-xNd alloys played an major role in corrosion behavior,which mainly depended on the volume fraction of Mg12(Y,Nd)and Mg24(Y,Nd)5phases.The relationship between microstructure and corrosion property can be confirmed by the type and distribution status of the precipitation.

Fig.10.Schematic of microstructure related to the corrosion mechanism:(a)Mg-7Y-0.5Nd alloy,(b)Mg-7Y-1.0Nd alloy,(c)Mg-7Y-1.5Nd alloy.

Fig.11.Schematics of precipitated phase unit cells:(a)Mg24(Y,Nd)5(b)Mg12(Y,Nd).
Fig.1(a),(c)and(e)shows that particle Mg24(Y,Nd)5phases that precipitated were in the grain and along the grain boundary,which served as the cathode of electrochemical reactions to accelerate the corrosion.However,with increased of Nd-content,the Mg12(Y,Nd)phases of Mg-7Y-1.5Nd alloys were along the grain boundary,and an amount of the precipitations formed a continuous distribution in many areas on the grain boundaries,which served as the corrosion barrier.Therefore,the precipitations played an important role in corrosion resistance.
4.Conclusion
(1)Mg-7Y-xNd alloys(x=0.5,1.0,1.5wt%)were prepared to study the microstructure and corrosion behavior with the increasing Nd-content.The Mg12(Y,Nd)and Mg24(Y,Nd)5precipitations were found in the Mg-7Y-xNd alloys.The quantity of the Mg12(Y,Nd)precipitations became larger with the increasing Ndcontent,whereas that of the Mg24(Y,Nd)5precipitations showed the opposite trend.
(2)The weight loss rate decreased from 17.5020 mg cm-2d-1(36.7542 mm y-1)to 9.3744 mg cm-2d-1(19.6862 mm y-1)with the increasing Nd-content from 0.5wt%to 1.5wt%.The electrochemical measurement also demonstrated the similar tendency.The Mg-7Y-1.5Nd sample showed a lowest electrochemically activity than other samples.The corrosion current density suggested that degradation rate of α-Mg matrix the followed the order:Mg-7Y-0.5Nd>Mg-7Y-1.0Nd>Mg-7Y-1.5Nd.
(3)Mg-7Y-1.5Nd samples had a better corrosion resistance and mechanical property.The phases played dual roles that depended on amount and distribution.The amount of particle-phase Mg24(Y,Nd)5as the cathode of the electrochemical reaction accelerated the corrosion,whereas the continuous distribution of Mg12(Y,Nd)precipitations formed the corrosion barrier to protect the Mg matrix.
Acknowledgment
The authors gratefully acknowledge the National Natural Science Foundation of China(Grant No.51501181),the Fundamental Research Project of Technology Program of Qingdao(17-1-1-76-JCH),the Key Research and Development Program of Shandong Province(2017GGX20139)for providing support for this work.
 Journal of Magnesium and Alloys2018年4期
Journal of Magnesium and Alloys2018年4期
- Journal of Magnesium and Alloys的其它文章
- Review on friction stir welding of magnesium alloys
- Rietveld re finement of powder X-ray diffraction,microstructural and mechanical studies of magnesium matrix composites processed by high energy ball milling
- Effect of Si addition on microstructure and wear properties of Mg-Sn as-cast alloys
- Atomistic calculations of surface and interfacial energies of Mg17Al12-Mg system
- Effect of process parameters on depth of penetration and topography of AZ91 magnesium alloy in abrasive water jet cutting
- Corrosion protection of AZ91D magnesium alloy by acerium-molybdenum coating-The effect of citric acid as an additive
