Associations between thromboxane A synthase 1 gene polymorphisms and the risk of ischemic stroke in a Chinese Han population
Lei Li, Zhi-yi He, Yan-zhe Wang, Xu Liu, Li-ying Yuan
Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China
Introduction
Worldwide, stroke is the main cause of disability and death(Ikram et al., 2009; Malik et al., 2014). Unlike in western countries, stroke is the leading cause of death in China (Wu et al., 2001; Yang et al., 2013). In China, ischemic stroke accounts for approximately 70% of all stroke cases (Wang et al., 2012), with the incidence exhibiting substantial geographic variation. Northern China has the highest incidence(486 per 100,000 person-years), whereas Southern China has a much lower incidence (136 per 100,000 person-years) (Wu et al., 2013). Although age, hypertension, diabetes mellitus,and environmental factors are well-known risk factors for ischemic stroke, it is a multifactorial and multigenic disorder (Matarin et al., 2009; Hachiya et al., 2017). Identifying gene variants that are associated with the risk of ischemic stroke could elucidate the pathogenesis of stroke and lead to new approaches to the prevention and management of this complicated disease. Although several genome-wide association studies have assessed various polymorphisms that may contribute to ischemic stroke (Ikram et al., 2009; Yamada et al., 2009; Bellenguez et al., 2012; Holliday et al., 2012; Lee et al., 2016), the genetic variants associated with predisposition to ischemic stroke have not been unequivocally determined in Chinese individuals.Thromboxane A synthase 1 (TBXAS1) is a downstream enzyme of arachidonic acid metabolism and is the obligate enzyme required to synthesize thromboxane A2 (TXA2) (Hsu et al., 2000; Iñiguez et al., 2008). rs2267682 and rs10487667 are two intronic single-nucleotide polymorphism (SNP)variations in the TBXAS1 gene (Miyata et al., 1994; Chevalier et al., 2001) that may influence the structure and stability of TBXAS1 mRNA, thus affecting the metabolite production of TXA2 (Wang et al., 2010). It is thought that TXA2, a prothrombotic lipid mediator, is implicated in the development and thrombogenicity of atherosclerotic lesions(Ishizuka et al., 1998; Wang et al., 2001; Sellers and Stallone,2008; Calder, 2009; Kim et al., 2010). Considering that atherosclerosis is a well-known risk factor for ischemic stroke,we hypothesized there may be a relationship between these TBXAS1 SNPs and ischemic stroke.
Several studies have revealed the associations the rs2267682 and rs10487667 TBXAS1 SNPs and the risk of cardiovascular diseases in Caucasians, a Korean population and a Uyghur population in Xinjiang (Lemaitre et al., 2009;Park et al., 2009; Wang et al., 2010). However, the results are inconsistent in different diseases and ethnic populations.Currently, the association between TBXAS1 and ischemic stroke in the Chinese Han population is not known.
This study investigated the associations rs2267682 and rs10487667 and susceptibility to ischemic stroke in a Northern Chinese Han population, which has a high incidence of ischemic stroke (Liu et al., 2007).
Participants and Methods
Participants
Ischemic stroke patients were enrolled from the First Affiliated Hospital of China Medical University in Shenyang city and the First Affiliated Hospital of Liaoning Medical University in Jinzhou city of China between October 2010 and May 2011. Healthy control participants were selected from the Health Check Center at the First Affiliated Hospital of China Medical University in China during the same period.All participants were unrelated members of the Chinese Han population in northern China. This study consecutively enrolled 404 ischemic stroke patients and 340 healthy controls. The study protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of China Medical University in Shenyang city and the First Affiliated Hospital of Liaoning Medical University in Jinzhou city. Written informed consent was provided by all participants. The protocol has been registered with the Chinese Clinical Trial Registry (registration number: Chi-CTRCOC-17013559).
The inclusion criteria for the ischemic stroke group were:patients with ischemic stroke diagnosed according to clinical features and neuroimaging criteria that included the sudden onset of a non-conclusive and focal neurological de ficit with corresponding infarction on brain imaging with computed tomography or magnetic resonance imaging, echocardiography, transcranial Doppler ultrasound, or carotid duplex imaging. Magnetic resonance angiography and computed tomography angiography were performed when necessary.
The exclusion criteria for the ischemic stroke group were:patients with severe cardiac, renal or hepatic diseases and those with cancer.
The inclusion criteria for the control group were: unrelated healthy controls with no clinical or radiological evidence of stroke or cerebrovascular diseases matched with the ischemic stroke patients in terms of area of residence, ethnic origin, gender, and age.
The exclusion criteria for the control group were: patients with cancer, autoimmune disease, chronic inflammation,renal or liver insufficiency, and hematopathy.
Data collection
Demographic and risk factor information was collected using a structured questionnaire. Measurements of body weight, height, blood pressure, plasma glucose, total plasma cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol are obtained.The body mass index was calculated as the body weight (kg)divided by the squared height (m2). Blood pressure measurements were acquired at least twice with the patient in the supine position after 15 minutes of rest. Hypertension was de fined by a systolic blood pressure of ≥ 140 mmHg, a diastolic blood pressure of ≥ 90 mmHg (1 mmHg = 0.133 kPa),or the use of antihypertensive medications. Diabetes mellitus was de fined by a fasting plasma glucose concentration of≥ 7.00 mM, a hemoglobin A1c content of ≥ 6.5%, or the use of antidiabetes medication. Hyperlipidemia was de fined by a total plasma cholesterol level ≥ 5.72 mM and/or a plasma triglyceride level ≥ 1.7 mM, or current use of lipid-lowering drugs.
After overall evaluation of the clinical data, patients with cardioembolic stroke, stroke of other determined aetiology,or stroke of undetermined aetiology according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classi fication were excluded from the analysis (Adams et al., 1993).Ischemic strokes were classi fied as either the large-artery atherosclerosis (LAA) or small-artery occlusion (SAO) subtypes.
Genotype determination
SNP ID numbers and detailed sequence information for rs2267682 and rs10487667 were obtained from the public dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/). These SNPs have minor allele frequencies of at least 5% in Han Chinese.
Peripheral blood (10 mL) samples were collected from each participant, and genomic DNA was extracted using the Wizard Genomic DNA Puri fication Kit (Promega, Sunnyvale, CA, USA) according to the manufacturer’s instructions. DNA purity and quantity were assessed using absorbance values obtained using a spectrophotometer (Thermo Scientific, Waltham, MA, USA). The DNA samples were stored at −20°C until use.
Genotyping was performed using the Multiplex SNaPshot sequencing method (Bujalkova et al., 2008; Di Cristofaro et al., 2010). Genomic DNA was first amplified by multiplex polymerase chain reaction (PCR) using speci fic primers. The sequences of the primers are shown inTable 1.
The PCR reactions (10 μL/tube) consisted of 10 ng of ge-nomic template, 1 μM of each primer, 10 μL 1× GC buffer I(Takara, Otsu, Shiga, Japan), 3.0 mM Mg2+(Takara), 0.3 mM dNTPs (Generay Biotech, Shanghai, China), and 1 U Hot-Star Taq polymerase (Qiagen, Hilden, Germany). PCR was performed in duplicate at 95°C for 15 minutes and subjected to 11 cycles of 94°C for 20 seconds, 67.5°C for 40 seconds,and 72°C for 1.5 minutes. Subsequently, PCR was performed using 24 cycles of 94°C for 20 seconds, 63°C for 30 seconds,and 72°C for 110 seconds, followed by an extension at 72°C for 2 minutes. The PCR products were then characterized using SNaPshot Multiplex sequencing and GeneMapper 4.0(Applied Biosystems, Princeton, NJ, USA). Additionally, we randomly selected 10% of the positive samples for repeated genotyping to assess experimental quality, and obtained the same results.
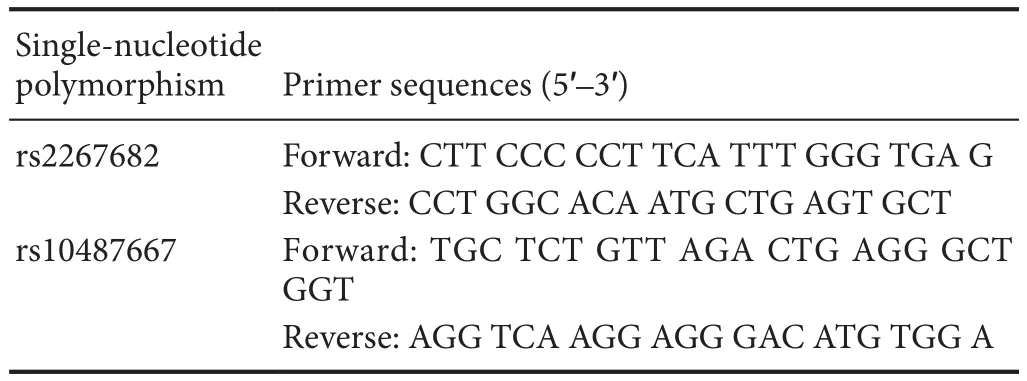
Table 1 Primer sequences for rs2267682 and rs10487667 ampli fication
Outcome measures
Primary outcome measures
The primary outcome measures were the genotype frequencies of the two TBXAS1 SNPs, rs2267682 and rs10487667, in the patients and controls.
Secondary outcome measures
The secondary outcome measures were the allele frequencies of rs2267682 and rs10487667 in patients and controls, multivariate logistic analysis for risk factors, and haplotype analysis.
Statistical analysis
Means ± standard deviations (SD) and percentages were used to assess continuous and categorical variables, respectively. Statistical analyses were performed using SPSS 13.0 software (IBM Corporation, Armonk, NY, USA).
The allele frequencies were calculated based on the genotypes of all participants. The genotype distributions were checked for Hardy-Weinberg equilibrium using chi-square tests. The distributions of the demographic variables were examined, and the differences in risk factors between cases and controls were reassessed using Pearson’s chi-square tests and Student’s t tests. Differences in the allele and genotype frequencies of TBXAS1 SNPs between the patients and controls were evaluated with chi-square tests and with odds ratios (ORs) and 95% con fidence intervals (95% CIs), respectively, using the most common genotype as the reference group. Multivariable logistic regression analysis was used to evaluate the relationships between TBXAS1 polymorphisms and ischemic stroke by adjusting for confounding variables.

Figure 1 Test intervention flow chart.
The linkage disequilibrium index (D-prime andr2) and the inferred haplotypes of these two SNPs were performed using the SHEsis analysis platform as described previously(Shi and He, 2005; Li et al., 2009). A P value < 0.05 was considered statistically signi ficant.
Results
Clinical characteristics of the participants
To determine the potential associations of TBXAS1 SNPs with the development of ischemic stroke, 404 ischemic stroke patients and 340 controls were recruited for this study at the beginning, but only 370 patients continued into the complete study (Figure 1). Twenty-six patients were excluded for cardiogenic cerebral embolisms. One patient was excluded for cerebral arteritis. Five patients were excluded for ischemic stroke caused by unknown factors. Two individuals were excluded for decline to participate in the study. This study population finally consisted of 370 patients with ischemic stroke (220 males and 150 females) and 340 healthy controls (193 males and 147 females). The general characteristics and biochemical parameters of the patients and controls are summarized inTable 2.
There were no significant differences in mean age, body mass index, or serum total cholesterol, high-density lipoprotein cholesterol, or total triglyceride levels between the patients and controls (Table 2). The risk factor pro file revealed that hypertension, diabetes, smoking, and alcohol use were common risk factors in the patients. The ischemic stroke patients also exhibited signi ficantly higher systolic and diastolic blood pressure and higher serum levels of low-density lipoprotein cholesterol and fasting plasma glucose (P < 0.05).
Ischemic stroke subtype stratification revealed that the percentages of patients with hypertension and type 2 diabetes mellitus, smokers, and elevated systolic and diastolic blood pressure and serum low-density lipoprotein cholesterol and fasting plasma glucose concentrations were markedly higher in the LAA and SAO patients than in the controls. However,the percentage of alcohol consumers was only markedly increased in the SAO patients compared with that in the controls.
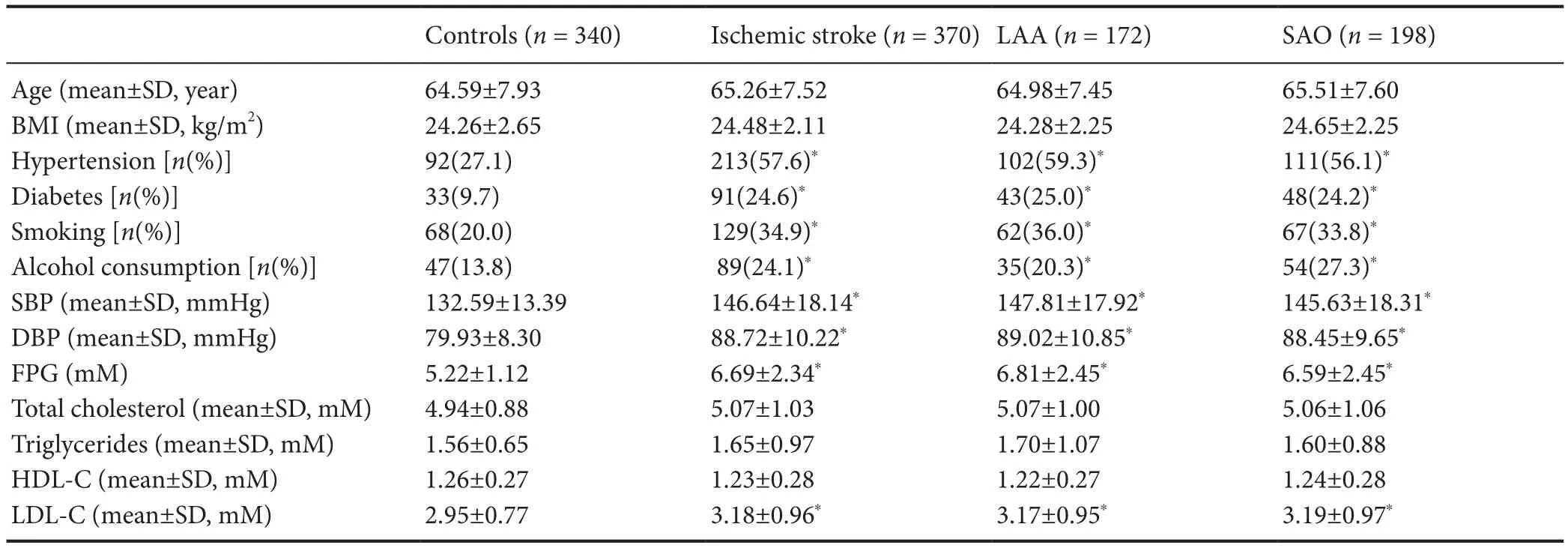
Table 2 Clinical characteristics of the study participants
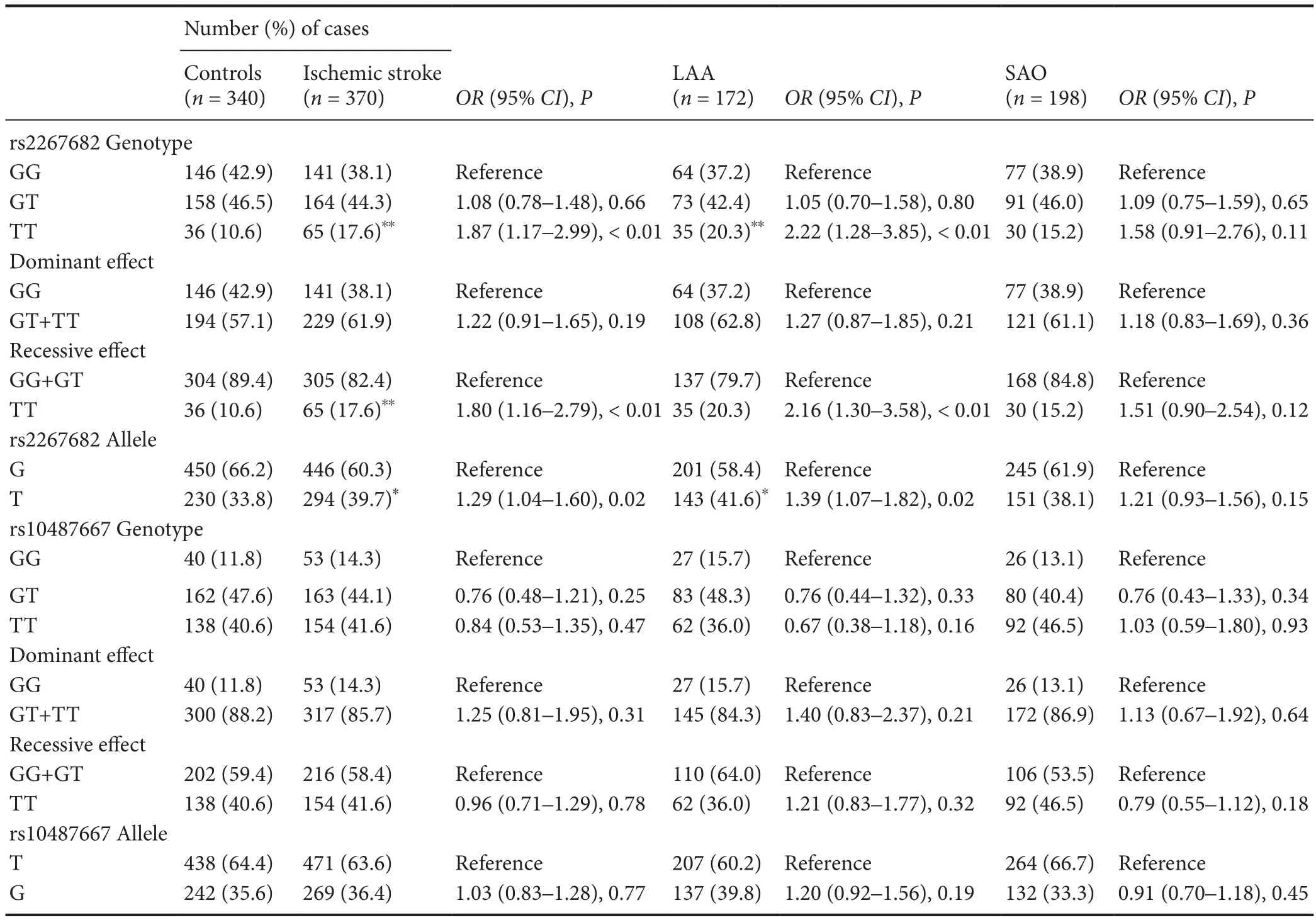
Table 3 Genotype and allele distributions in patients with ischemic stroke and controls
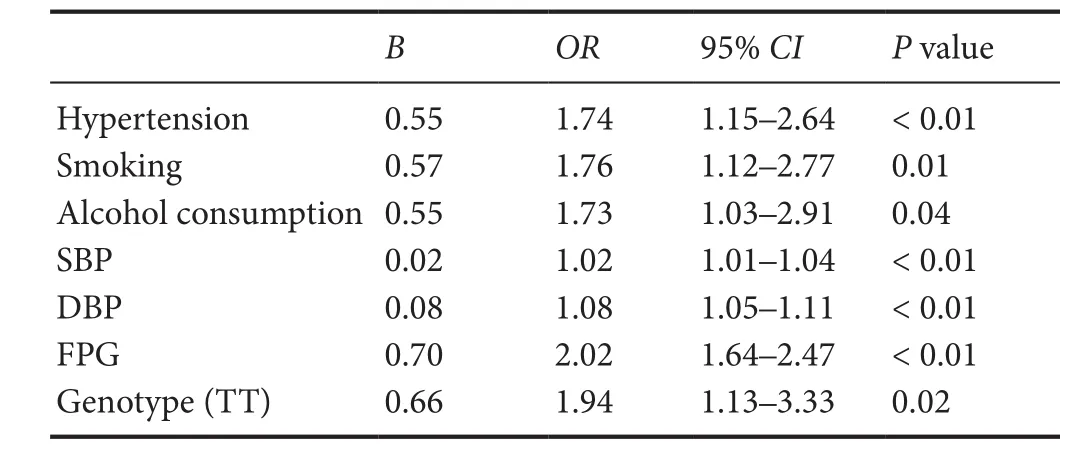
Table 4 Multivariable logistic regression analysis of ischemic stroke

Table 6 Haplotype analysis of the TBXAS1 gene in ischemic stroke patients and controls
Genotype analysis
Table 3presents the genotype and allele frequencies of the TBXAS1 SNPs in the patients and controls. The genotype distributions of the two SNPs were in Hardy—Weinberg equilibrium in both patients and controls (P > 0.05). As presented inTable 3, there were no signi ficant differences in the rs10487667 genotype or the allele distributions between ischemic stroke patients and controls. In contrast, signi ficant differences were observed in the distributions of the TT genotype and the T allele of rs2267682 between ischemic stroke patients and controls (P < 0.01 and P = 0.02, respectively).Further analyses of the LAA or SAO subgroups revealed that the rs2267682 TT genotype frequency and T allele frequency in patients with LAA were signi ficantly greater than those in controls (P < 0.01 and P = 0.02, respectively), but this difference was not observed in patients with SAO.
Multivariate logistic regression analysis was used to evaluate the relationships between the rs2267682 polymorphism and ischemic stroke. After adjustments for confounding variables, the TT genotype of rs2267682 remained significantly associated with an increased risk of ischemic stroke(OR = 1.94, 95% CI: 1.13—3.33, P = 0.02;Table 4). Similarresults were observed in patients with the LAA subtype (OR= 2.20, 95% CI : 1.16—4.18, P = 0.02;Table 5).
(2)形声字的教学。汉字是音、形、义的统一体。因此,在对形声字进行教学的时候要将汉字的形与声结合起来,帮助学生记忆。例如:在教授“胎”字时,可以跟学生介绍“月”字旁是与人体有关,而台与“胎”与“台”音近,台字是用来表音的,而“胎”字又可以引出“腭”、“肺”字的学习。
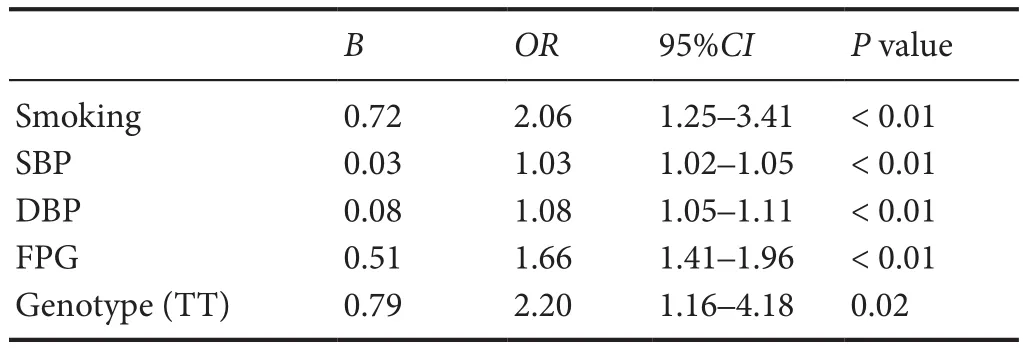
Table 5 Multivariable logistic regression analysis of the LAA subtypes
Using the SHEsis program platform, our data revealed that these two TBXAS1 SNPs were in linkage disequilibrium in the Chinese population. Four haplotypes with frequencies greater than 3% among both cases and controls were included in the haplotype analysis.Table 6presents the distribution of the individual haplotypes constructed with rs2267682 and rs10487667. The overall haplotype distributions were significantly different between the cases and controls (global test, P = 0.03). The frequency of the T-G haplotype constructed with rs22676821 and rs10487667 in patients with ischemic stroke was signi ficantly greater than that in controls (OR = 1.49, 95% CI: 1.10—2.00, P < 0.01).
Discussion
Ischemic stroke is a multigenic and multifactorial disease.Environmental, cultural, and genetic factors may participate in its development. Identification of the genetic factors for stroke plays an important role in the early recognition of susceptibility to stroke in people at a high risk, before symptoms are found, and early intervention. To our knowledge, this report is the first to reveal the correlation between TBXAS1 and susceptibility to ischemic stroke in a Chinese Han population.
In line with our results, Park et al. (2009) reported that the TT genotype of rs2267682 and a specific haplotype of TBXAS1 exhibit significant associations with increased susceptibility to non-cardiogenic stroke in a Korean population. However, the molecular mechanism underlying the association between this SNP with ischemic stroke remains unclear. One possible reason is the effect on TXA2 synthesis. TXA2 results from the isomerization of prostaglandin H2 (PGH2) by TBXAS1 (Wang and Kulmacz, 2002) and is formed in different cell types in the blood and vascular wall in the presence of various physiological and pathological stimuli (Needleman et al., 1976; Pyo et al., 2007; Gabrielsen et al., 2010; Muzaffar et al., 2011). TXA2 is a potent platelet activator and vasoconstrictor, and may play a key role in acute coronary syndromes and atherosclerosis (Koudstaal et al., 1993; Davi and Patrono, 2007; Calder, 2009; Borow et al.,2015). A previous study suggested that the TBXAS1 binding sites for transcriptional regulatory factors are located in in-tron 1, including the cytosine-cytosine-adenosine-adenosine-thymidine box, polyoma enhancer activator 3, cyclic adenosine monophosphate-response element, and lymphocyte function-associated antigen 1 (Miyata et al., 1994). These observations suggest that SNPs in intron 1 may influence promoter/enhancer activity. The TT genotype and T allele of rs2267682 may enhance promoter activity and lead to up-regulation of the expression of TBXAS1. The observation that the TT genotype of the TBXAS1 rs2267682 SNP increases susceptibility to ischemic stroke might be explained,at least in part, by the role of TBXAS1 in synthesizing TXA2.
In contrast, the TBXAS1 rs2267682 SNP is independent of ischemic stroke incidence, according to a recent study of white participants in North America (Lemaitre et al., 2009).Combined with the results in a Korean population (Park et al., 2009), this phenomenon might be explained at least in part by differences in race and geographical groups, and these associations could be applied to Asian populations.Moreover, the study in an American population placed more emphasis on cardiogenic stroke (Lemaitre et al., 2009).Disparate mechanisms may be critical in the development of acute ischemic coronary and cerebrovascular events (Cheng et al., 2012). This disagreement could be due to differences in study design and sample selection, as well as sample size.
Ischemic stroke is a complicated disease that is classi fied into various subtypes according to different pathogeneses.The TOAST criteria have been widely used in studies to etiologically classify acute ischemic stroke. Jerrard-Dunne et al.(2003) suggested that the strongest genetic in fluences would be detected in strokes attributable to LAA or SAO. Therefore, only patients with these two subtypes were enrolled in our study. Based on the TOAST criteria, this study separately examined the association between the two TBXAS1 SNPs and the LAA and SAO subtypes. The present study found that rs2267682 may contribute to an increased risk for the LAA subtype. This signi ficant finding remained after adjusting for potential confounding risk factors.
Recently, Gabrielsen et al. (2010) found that TBXAS1 mRNA is significantly elevated in atherosclerosis with advanced lesions in rats. TBXAS1 is expressed in human carotid atherosclerotic lesions and is related to increases in inflammatory cells, particularly M2 polarized macrophages.Furthermore, these authors discovered that TBXAS1 mRNA levels are increased in the atherosclerotic plaques of patients with recent cerebral symptoms of plaque thrombus formation compared with those of patients without symptoms (Gabrielsen et al., 2010). This research shows a correlation between TBXAS1 expression in advanced atherosclerotic lesions and plaque instability. Another study revealed that a genetic variant of TBXAS1 was associated with carotid artery or intracranial arterial stenosis, carotid plaque vulnerability, platelet activation, and TXA2 levels (Yi et al., 2016a,b, 2017). It seems reasonable that TBXAS1 and TXA2 play important roles in atherosclerosis and thrombosis. These findings might help explain why the TT genotype and T allele of rs2267682 were associated with the development of LAA but not SAO.
In the haplotype analysis, four major haplotypes of these two SNPs with frequencies greater than 5% were identified.Interestingly, compared with the control group, the ischemic stroke group exhibited a remarkably higher frequency of the T-G haplotype comprising rs22676821-rs10487667. This suggests that this haplotype may be a genetic marker for ischemic stroke in Han Chinese.
However, this study is preliminary due to the relatively small sample size, lack of measurements of TBXAS1 mRNA and protein expression, absence of TXA2 measurements, and the assessment of only two SNPs in the TBXAS1 gene. Therefore, the results require con firmation with a larger sample size and further research including genotyping, expression, and translation. If the correlation is con firmed, it is possible that these two TBXAS1 SNPs could be used to indicate the risk of ischemic stroke in Chinese Han individuals.
In conclusion, the TT genotype of TBXAS1 and the T allele of the rs2267682 SNP increase the susceptibility to ischemic stroke in a Northern Chinese Han population. Furthermore,the T-G haplotype comprising rs22676821-rs10487667 confers a genetic risk factor for ischemic stroke in this Chinese population.
Author contributions:LL conceived and designed the study, performed the experiments, analyzed the data, drafted the paper and revised it critically. LL, LYY and YZW provided reagents/materials/analysis tools. XL analyzed the data. ZYH revised this paper critically. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Research ethics:The study protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of China Medical University and the First Affiliated Hospital of Liaoning Medical University in China.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form, participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Takatoshi Hara, the Jikei University School of Medicine, Japan.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL,Marsh EE 3rd (1993) Classi fication of subtype of acute ischemic stroke.De finitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24:35-41.
Bellenguez C, Bevan S, Gschwendtner A, Spencer CC, Burgess AI, Pirinen M,Jackson CA, Traylor M, Strange A, Su Z, Band G, Syme PD, Malik R, Pera J, Norrving B, Lemmens R, Freeman C, Schanz R, James T, Poole D, et al. (2012) Genome-wide association study identi fies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet 44:328-333.
Borow KM, Nelson JR, Mason RP (2015) Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis 242:357-366.
Bujalkova M, Zavodna K, Krivulcik T, Ilencikova D, Wolf B, Kovac M,Karner-Hanusch J, Heinimann K, Marra G, Jiricny J, Bartosova Z(2008) Multiplex SNaPshot genotyping for detecting loss of heterozygosity in the mismatch-repair genes MLH1 and MSH2 in microsatellite-unstable tumors. Clin Chem 54:1844-1854.
Calder PC (2009) Polyunsaturated fatty acids and in flammatory processes: New twists in an old tale. Biochimie 91:791-795.
Cheng YC, Anderson CD, Bione S, Keene K, Maguire JM, Nalls M, Rasheed A, Zeginigg M, Attia J, Baker R, Barlera S, BiffiA, Bookman E, Brott TG, Brown RD Jr, Chen F, Chen WM, Ciusani E, Cole JW,Cortellini L, et al. (2012) Are myocardial infarction-associated single-nucleotide polymorphisms associated with ischemic stroke? Stroke 43:980-986.
Chevalier D, Lo-Guidice JM, Sergent E, Allorge D, Debuysère H, Ferrari N, Libersa C, Lhermitte M, Broly F (2001) Identi fication of genetic variants in the human thromboxane synthase gene (CYP5A1). Mutat Res 432:61-67.
Davi G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357:2482-2494.
Di Cristofaro J, Silvy M, Chiaroni J, Bailly P (2010) Single PCR multiplex SNaPshot reaction for detection of eleven blood group nucleotide polymorphisms: optimization, validation, and one year of routine clinical use. J Mol Diagn 12:453-460.
Gabrielsen A, Qiu H, Bäck M, Hamberg M, Hemdahl AL, Agardh H,Folkersen L, Swedenborg J, Hedin U, Paulsson-Berne G, Haeggström JZ, Hansson GK (2010) Thromboxane synthase expression and thromboxane A2 production in the atherosclerotic lesion. J Mol Med (Berl)88:795-806.
Hachiya T, Kamatani Y, Takahashi A, Hata J, Furukawa R, Shiwa Y, Yamaji T, Hara M, Tanno K, Ohmomo H, Ono K, Takashima N, Matsuda K, Wakai K, Sawada N, Iwasaki M, Yamagishi K, Ago T, Ninomiya T,Fukushima A, et al. (2017) Genetic predisposition to ischemic stroke: a polygenic risk score. Stroke 48:253-258.
Holliday EG, Maguire JM, Evans TJ, Koblar SA, Jannes J, Sturm JW, Hankey GJ, Baker R, Golledge J, Parsons MW, Malik R, McEvoy M, Biros E, Lewis MD, Lincz LF, Peel R, Oldmeadow C, Smith W, Moscato P,Barlera S, et al. (2012) Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet 44:1147-1151.
Hsu PY, Tsai AL, Wang LH (2000) Identi fication of thromboxane synthase amino Acid residues involved in heme-propionate binding. Arch Biochem Biophys 383:119-127.
Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS,Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL,Rosamond WD, Rivadeneira F, et al. (2009) Genomewide association studies of stroke. N Engl J Med 360:1718-1728.
Iñiguez MA, Cacheiro-Llaguno C, Cuesta N, Díaz-Muñoz MD, Fresno M(2008) Prostanoid function and cardiovascular disease. Arch Physiol Biochem 114:201-209.
Ishizuka T, Kawakami M, Hidaka T, Matsuki Y, Takamizawa M, Suzuki K, Kurita A, Nakamura H (1998) Stimulation with thromboxane A2 (TXA2) receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol 112:464-470.
Jerrard-Dunne P, Cloud G, Hassan A, Markus HS (2003) Evaluating the genetic component of ischemic stroke subtypes: a family history study.Stroke 34:1364-1369.
Kim SR, Bae SK, Park HJ, Kim MK, Kim K, Park SY, Jang HO, Yun I, Kim YJ, Yoo MA, Bae MK (2010) Thromboxane A(2) increases endothelial permeability through upregulation of interleukin-8. Biochem Biophys Res Commun 397:413-419.
Koudstaal PJ, Ciabattoni G, van Gijn J, Nieuwenhuis HK, de Groot PG,Sixma JJ, Patrono C (1993) Increased thromboxane biosynthesis in patients with acute cerebral ischemia. Stroke 24:219-223.
Lee TH, Ko TM, Chen CH, Lee MT, Chang YJ, Chang CH, Huang KL,Chang TY, Lee JD, Chang KC, Yang JT, Wen MS, Wang CY, Chen YT, Hsieh CS, Chou SY, Liu YM, Chen HW, Liao HT, Wang CW, et al. (2016) Identification of PTCSC3 as a Novel locus for large-vessel ischemic stroke: a genome-wide association study. J Am Heart Assoc 5:e003003.
Lemaitre RN, Rice K, Marciante K, Bis JC, Lumley TS, Wiggins KL, Smith NL, Heckbert SR, Psaty BM (2009) Variation in eicosanoid genes,non-fatal myocardial infarction and ischemic stroke. Atherosclerosis 204:e58-63.
Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, He L, Shi Y (2009) A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res 19:519-523.
Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ (2007) Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 6:456-464.
Malik R, Bevan S, Nalls MA, Holliday EG, Devan WJ, Cheng YC, Ibrahim-Verbaas CA, Verhaaren BF, Bis JC, Joon AY, de Stefano AL, Fornage M, Psaty BM,Ikram MA, Launer LJ, van Duijn CM, Sharma P,Mitchel BD, Rosand J, Meschia JF, et al. (2014) Multilocus genetic risk score associates with ischemic stroke in case-control and prospectivecohort studies. Stroke 45:394-402.
Matarin M, Brown WM, Dena H, Britton A, De Vrieze FW, Brott TG,Brown RD Jr, Worrall BB, Case LD, Chanock SJ, Metter EJ, Ferruci L,Gamble D, Hardy JA, Rich SS, Singleton A, Meschia JF (2009) Candidate gene polymorphisms for ischemic stroke. Stroke 40:3436-3442.
Miyata A, Yokoyama C, Ihara H, Bandoh S, Takeda O, Takahashi E, Tanabe T (1994) Characterization of the human gene (TBXASl) encoding thromboxane synthase. Eur J Biochem 224:273-279.
Muzaffar S, Shukla N, Massey Y, Angelini GD, Jeremy JY (2011) NADPH oxidase 1 mediates upregulation of thromboxane A2 synthase in human vascular smooth muscle cells: inhibition with iloprost. Eur J Pharmacol 658:187-192.
Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B (1976) Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261:558-560.
Park SA, Park BL, Park JH, Lee TK, Sung KB, Lee YK, Chang HS, Park CS, Shin HD (2009)Association of polymorphisms in thromboxane A2 receptor and thromboxane A synthase 1 with cerebral infarction in a Korean population. BMB Rep 42:200-205.
Pyo MK, Kim JM, Jin JL, Chang KC, Lee DH, Yun-Choi HS (2007) Effects of higenamine and its 1-naphthyl analogs, YS-49 and YS-51, on platelet TXA2 synthesis and aggregation. Thromb Res 120:81-86.
Sellers MM, Stallone JN (2008) Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am J Physiol Heart Circ Physiol 294:H1978-1986.
Shi YY, He L (2005) SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15:97-98.
Wang BZ, Ma YT, Fu ZY, Xie X, Zhang XL, Chen BD, Liu F, Yu ZX (2010)Association of rs10487667 genetic polymorphism of thromboxane synthase with myocardial infarction in Uigur population of Xinjiang.Zhonghua Yu Fang Yi Xue Za Zhi 44:1032-1036.
Wang LH, Kulmacz RJ (2002) Thromboxane synthase: structure and function of protein and gene. Prostaglandins Other Lipid Mediat 68-69:409-422.
Wang LH, Tsai AL, Hsu PY (2001) Substrate binding is the rate-limiting step in thromboxane synthase catalysis. J Biol Chem 276:14737-14743.
Wang YJ, Zhang SM, Zhang L, Wang CX, Dong Q, Gao S, Huang RX,Huang YN, Lv CZ, Liu M, Qin HQ, Rao ML, Xiao Y, Xu YM, Yang ZH,Wang YJ, Wang CX, Wang JZ, Wang WZ, Wang J, et al. (2012) Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther 18:93-101.
Wu X, Zhu B, Fu L, Wang H, Zhou B, Zou S, Shi J (2013) Prevalence,incidence, and mortality of stroke in the chinese island populations: a systematic review. PLoS One 8:e78629.
Wu Z, Yao C, Zhao D, Wu G, Wang W, Liu J, Zeng Z, Wu Y (2001) Sino-MONICA project: a collaborative study on trends and determinants in cardiovascular diseases in China, Part i: morbidity and mortality monitoring. Circulation 103:462-468.
Yamada Y, Fuku N, Tanaka M, Aoyagi Y, Sawabe M, Metoki N, Yoshida H,Satoh K, Kato K, Watanabe S, Nozawa Y, Hasegawa A, Kojima T (2009)Identi fication of CELSR1 as a susceptibility gene for ischemic stroke in Japanese individuals by a genome-wide association study. Atherosclerosis 207:144-149.
Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y,Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ (2013) Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 381:1987-2015.
Yi X, Liao D, Wu L, Chen H, Li J, Wang C (2016b) CYP genetic variants,CYP metabolite levels, and symptomatic carotid stenosis in ischemic stroke patients. J Atheroscler Thromb 23:621-631.
Yi X, Lin J, Luo H, Wang C, Liu Y (2017) Genetic variants of PTGS2, TXA2R and TXAS1 are associated with carotid plaque vulnerability, platelet activation and TXA2 levels in ischemic stroke patients. PLoS One 12:e0180704.
Yi XY, Liao DX, Wang C, Cheng W, Fu XQ, Zhang B (2016a) Cytochrome P450 genetic variants and their metabolite levels associated with plaque stability in ischemic stroke patients. J Atheroscler Thromb 23:330-338.
- 中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- Regulatory role of calpain in neuronal death
- The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells
- Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation
- Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury

