Overview of Graphene as Anode in Lithium-Ion Batteries
Ri-Peng Luo, Wei-Qiang Lyu, Ke-Chun Wen, and Wei-Dong He
1.Introduction
With high energy density and favorable cyclic stability,lithium-ion batteries (LIBs) have been widely applied as the main power supplies of portable electrical devices, such as mobile phones, laptops, and other digital products. However,the limitations of traditional anode/cathode materials including the poor energy capacity, short cyclic life, and low power density, have impeded the development of high-performance LIBs for electrical vehicles and energy storage systems[1]-[4].During cycling, the graphite-based anode is likely to undergo layers exfoliation and mechanical fracture due to constant volumetric changes and the formation of lithium dendrite,detrimental to the cyclic performance of the batteries. Constant decomposition and recovery of solid-electrolyte-interface (SEI)films are major consumption of recyclable lithium and electrolyte, leading to capacity fading, especially at high temperature. Under decades of development, the new anode materials are required to meet the demand for further application or marketization, in following aspects: 1) high specific capacity for the development of high cruising batteries for electrical vehicles; 2) high safety performance with high thermal stability and structural stability; 3) high rate performance with moderate electrochemical reactivity from kinetics perspective. Graphene with attractive behaviors is promising to make revolutionary breakthroughs in the field of electrochemical energy storage.
Since found in 2004 by Novoselov et al.[5], graphene formed by a flat monolayer of carbon atoms with the structure of two-dimensional (2D) honeycomb lattice, has drawn extensive attention. Plentiful efforts have been devoted to exploring its properties and applications. As a basic building block for graphitic material, graphene can be processed into the 0-dimensional fullerenes, 1-dimensional nanotubes or even 3-dimensional graphite[5]-[9]. In comparison with graphite,graphene has larger specific surface area, higher electronic and thermal conductivity[10]-[12]. The performance of graphene-based LIBs is promoted significantly due to its inherent excellent properties. For instance, the exceptional high electronic conductivity enables the electron to move freely and thus decreases electrical impedance and polarization during the electrochemical process, accelerating the electrochemical process[10],[13],[14]. Substantial heat release can be significantly reduced during cycling on account of the relatively low resistance, guaranteeing the moderate working temperature for the battery pack. In addition, during lithium insertion and exertion upon electrode, the inevitable swell and flex may possibly lead to destruction and pulverization of morphological topology and microstructure, degrading the cyclic stability and service life. The desirable mechanical behaviors of graphene[10],[13],[15], can maintain the structural stability of functional materials and address the problems mentioned above[13], In addition, the large specific area of graphene provides mass channels for ionic transport and intercalation,promoting the rate performance of LIBs in theoretical dynamics[5],[13]. Hence, graphene is promising to assist in developing significantly enhanced LIBs from all-round dimensions.
The rise of graphene contributes to the increasing investigations of other 2D inorganic materials, including transition metal oxides, transition metal sulfides, and other graphene analogues (silicene, phosphene, borophene)[16]. The success in the exfoliation of these 2D functional materials leads to the applications in high-efficiency energy storage devices.Layered silicon-nanosheets with high theoretical capacity are thermally unstable upon active interaction, which is the major disadvantage in compared with graphene. Unlike graphene, the empty d-orbits make silicon-based nanosheets perturbed under exterior disturbance, such as light radiation. Layered black phosphorus can be mechanically removed from the bulk volume. Its anisotropy presents desirable properties in optical,electronic, chemical application. Layered black phosphorus shows wide bandgap of black phosphorus (0.76 eV to 2.1 eV).However, black phosphorus suffers from environmental instability and poor conductivity[17], 2D transition metal oxide and sulfide have attractive applications in LIBs,supercapacitors, solar cell and other devices, due to their highly-active catalytic property. However, in comparison with graphene, they are inferior in the charge mobility and specific surface area and cost-efficiency.
To date, the development of graphene in LIBs has been greatly promoted, but still with problems and challenges to deal with, such as poor power density[18], cost concerns, impurities,defects, scalability, etc. Scientists have achieved the flawless single layers in the experiments by mechanical exfoliation,which is laborious and impractical for graphene to be industrially scalable. And other commonly-utilized methods include solvent-based approaches, the reduction of grapheneoxide, substrate- based approaches and total organic synthesis.But none is perfect and needed to be modified to handle either quality or quantity concerns. Whether the mass-produced application is realized in the industrial manufacture and whether the quality of industrial-produced one equates with that of in laboratories, determine whether graphene could attain great success in many fields, especially in energy storage. Thus,simultaneous efforts should be placed in exploring efficient applications as well as finding the way to boost the throughput of high-quality graphene[9].
So far, profuse investigations have been devoted to applying graphene as components in LIBs. And attempts have been made to produce new types of batteries by adding graphene, including the graphene nanosheets battery, the reduced-graphene oxide/SnO2composite battery, the reducedgraphene oxide/Si composite battery, the reduced-graphene oxide/transition metal oxide composite battery, etc. And each research brings the enhancement of LIBs performance.Additionally, it seems to be inherently perfect for graphene to be used in highly-anticipated wearable electronic equipment with a tendency of thinner, lighter, flexible, stretchable and transparent. Currently, the upsurge of metallic lithium anode has been provoked due to its high power and high capacity.However, intrinsic major hurdles of metallic lithium largely hinder its applications. Zhang et al.[19]applied chemiadsorption with lithiophilic sites in N-doped graphene to guide uniform lithium deposition for dendrite-free lithium surface. Lin et al.[20]designed stable host, reduced graphene oxide with molten lithium infusion, to accommodate the constant dimensional change in metallic lithium.
Based on the situation discussed above, this review will focus on the analysis of advantages and the drawbacks of graphene of current synthesis methods and some common applications in LIBs and discuss some insightful perspectives on the prospect of graphene in LIBs for energy storage.
2.Alternatives to Mechanical Exfoliation
Graphene is a two-dimensional carbon sheet with a honeycomb structure, resulted from the hybridization of three energy-closing atomic orbitals (s, px, and py). The strong covalent sp2bonds bridge the interconnections of each carbon atom and form uniform 120° atomic bond angle. The leftover pzorbitals overlap together generates a delocalized π*band,strengthening the structure stability and allowing the delocalized electrons to transport rapidly. However, despite the general outstanding properties of graphene, the characterization and morphology, such as the number of layers and defects density, vary with different synthesis methods, leading to various electrochemical properties. Therefore, the importance of synthesis methods should be never overemphasized.
Tremendous alternative methods to prepare single nanosheets have been investigated. A few of them have brought some enlightenment for the future work for promising devices. For the time being, the alternatives to mechanical exfoliation mainly consist of four practical total methods:chemically reduction of graphene from graphite oxide,exfoliating and stabilizing nanosheets in solution in a chemical way, catalyzing the in-situ generating of graphene on a substrate, directly growing the single layer from organic precursors from the bottom. However, neither of these approaches is perfect with different issues, which will be discussed in detail as follows.
Despite tremendous new approaches have been put forward, mechanical exfoliation with cellophane tape is the best option to obtain highest quality flake combined with the merits of low-cost accounting and simple synthesis process. But with this approach, it is impractical to be industrially scalable and the size of graphene cannot be automatically controlled.Therefore, to realize the utilization and commercialization of graphene, efforts should be focused on exploring more efficient and feasible methods of mass-produced and high-quality graphene for industrial manufacture.
2.1 Chemically/Electrochemically Reduced Graphene from Graphite Oxide
As the most commonly-used synthesis method of graphene oxide (GO), Hummers’ method, as shown in Fig. 1[9],is developed several decades ago and still in wide use today with slight modification[21]-[22]. Layered stack of graphene-oxide is obtained with AB stacking structure. The reduction methods adopted for the reduction of GO primarily consist of chemical ways with reductants, electron beam, and thermal reduction.The deposited carbon obtained by the method with hydrazine hydrate as reductants generally has a high surface area, a high ratio of C/O as well as an exceptional high electrical conductivity, consistent with highly-reduced morphology.However, with the application of reductants, these chemical methods often introduce impurities, as shown by kinds of spectroscopic techniques such as element analysis and X-ray photoelectron spectroscopy (XPS). The quality of the samples produced by thermal reduction is often close to those obtained by chemical approaches discussed above. The product of thermal reduction process is in high consistency with that of reducing with reductants, but it generally avoids introducing heteroatom impurities. The recently-developed electrochemistry methods with a simple process can evidently save the time of reduction and get rid of aggressive reductants in contrast with the pristine methods[24].
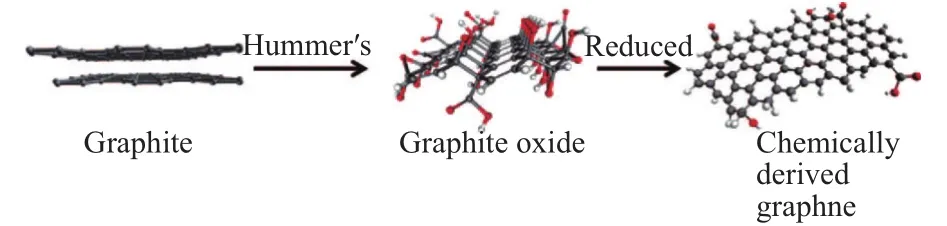
Fig. 1. Molecular models show the conversion process from graphite to chemically derived graphene[9].
The most extraordinary merits of the GO method lie in the low expense and considerate output, based on which the reduction approach is most commonly applied to massive production. Further, with this synthesis method, versatile graphene with a variety of morphologies are produced to be applied in tremendous composite materials[6],[7]. However, there is no way to produce high-quality graphene sheets without crystal boundary as well as crystal defects[7],[8]. The inevitable defects aroused amid oxidation and exfoliation processes are attributed to higher levels of electrode resistance, which lower the performance of lithium-ion batteries. So efforts should be made to improve the flatness of graphene sheet[7].
2.2 Liquid-Phase Synthesis Method
Another most widely-utilized synthesis method is liquidphase synthesis method. In this process, graphite is dispersed and exfoliated in aqueous solution to prepare suspensions of small graphitic flakes, ultrasonication serves as a powerful tool to separate the graphitic building block. Shown by the transmission electron microscopy (TEM), the graphene nanosheet obtained by the liquid-based method is defect-free and slightly oxidized, which is fairly favourable for the utilization in electrode material, as shown in Fig. 2[25]. However,the challenge remains in the relatively low yield and output,since it is difficult to overcome the surface energy in aqueous solution. To solve this challenge, the adoption of organic solvent, such as N-methyl-pyrrolidone, is put forward,combined with the disadvantages of high cost and high boiling points, which make it hard to deposit single layers by drying the solvent[6],[25].

Fig. 2. Selected TEM images of flakes prepared by surfactant processing: (a) a monolayer (albeit with a small piece of square debris close to its left-hand edge), (b) a bilayer, (c) a trilayer, (d) a disordered multilayer, (e) a very large flake, inset: A closeup of an edge of a very large flake showing a small multilayer graphene flake protruding, and (f) a monolayer from a sample prepared by sediment recycling[25].
2.3 Epitaxial Synthesis and Chemical Vapor Deposition of Graphene
As two main substrate-based approaches, epitaxial synthesis and chemical vapor deposition (CVD) allow the growth of graphene with high quality and large area. In the epitaxial method, graphene results from the reduction of silicon carbide at elevated temperature (1000 °C) in ultrahigh vacuum.After the desorbing and subliming of the silicon wafer, there leaves behind granular graphitized carbon on the substrate. As shown in Fig. 3[26],[27], an ideal density of defects is observed but it is hard for layer control upon this method. Furthermore, since silicon carbide substrates are expensive and the graphene formed on the original substrate is difficult to transfer,therefore, this method is not widely applied.
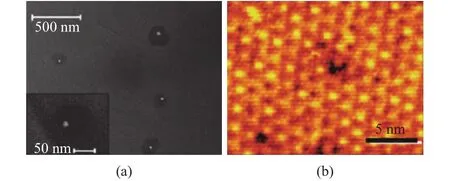
Fig. 3. Silicon carbide is reduced to graphene as silicon sublimes at high temperature: (a) scanning electron microscope (SEM) image shows small hexagonal crystallites and (b) STM image shows long-range order and a low density of defects[26],[27].
Another substrate-based approach is CVD, where graphene grows on the substrate of the transition metal. In general, nickel and copper films are used as the substrate and CH4acts as a carbon source. At elevated temperature, transition metal with high carbon-saturation absorbs the maximum amount of carbon as contacted with hydrocarbon gas. During the cooling process,the solubility of carbon decreases; a thick film of carbon is deposited on the surface of the substrate. After etching the underlying metal, the graphene sheet is free to be transferred to other targeted substrates. A large surface area can be achieved by this synthesis approach. The high quality and purity could pronouncedly improve the performance of graphene, as shown in Fig. 4[27]. However, the existing challenges are lacking applicable control over film thickness as well as avoiding secondary crystal formation[9]. Unless the price concerns eliminated, this approach is not suitable for massive marketization.
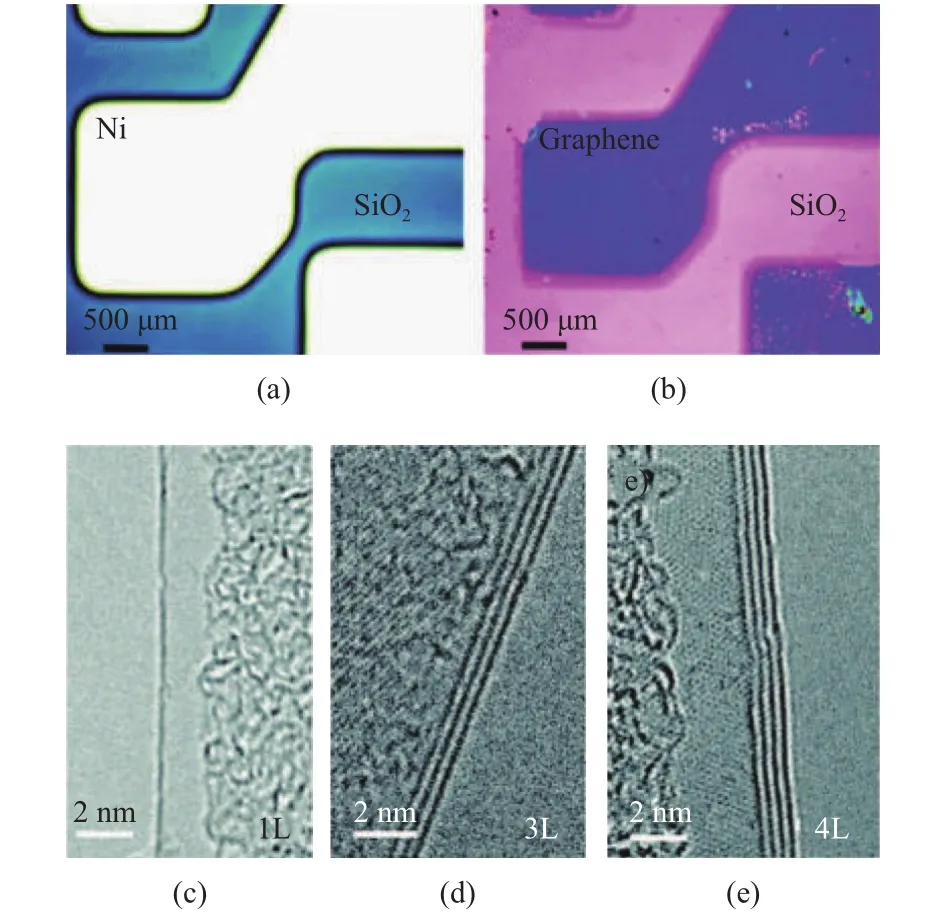
Fig. 4. Chemical vapor deposition of graphene on transition metal substrates. Optical microscope image of (a) the nickel catalyst and(b) the resulting graphene film. TEM images show the nucleation of (c) one, (d) three, or (e) four layers during the growth process[27].
2.4 Total Organic Synthesis
With unique structure similar between “molecular” and“macromolecular”, graphene-like polycyclic hydrocarbons(PAHs) attracts great interest in preparing graphene due to their exceptionally versatile properties and high purity.Additionally, the plane, graphene-like, two-dimensional material wins the name of “porous graphene”. The pores are under well control to achieve expected separation of gases and molecules. Organic synthesis approach provides highquality graphene as the electrode material in lithium-ion batteries[9]. However, the drawback lies in the limitation of the size range. As the molecular weight increases, the decreasing of solubility and the arising of side-reactions, have hindered the fabrication of large-area graphene nanosheet. It is a challenge to preserve the dispersibility and planar morphology of large PAHs, as shown in Fig. 5[28]. The high cost and low productivity limit the use of lithium-ion batteries. So it is vital to exploit new methods to further enlarge the size of PAHs lower the cost in the future[6].
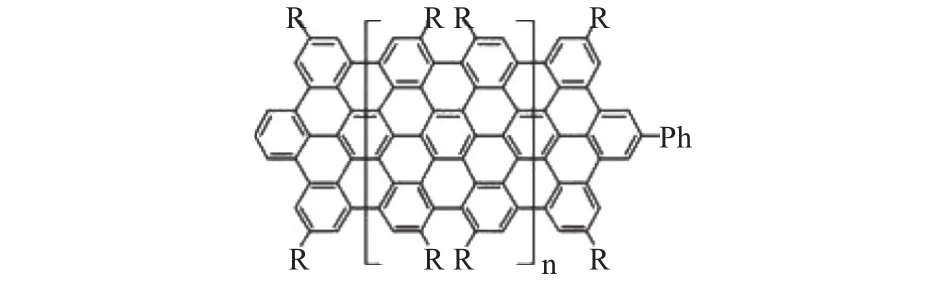
Fig. 5. Polycyclic aromatic hydrocarbons (PAHs) may offer a ground-up synthesis of graphene[28].
To sum up, the synthesis methods of graphene, either qualitative or financial concerns should be addressed and worked out amongst the methods aforementioned, and a comprehensive analysis is made and shown in Fig. 6. Amongst these synthesis strategies, reduction of graphene oxide and liquid-based methods are the most common and feasible methods due to their low cost and mass production.Additionally, the versatile reduced graphene oxide is suitable to prepare different composite-based anodes. Therefore, reduction methods seem to have highest practical applicability and are widely applied.
3.Graphene-Based Anode Materials
LIBs have been widely utilized as the main power source of electrical devices[4],[29]-[32]. Graphite, as the dominant material of the anode, is facing tremendous challenges and not sufficient enough to satisfy the expanding market demand for advanced lithium-ion batteries of better performance[22],[33],[34]. Moreover,since the intercalation potential is low, dendrite-like lithium precipitation may exist and accounts for safety problems[35]-[37].New substitution or modification methods for the current anode is in urgent need to enhance the performance of LIBs.Graphene with the large specific surface area and desirable mechanical property is promising to accomplish the mission.Theoretically, the application of graphene is capable of improving the capability of the anode and accelerating the process of charge-discharge. However, the disadvantages of graphene as anode lie in low coulombic efficiency in the first charge-discharge cycle, low potential and poor cyclic stability[38],[39]. Graphene is not practical to be used to replace graphite as integral anode material at present owing to its high cost. To the credit, researchers have been carried out to measure the performance of graphene-based LIBs. Graphene mainly functions as the composite material to cope with issues like huge volume change and active material loss[23],[30],[32],[40]-[43].
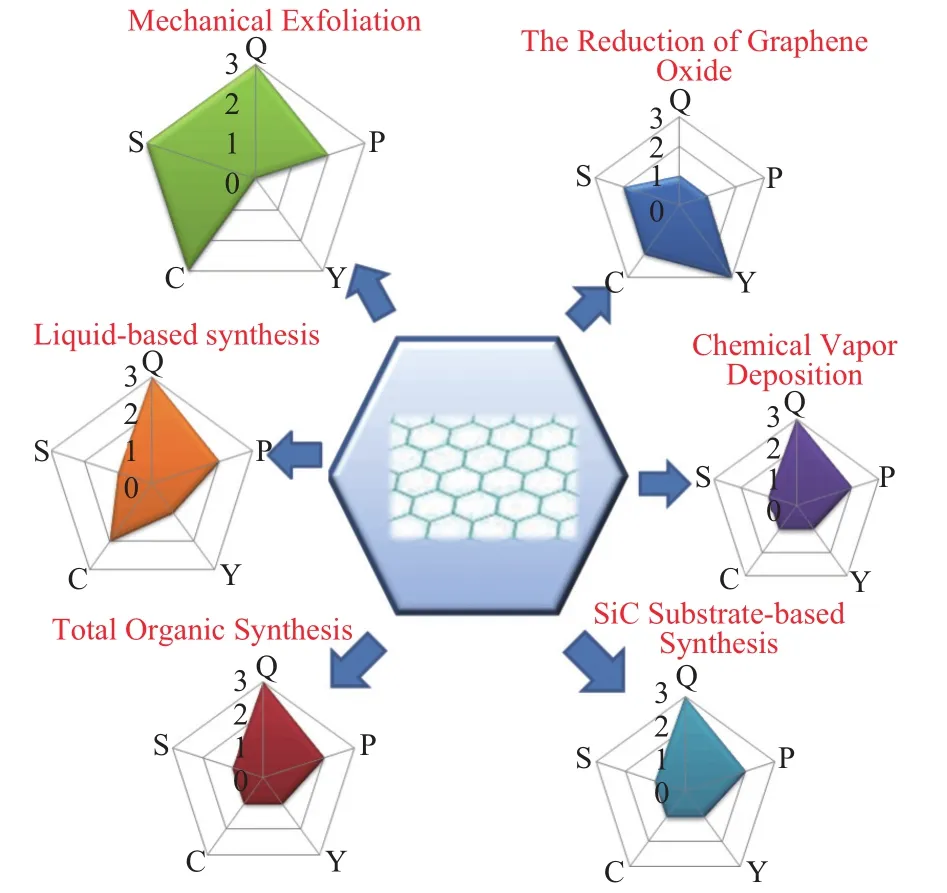
Fig. 6. Comparison between some common synthesis methods of graphene. In the schema, advantages and disadvantages are evaluated in different degree in terms of quality, purity, yield, low cost, simplicity of process, represented by character Q, P, Y, C, S.
3.1 Graphene as Anode Material in LIBs
By using a soft chemical synthesis approach, Wang et al.[40]synthesized graphene nanosheet in large scale. Through observation by an X-ray diffraction spectra (XRD), a field emission electron microscopy (FEM), and transmission electron microscopy (TEM), as shown in Fig. 7[40], they found that both multilayers and individual single graphene nanosheets are likely to scroll, which leads to the fact that graphene with 2D structure thermodynamically stable through corrugation. By observing HRTEM images, of different magnification, they further analyzed the structure of graphene nanosheets and found that the interplanar distance of graphene (0.34 nm) is larger than that of graphite (0.37 nm)[40]. Moreover, the data by Raman spectroscopy and ultraviolet-visible (UV-Vis)spectroscopy, reveals the partially crystal structure in disorder of graphene and similar structure to graphite.

Fig. 7. Graphene nanosheet: (a) low magnification FEG-SEM image of loose graphene nanosheet powders and (b) high magnification FEGSEM view of graphene nanosheet petals[40].
Constant current and charge/discharge cycling is also carried out to examine the electrochemical behaviors of graphene anode. Cyclic voltammograms of graphene nanosheet anodes indicate desirable cyclic performance and reversibility,as shown in Fig. 8[40]. Wang et al. proposed that the great performance stems from faradic capacitance both on surface and two sides due to the unique scrolling and crumpling structure, and thus enhance the storage of lithium-ion.

Fig. 8. Charge-discharge curves at the initial and 100th cycle of graphene nanosheets as the anode in lithium-ion batteries. The inset is the cyclic voltammograms of graphene nanosheet electrode[40].
Due to relatively poor conductivity, graphene nanostructure with increased defects and disorder is not desired. However,Pan et al.[44]suggested that the additional high capacity can be ascribed to the vast amount of disorder and defects induced by oxidation and reduction process on the surface, edge, and vacancies. To make clear the mechanism of Li storage and the key structure parameters, they adopted different reduction processes, such as hydrazine reduction, hypothermia pyrolysis,as well as electron beam irradiation, to prepare graphene nanosheets with the divided level of disorder and defects.Pyrolytic GO at low temperature (300 °C) exhibits lower extent of disorder. The data obtained also indicates that electron beam-rGO has less ordered sp2domains and higher additional reversible storage than the chemically reduced one. Therefore,the rapid electron-beam reduction can take the place of the slow hydrazine reduction to produce a larger amount of disordered graphene with smaller sp2domains. Graphene with high disorder synthesized by both electron beam reduction and pyrolysis at 300 °C shows higher conductivity than pristine graphene produced by hydrazine reduction with a smaller degree of defects. Moreover, the graphene anodes also show better cyclic performance and reversibility compared with pristine graphene and natural graphite, as shown in Fig. 9[44].

Fig. 9. Cyclic performance and reversibility comparison: (a)Charge/discharge profiles of (i) natural graphite, (ii) pristine GO,and (iii) hydrazine-reduced GO; (b) charge/discharge profiles of(iv) 300 °C pyrolytic GO, (v) 600 °C pyrolytic GO, and (vi)electron-beam-reduced GO; (c) reversible (charge) capacity verse cycle numbers at a current density of 0.05 A/g[44].
Although graphene and its derivatives are promising with great properties, there still remain problems like rapid energy loss and high cost. It is proposed that the energy loss takes place in the first cycle may be caused by the formation of solid electrolyte interface (SEI) and the variation of morphology, as shown in Fig. 10.
3.2 Silicon-Graphene Composite and SnO2-Graphene Composites as Anode Materials
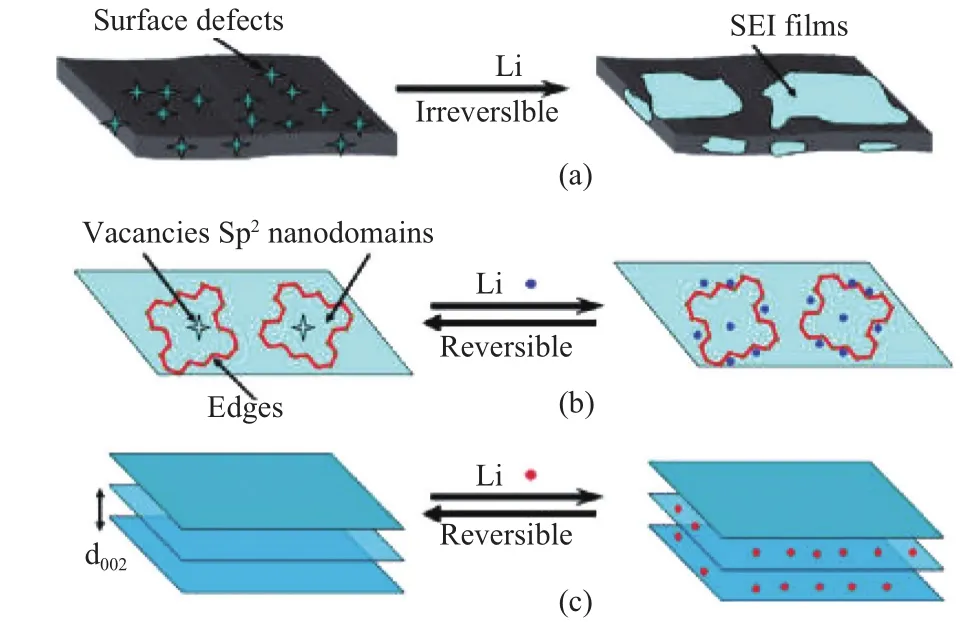
Fig. 10. Diagrammatic representation of surface interaction between graphene nonosheets and electrolyte: (a) Irreversible Li storage at the interface between the graphene nanosheets and electrolyte, (b)reversible Li storage at edge sites and internal defects (vacancies etc.) of nanodomains embedded in graphene nanosheets, and (c) reversible Li storage between (002) planes[44].
Silicon is viewed as promising candidates as new substitution of anode materials for their high capacity of 4200 mAh/g[45]-[48]. However, despite admirable behaviors, issues accompanied also largely prevent the commercialization of silicon-based LIBs, such as capacity loss due to substantial volume expansion and contraction resulted from intercalation and deintercalation of Li-ion in the Si anode[30],[39],[46],[49],[50]. Owing to severe drawbacks of the structure, the detrimental to the sustainability of the batteries can lead to tremendous problems like the instability of solid electrolyte interphase (SEI), rapid aging, devastation of silicon electrodes, and disconnection of the current collector and active material. A strategy of applying graphene coating is effective to form stable SEI as well as to enhance electron conductivity of silicon-based anode.
By incorporating graphene, Si composite exhibits a lower level of volume expansion, where Si evenly dispersed within the graphene nanosheet. Chabot et al.[46]prepared Si-graphene composite with a freeze-drying method. The freeze-drying induced 3-D structure not only enhances lithium ion movement and accelerates electrolyte diffusion, but also attains better retention of anode structure while cycling. Different ratios of Si and graphene have been tested in the aspect of nanosize morphology and electrochemistrical properties. The optimal composite greatly enhances the sustainability of the batteries.The facile and environmental-friendly manufacturing process allows for mass production.
Zhao et al.[49]adopted a new way to obtain the homogeneous distribution of Si nanoparticles on the graphene.By utilizing covalent immobilization and subsequent heating to prepare Si nanoparticle graphene hybrids, they obtained Sibased anode with better cyclic stability and higher power density, as shown in Fig. 11[49]. Generally, due to a higher ratio of Si, pristine Si-nanosheets have higher initial capacity compared with Si/graphene mixture, but the capacity degrades significantly as cyclic number increases. The large specific surface area provides ample internal space to accommodate volume variation during cycling (Li+insertion/exertion). Hence,the cyclic stability of Si anode is well promoted with the good distribution of Si on graphene nanosheets.

Fig. 11. Cyclic performance (Li+ insertion capacities) of pristine Si-nanosheets and Si/Graphene mixture anode[49].
Similar to Si-based anode material, SnO2-based anode also exhibits high theoretical capacity (782 mAh/g). However, this type of anode is not widely commercialized since the capacity decays rapidly during cycling[2],[51]due to volume expanding and the anode is thus wildly damaged, causing a short circuit and shortening the cyclic life[52]-[57]. To cope with these situations,many efforts have been made to modify the SnO2-based anode with graphene. Li et al.[56]adopted a one-pot route for the production of SnO2-reduced graphene oxide-based anode. The modified anode displays extraordinary high Li-ion storage and high-speed performance. More importantly, cycling performance has been greatly promoted, since volume change and pulverization of anode composite has been pronouncedly diminished, avoiding the issues like the short circuit and losing contact with the current collector. Electrochemical performance of SnO2-supercritical reduced graphene nanosheets (SRGO)electrode is estimated by the charge-discharge test in aspects of the specific capacity and cyclic lifetime as shown in Fig. 12[56].The result reveals that the mixture of SnO2and SRGO has a better cyclic performance compared with bare SnO2or SRGO.The curves show that the specific capacity is elevated with increasing proportion of SRGO, combined with a higher declining rate of capacity, which probably stemmed from electrode destructure caused by volume variation and particles aggregated. The SEM and TEM images show a higher degree of aggregation and highly uniform dispersion of SnO2on reduced graphene oxide, possibly ascribing to the slowdown of the volume expansion and capacity fading, as shown in Fig. 13.
3.3 Transition Element Oxide-graphene Composites as Anode Materials for LIBs
To obtain high storage capacity of lithium-ion batteries,varieties of transition metal oxide with extraordinary capability of resemble Li+insertion/extraction, like TiO2, Fe2O3, Fe3O4,CuO, MoO2, MnO2, VO2, Co2O3, have been regarded as the advanced substitution of graphite anode[22],[37],[58]-[67]. However,despite the high energy density provided, using different material has its drawbacks, like low ionic and electronic conductivity. Graphene can serve as a conductive network to compensate the shortness of transition metal-based anode[35],[36],[41],[67]-[73].
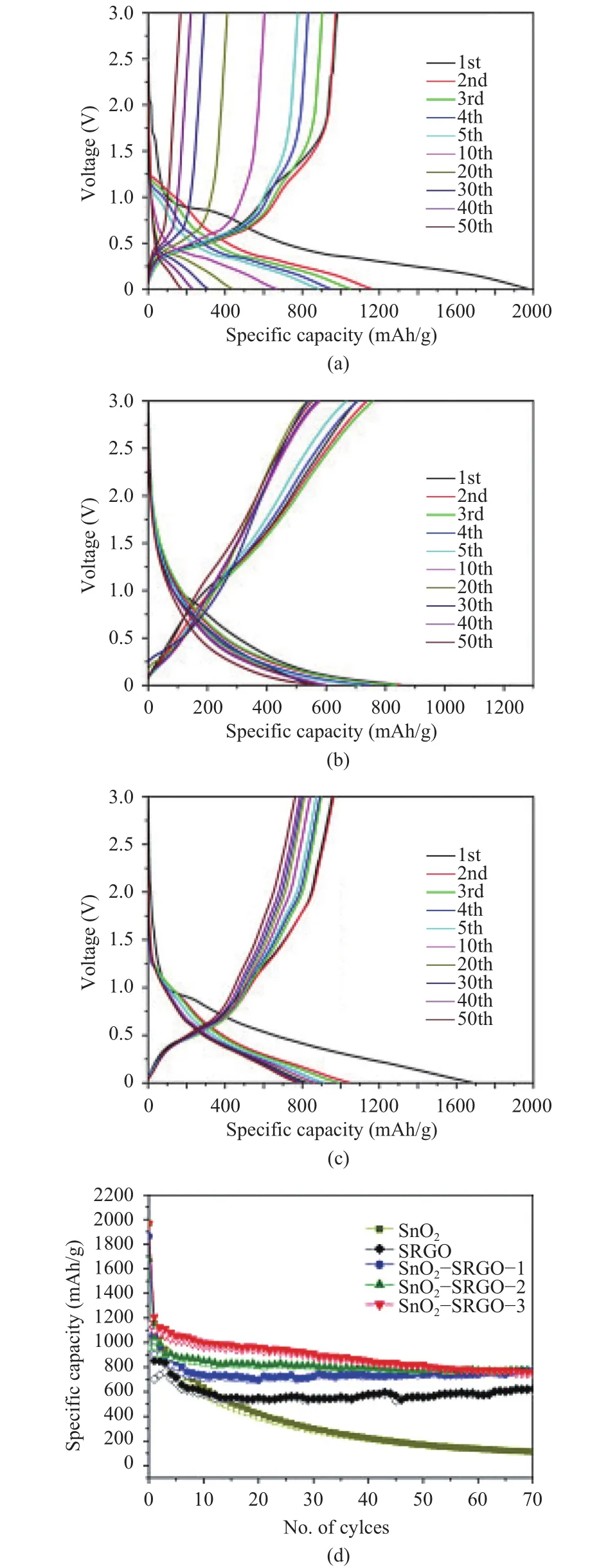
Fig. 12. Charge-discharge curves of (a) bare SnO2, (b) bare SRGO,(c) SnO2-SRGO-2 at a constant current density of 0.1 A/g between 0.01 V and 3.0 V, (d) Cycling stability of bare SnO2, SRGO, and SnO2-SRGO composites at 0.1 A/g. Filled symbols: Charge;outlined symbols: Discharge[56].

Fig. 13. SEM and TEM images of (a) bare SRGO, (b) SnO2-SRGO-1,and (c) SnO2-SRGO-2[56].
By using an in-situ synthesis method, Tao et al.[68]produced TiO2nanoparticles with homogeneously dispersed on graphene nanosheets (GNS). The obtained composite shows high initial reversible capacity and good cyclic ability. Moreover, their unique crystallite structure with negligible electrode volume change during cycling, result in outstanding structure stability and long cycling life of the anodes. Additionally, the coating of graphene not only decreases the resistance upon the contact of the current collector and improve the electronic conductivity,but also diminishes the aggregation of nanoparticles. They also proposed that the switch of structure from rutile to anatase can lead to the enhancement of the electrochemical properties. In Fig. 14 (a), TiO2electrode mixed with graphene nanosheet exhibits extended plateau and higher capacity compared with neat TiO2or graphene nanosheets. And the apparent decrease in peak separation reveals that the addition of GNS greatly suppresses the polarization. Moreover, the current density and reversible capacity of TiO2/GNS composite are evidently promoted over 50 cycles, shown in Fig. 14 (b)[68].
Bhaskar et al.[58]carried out a low-temperature phase reduction method to obtain the MoO2/graphene composite.They studied the structure and electrochemical properties of the neat graphene, neat MoO2, MoO2/graphene composite, by adopting XRD patterns, Raman spectra, SEM, TEM, XPS and cyclic voltammograms. The TEM images show a homogeneous diversion of MoO2on graphene nanosheets and a coverage of MoO2nanosheets is continuously and uniformly formed on the surface of the composite. The electronic conductivity of the anode is dramatically improved. The addition of graphene also decreases the aggregation of MoO2nanoparticles, resulting in the decrease of cyclic volume change. As shown in Fig. 15 (a), MoO2/graphene composite electrode suffers from a great level of irreversible capacity loss of 52% (1450 mAh/g of the initial cycle to 703.7 mAh/g),which is speculatively related to incomplete removal of graphene-oxide. However, the examined composite electrode exhibits an admirable coulombic efficiency even if at the repetitive cycle of 1000 combined with 75.4% retention of the first cycle, as shown in Fig. 15 (b). Additionally, the pronounced rate capacity of MoO2/graphene composite is also reported at different current rates of 540 mA/g, 1042 mA/g, and 2045 mA/g, as shown in Fig. 15 (c). By comparing the cyclic performance of MoO2/graphene composite to neat MoO2, neat graphene, it shows higher maximum capacity and long cyclic life, as shown in Fig. 15 (d). The result can be attributed to the increase in the active side, due to a large specific area of graphene[58].

Fig. 14. Electrochemical performances of TiO2/GNS composite and comparison electrodes: (a) the first charge-discharge curves cycled at a current density of 50 mA/g, and (b) cycle performances and rate capabilities of as-prepared TiO2-GNS composite from TiCl4/GO with weight ratio=12 and 6, GNS, and pure TiO2 obtained from the hydrolysis of TiCl[66].4
4.Summaries and Prospectives
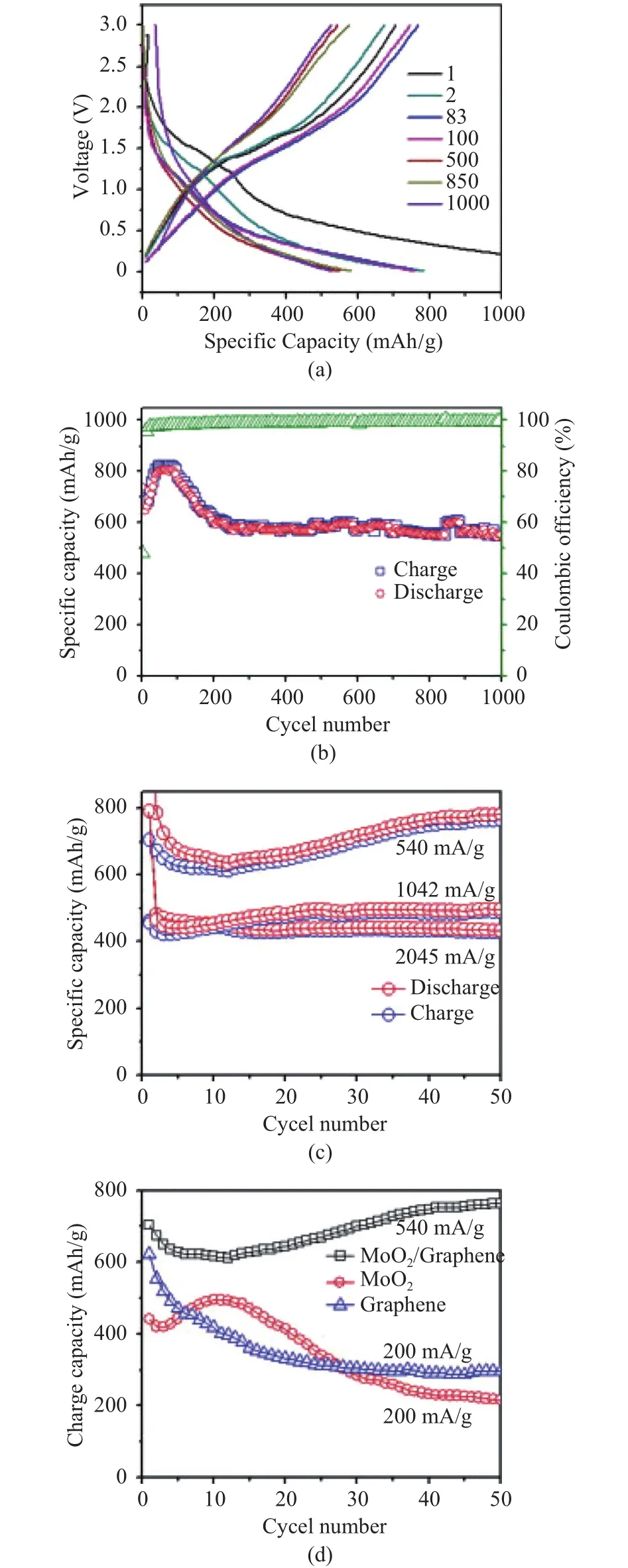
Fig. 15. Electrochemical performances of MoO2/Graphene composite and comparison electrodes: (a) Galvanostatic discharge–charge curves of the MoO2/Graphene composite electrode at a current density 540 mA/g in the voltage range of 0.01 V to 3.00 V, (b) the cycling performance of the MoO2/Graphene at current density of 540 mAh/g; (c) rate performance of the MoO2/Graphene composite, and (d) the cycling performance of MoO2/Graphene, neat MoO2 and neat graphene electrodes at different current densities[58].
In this review, we have summarized the properties, state of art and the applications as the anode material in lithium-ion batteries of graphene. The superior properties of graphene can be roughly summed up, including high mechanical robustness,large specific surface area, desirable flexibility, high electronic conductivity. So far, the graphene composite as the anode is viewed as the primary strategy for practical improvement of LIBs performance. Several applications of graphene as a part of LIBs, acting as active substance, interfacial layers, robust architecture, have been studied. It is believed that graphene can largely enhance the performance of lithium-ion batteries, in aspects of reversible capacity, cyclic performance, rate performance, etc. Moreover, graphene can serve as a great conductive matrix to improve electronic conductivity. And issues like excessive volume variation and particles aggregation can be decreased. Thus, existing safety concerns and cyclic instability can be addressed with the adoption of graphene.However, due to the high expense and a lack of feasible synthesis methods to be utilized in industrial production, there is a long way to go for graphene to attain large-scale marketization. The future work could focus on 1) desirable synthesis methods of producing high-quality graphene in mass production should be exploited to lower the cost price. Only when the cost concerns no longer be a problem can graphene thrive and fulfill the potential of its application and commercialization. 2) More effective designs for application of graphene in LIBs should be conducted and be examined theoretically and practically.
[1]S. H. Yeon, H. Yoon, S. H. Lee,et al., “Enhanced anode performance of micro/meso-porous reduced graphene oxide prepared from carbide-derived carbon for energy storage devices,”Carbon, vol. 91, pp. 241-251, 2015, DOI:10.1016/j.carbon.2015.04.087
[2]A. Birrozzi, F. Maroni, R. Raccichini,et al., “Enhanced stability of SnSb/graphene anode through alternative binder and electrolyte additive for lithium ion batteries application,”Journal of Power Sources, vol. 294, no. 30, pp. 248-253,2015.
[3]Y.-H. Shang, X-J Lin, X Lu,et al., “Nano-TiO2(B) coated LiMn2O4as cathode materials for lithium-ion batteries at elevated temperatures,”Electrochimica Acta, vol. 156, no.20, pp. 121-126, 2015.
[4]N. Atar, T. Eren, and M. L. Yola, “Ultrahigh capacity anode material for lithium ion battery based on rod gold nanoparticles decorated reduced graphene oxide,”Thin Solid Films, vol. 590, pp. 156-162, Sept. 2015.
[5]A. K. Geim and K. S. Novoselov, “The rise of graphene,”Nature Materials, vol. 6, no. 3, pp. 183-191, 2007.
[6]D. R. Dreyer, R. S. Ruoff, and C. W. Bielawski, “From conception to realization: An historial account of graphene and some perspectives for its future,”Angewandte Chemie-Intl. Edition, vol. 49, no. 49, pp. 9336-9344, 2010.
[7]O. C. Compton and S. T. Nguyen, “Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials,”Small, vol. 6, no. 6, pp.711-723, 2010.
[8]J.-S. Wu, W. Pisula, and K. Mullen, “Graphenes as potential material for electronics,”Chemical Reviews, vol. 107, no. 3,pp. 718-747, 2007.
[9]M. J. Allen, V. C. Tung, and R. B. Kaner, “Honeycomb Carbon: A Review of Graphene,”Chemical Reviews, vol.110, no. 1, pp. 132-145, 2010.
[10]E. Yoo, J. Kim, E. Hosono,et al., “Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries,”Nano Letters, vol. 8, no.8, pp. 2277-2282, 2008.
[11]C.-X. Gao, D.-W. He, M. Fu,et al., “Graphene based electrode using in rechargeable lithium ion batteries,”Advanced Technologies in Manufacturing, Engineering and Materials, vol. 774-776, no. 8, pp. 640-645, 2013.
[12]M. M. Atabaki and R. Kovacevic, “Graphene composites as anode materials in lithium-ion batteries,”Electronic Materials Letters, vol. 9, no. 2, pp. 133-153, 2013.
[13]X.-Y. Lu, X.-H. Jin, and J. Sun, “Advances of graphene application in electrode materials for lithium ion batteries,”Science China-Technological Sciences, vol. 58, no. 11, pp.1829-1840, 2015.
[14]M. Agostini, L. G. Rizzi, G. Cesareo,et al., “Characteristics of a graphene nanoplatelet anode in advanced lithium-Ion batteries using ionic liquid added by a carbonate electrolyte,”Advanced Materials Interfaces, vol. 2, no. 8, 2015, DOI:10.1002/admi.201500085
[15]L.-Z. Bai, D.-L. Zhao, J.-M. Zhang, and F. Li, “Large reversible capacity of graphene electrodes used in lithiumion batteries,”China Functional Materials Technology and Industry Forum, vol. 320, pp. 114-118, 2013, DOI:10.4028/www.scientific.net/AMM.320.114
[16]L. Shi and T. Zhao, “Recent advances in inorganic 2D materials and their applications in lithium and sodium batteries,”Journal of Materials Chemistry A, vol. 5, no. 8,pp. 3735-3758, 2017.
[17]O. I. Joshua, G. A. Steele, H. S. J. van der Zant, and A. C.Gomez, “Environmental instability of few-layer black phosphorus,”2D Materials, vol. 2, no. 1, p. 011002, 2015.
[18]J. H. Jeong, D. W. Jung, B. S. Kong, C. M. Shin, and E. S.Oh, “The effect of graphene nanosheets as an additive for anode materials in lithium ion batteries,”Korean Journal of Chemical Engineering, vol. 28, no. 11, pp. 2202-2205, 2011.
[19]R. Zhang, X-R. Chen, X-B. Cheng,et al., “Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes,”Angewandte Chemie Intl. Edition, no. 56, pp. 7764-7768, May 2017.
[20]D-C. Lin, Y-Y. Liu, Z. Liang,et al., “Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes,” Nature Nanotechnology, vol.11, p. 626, 2015.
[21]S. H. Choi and Y. C. Kang, “Crumpled graphenemolybdenum oxide composite powders: preparation and application in lithium-ion batteries,”Chemsuschem, vol. 7,no. 2, pp. 523-528, 2014.
[22]S.-T. Li, Z. Wei, Y. Tang,et al., “Synthesis of reduced graphene oxide carried VO2(A) nanorods and their application in lithium-ion batteries,”Science of Advanced Materials, vol. 6, no. 6, pp. 1293-1297, 2014.
[23]D.-B. Xiong, X. Li, H. Shan,et al., “Oxygen-containing functional groups enhancing electrochemical performance of porous reduced graphene oxide cathode in lithium ion batteries,”Electrochimica Acta, vol. 174, no. 20, pp. 762-769, 2015.
[24]A. M. Abdelkader, A. J. Cooper, R. A. Dryfe, and I. A.Kinloch, “How to get between the sheets: a review of recent works on the electrochemical exfoliation of graphene materials from bulk graphite,”Nanoscale, vol. 7, no. 16, pp.6944-6956, 2015.
[25]M. Lotya, Y. Hernandez, P. J. King,et al., “Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions,”Journal of the American Chemical Society, vol. 131, no. 10, pp. 3611-3620, 2009.
[26]E. Rollings, G. H. Gweon, S. Y. Zhou,et al., “Synthesis and characterization of atomically thin graphite films on a silicon carbide substrate,”Journal of Physics and Chemistry of Solids, vol. 67, no. 9-10, pp. 2172-2177, 2006.
[27]A. Reina, X. Jia, J. Ho,et al., “Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition,”Nano Letters, vol. 9, no. 1, pp. 30-35, 2009.
[28]X.-Y. Yang, X. Dou, A. Rouhanipour,et al., “Twodimensional graphene nanoribbons,”Journal of the American Chemical Society, vol. 130, no. 13, pp. 4216-4217,2008.
[29]L.-L. Liu, M.-Z. An, P.-X. Yang,et al., “Few-layer graphene prepared via microwave digestion reduction and its electrochemical performances in lithium ion batteries,”Intl.Journal of Electrochemical Science, vol. 10, no. 2, pp. 1582-1594, 2015.
[30]W. H. Jang, C. K. Min, S. N. Lee,et al., “Enhanced elevated temperature performance of LiFePO4modified spinel LiNi0.5Mn1.5O4cathode,”Journal of Alloys and Compounds,vol. 612, no. 5, pp. 51-55, 2014.
[31]S. J. R. Prabakar, Y. H. Hwang, E. G. Bae,et al., “Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries,”Carbon, vol. 52, no. 2, pp.128-136, 2013.
[32]J. C. Pramudita, D. Pontiroli, G. Magnani,et al., “Graphene and selected derivatives as negative electrodes in sodiumand lithium-ion batteries,”Chemelectrochem, vol. 2, no. 4,pp. 600-610, 2015.
[33]H.-B. Liu, C. Miao, Y. Meng,et al., “Effect of graphene nanosheets content on the morphology and electrochemical performance of LiFePO4particles in lithium ion batteries,”Electrochimica Acta, vol. 135, no. 22, pp. 311-318, 2014.
[34]X.-W. Liu, H. Zao, X. Huang,et al., “Fabrication of flexible graphene paper and its electrochemical properties used in lithium ion batteries,”European Physical Journal-Applied Physics, vol. 66, no. 3, p. 30301, 2014.
[35]G.-H. Qin, H.-J. Zhang, and C.-Y. Wang, “Ultrasmall TiO2nanoparticles embedded in nitrogen doped porous graphene for high rate and long life lithium ion batteries,”Journal of Power Sources, vol. 272, no. 272, pp. 491-500, 2014.
[36]B.-C. Qiu, M.-Y. Xing, and J.-L. Zhang, “Mesoporous TiO2nanocrystals grown in situ on graphene aerogels for high photocatalysis and lithium-ion batteries,”Journal of the American Chemical Society, vol. 136, no. 16, pp. 5852-5855,2014.
[37]M. Srivastava, J. Singh, T. Kuila,et al., “Recent advances in graphene and its metal-oxide hybrid nanostructures for lithium-ion batteries,”Nanoscale, vol. 7, no. 11, pp. 4820-4868, 2015.
[38]X. Tong, H. Wang, G. Wang,et al., “Controllable synthesis of graphene sheets with different numbers of layers and effect of the number of graphene layers on the specific capacity of anode material in lithium-ion batteries,”Journal of Solid State Chemistry, vol. 184, no. 5, pp. 982-989, 2011.
[39]F. Xia, S. Kwon, W. W. Lee,et al., “Graphene as an interfacial layer for improving cycling performance of Si nanowires in lithium-ion batteries,”Nano Letters, vol. 15,no. 10, pp. 6658-6664, 2015.
[40]G.-X. Wang, X. Shen, J. Yao, and J. Park, “Graphene nanosheets for enhanced lithium storage in lithium ion batteries,”Carbon, vol. 47, no. 8, pp. 2049-2053, 2009.
[41]J. Wang, L. Shen, P. Nie,et al., “Synthesis of hydrogenated TiO2-reduced-graphene oxide nanocomposites and their application in high rate lithium ion batteries,”Journal of Materials Chemistry A, vol. 2, no. 24, pp. 9150-9155, 2014.
[42]D.-H. Wu, Y.-F. Li, and Z. Zhou, “First-principles studies on doped graphene as anode materials in lithium-ion batteries,”Theoretical Chemistry Accounts, vol. 130, no. 2-3, pp. 209-213, 2011.
[43]Y. Xu, X. Zhu, X. Zhou,et al., “Ge nanoparticles encapsulated in nitrogen-doped reduced graphene oxide as an advanced anode material for lithium-ion batteries,”Journal of Physical Chemistry C, vol. 118, no. 49, pp. 28502-28508,2014.
[44]D.-Y. Pan, S. Wang, B. Zhao,et al., “Li storage properties of disordered graphene nanosheets,”Chemistry of Materials,vol. 21, no. 14, pp. 3136-3142, 2009.
[45]J.-K. Feng, Z. Zhang, L.-J Ci,et al., “Chemical dealloying synthesis of porous silicon anchored by in situ generated graphene sheets as anode material for lithium-ion batteries,”Journal of Power Sources, vol. 287, no. 1, pp. 177-183,2015.
[46]V. Chabot, K. Feng, H. W. Park,et al., “Graphene wrapped silicon nanocomposites for enhanced electrochemical performance in lithium ion batteries,”Electrochimica Acta,vol. 130, no. 4, pp. 127-134, 2014.
[47]X. Zhao, C. M. Hayner, M. C. kung, and H. H. Kung, “Inplane vacancy-enabled high-power Si-graphene composite electrode for lithium-ion batteries,”Advanced Energy Materials, vol. 1, no. 6, pp. 1079-1084, 2011.
[48]X.-S. Zhou, Y.-X. Yin, L.-J. Wang, and Y.-G. Guo, “Selfassembled nanocomposite of silicon nanoparticles encapsulated in graphene through electrostatic attraction for lithium-ion batteries,”Advanced Energy Materials, vol. 2,no. 9, pp. 1086-1090, 2012.
[49]G.-Y. Zhao, L. Zhang, and Y. Meng, “Decoration of graphene with silicon nanoparticles by covalent immobilization for use as anodes in high stability lithium ion batteries,”Journal of Power Sources, vol. 240, no. 6, pp.212-218, 2013.
[50]M.-S. Wang, W.-L. Song, and L.-Z. Fan, “Three-dimensional interconnected network of graphene-wrapped silicon/carbon nanofiber hybrids for binder-free anodes in lithium-ion batteries,”Chemelectrochem, vol. 2, no. 11, pp. 1699-1706,2015.
[51]X.-S. Zhou, L.-J. Wan, and Y.-G. Guo, “Binding SnO2nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries,”Advanced Materials, vol.25, no. 15, pp. 2152-2157, 2013.
[52]B. Wang, D. Su, P. Jinsoo,et al., “Graphene-supported SnO2nanoparticles prepared by a solvothermal approach for an enhanced electrochemical performance in lithium-ion batteries,”Nanoscale Research Letters, vol. 7, no. 1, p. 215,2012.
[53]H.-J. Zhang, P. Xu, Y. Ni,et al., “In situ chemical synthesis of SnO2/reduced graphene oxide nanocomposites as anode materials for lithium-ion batteries,”Journal of Materials Research, vol. 29, no. 5, pp. 617-624, 2014.
[54]J. Yao, P. Xu, Y. Ni,et al., “In situ chemical synthesis of SnO2-graphene nanocomposite as anode materials for lithium-ion batteries,”Electrochemistry Communications,vol. 11, no. 10, pp. 1849-1852, 2009.
[55]W.-B. Yue, S. Yang, and Y. Ren, “In situ growth of Sn, SnO on graphene nanosheets and their application as anode materials for lithium-ion batteries,”Electrochimica Acta, vol.92, no. 1, pp. 412-420, 2013.
[56]W. Li, D. Yoon, J. Hwang,et al., “One-pot route to synthesize SnO2-Reduced graphene oxide composites and their enhanced electrochemical performance as anodes in lithium-ion batteries,”Journal of Power Sources, vol. 293,no. 20, pp. 1024-1031, 2015.
[57]E. Choi, D. Kim, I. Lee,et al., “SnO2-Graphene nanocomposite free-standing film as anode in lithium-ion batteries,”Electronic Materials Letters, vol. 11, no. 5, pp.836-840, 2015.
[58]A. Bhaskar, M. Deepa, T. N. Rao, and U. V. Varadaraju,“Enhanced nanoscale conduction capability of a MoO2/Graphene composite for high performance anodes in lithium ion batteries,”Journal of Power Sources, vol. 216,no. 11, pp. 169-178, 2012.
[59]H. Chen, F. Feng, F.-S. Liu,et al., “Preparation of uniform flower-like CuO and flower-like CuO/graphene composite and their application in lithium ion batteries,”Trans. of Nonferrous Metals Society of China, vol. 22, no. 10, pp.2523-2528, 2012.
[60]J.-W. Deng, L. Chen, Y. Sun,et al., “Interconnected MnO2nanoflakes assembled on graphene foam as a binder-free and long-cycle life lithium battery anode,”Carbon, vol. 92, pp.177-184, 2015, DOI: 10.1016/j.carbon.2015.04.021
[61]L.-S. Fan, B. Li, D. W. Rooney, N. Zhang, and K. Shun, “In situ preparation of 3D graphene aerogels@hierarchical Fe3O4nanoclusters as high rate and long cycle anode materials for lithium ion batteries,”Chemical Communications, vol. 51,no. 9, pp. 1597-1600, 2015.
[62]C.-J. Fu, G. Zhao, H. Zhang, and S. Li, “A facile route to controllable synthesis of Fe3O4/graphene composites and their application in lithium-ion batteries,”Intl. Journal of Electrochemical Science, vol. 9, no. 1, pp. 46-60, 2014.
[63]J. Hou, R. Wu, P. Zhao,et al., “Graphene-TiO2(B) nanowires composite material: Synthesis, characterization and application in lithium-ion batteries,”Materials Letters, vol.100, no. 6, pp. 173-176, 2013.
[64]H.-Q. Liu, K. Cao, X. Xu,et al., “Ultrasmall TiO2nanoparticles in situ growth on graphene hybrid as superior anode material for sodium/lithium ion batteries,”Acs Applied Materials and Interfaces, vol. 7, no. 21, pp. 11239-11245,2015.
[65]D.-F. Qiu, D. Qiu, L. Ma,et al., “MnO nanoparticles anchored on graphene nanosheets via in situ carbothermal reduction as high-performance anode materials for lithiumion batteries,”Materials Letters, vol. 84, no. 21, pp. 9-12,2012.
[66]H.-C. Tao, L.-Z. Fan, X. Yang, and X. Qu, “In situ synthesis of TiO2-graphene nanosheets composites as anode materials for high-power lithium ion batteries,”Electrochimica Acta,vol. 69, no. 69, pp. 328-333, 2012.
[67]J.-Z. Wang, C. Zhong, and D. Wexler, “Grapheneencapsulated Fe3O4nanoparticles with 3D laminated structure as superior anode in lithium ion batteries,”Chemistry—A European Journal, vol. 17, no. 2, pp. 661-667,2011.
[68]D.-D. Cai, P. Lian, X. Zhu,et al., “High specific capacity of TiO2-graphene nanocomposite as an anode material for lithium-ion batteries in an enlarged potential window,”Electrochimica Acta, vol. 74, no. 4, pp. 65-72, 2012.
[69]Y. Jiang, Z. Jiang, L. Ynag,et al., “A high-performance anode for lithium ion batteries: Fe3O4microspheres encapsulated in hollow graphene shells,”Journal of Materials Chemistry A, vol. 3, no. 22, pp. 11847-11856,2015.
[70]H. K. Kim, K. C. Roh, and K. B. Kim, “In situ electrochemical dilatometric study of Fe3O4/reduced graphene oxide nanocomposites as anode material for lithium ion batteries,”Journal of the Electrochemical Society, vol.162, no. 12, pp. A2308-A2312, 2015.
[71]X.-Y. Li, Y. Ma, L. Qin,et al., “A bottom-up synthesis of alpha-Fe2O3nanoaggregates and their composites with graphene as high performance anodes in lithium-ion batteries,”Journal of Materials Chemistry A, vol. 3, no. 5,pp. 2158-2165, 2015.
[72]Y. Li, Q. Meng, S.-M. Zhu,et al., “A Fe/Fe3O4/ N-carbon composite with hierarchical porous structure and in situ formed N-doped graphene-like layers for high-performance lithium ion batteries,”Dalton Trans., vol. 44, no. 10, pp.4594-4600, 2015.
[73]E.-Z. Liu, J. Wang, C. Shi,et al., “Anomalous interfacial lithium storage in graphene/TiO2for lithium ion batteries,”Acs Applied Materials and Interfaces, vol. 6, no. 20, pp.18147-18151, 2014.
 Journal of Electronic Science and Technology2018年1期
Journal of Electronic Science and Technology2018年1期
- Journal of Electronic Science and Technology的其它文章
- Message from JEST Editorial Committee
- Modeling TCP Incast Issue in Data Center Networks and an Adaptive Application-Layer Solution
- UEs Power Reduction Evolution with Adaptive Mechanism over LTE Wireless Networks
- Multi-Reconfigurable Band-Notched Coplanar Waveguide-Fed Slot Antenna
- High Power Highly Nonlinear Holey Fiber with Low Confinement Loss for Supercontinuum Light Sources
- Pairing-Free Certificateless Key-Insulated Encryption with Provable Security
