茶叶调节SREBPs的降脂作用
潘联云,鹿颜,龚雨顺
茶叶调节SREBPs的降脂作用
潘联云,鹿颜,龚雨顺*
湖南农业大学园艺园林学院,国家植物功能成分利用工程技术研究中心,茶学教育部重点实验室,湖南省植物功能成分利用协同创新中心,湖南 长沙 410128
茶叶可调节不同组织的脂质代谢,抑制肠道消化吸收脂质,起到降脂减肥作用。茶叶对脂质代谢途径具有显著影响,主要通过调控固醇调节元件结合蛋白(Sterol Regulatory Element Binding Proteins)及其上下游因子表达,影响脂质合成和分解,从而降低脂肪积累。
茶;肥胖;脂质代谢;SREBP
肥胖与糖尿病、高脂血症、动脉粥样硬化和某些癌症等密切相关[1-2]。茶叶可以调控脂质代谢途径中的关键基因或限制酶,如(同源哺乳动物)或硬脂酰辅酶A去饱和酶(Stearyol-Co A desaturase, SCD),降低肥胖和相关疾病发生率[3-7]。探索茶叶降脂作用及其功能机理,有利于深入研究茶叶的保健功能。
1 茶叶降脂研究现状
流行病学研究表明,茶叶具有降脂减肥作用[8-9],长期喝茶人群的脂肪堆积明显降低,腰、臀围比显著下降[10]。茶叶及茶叶生物活性成分(茶多酚和儿茶素等)可以降低体重、减少脂肪含量、抑制高血脂症与糖尿病、降低动脉粥样硬化指数、促进低密度脂蛋白受体表达,以及降低血浆甘油三酯、胆固醇和低密度脂蛋白水平[11-13]。
1.1 茶叶降低脂肪含量
绿茶可以降低Wistar大鼠脂肪组织总重量,不影响肌肉组织和结蹄组织重量[14]。普洱茶可以降低C57BL/6J小鼠脂肪含量[15],改变秀丽线虫脂滴的大小和数量[16]。茯砖茶特有的冠突散囊菌提取液能够降低3T3-L1脂肪细胞和秀丽线虫的脂质含量,抑制脂肪沉积[17]。除此之外,红茶和乌龙茶也能够降低C57BL/6J小鼠内脏脂质积累[18]。
1.2 茶叶调节肝脏、胰脏、骨骼肌和脂肪组织的脂质代谢
茶叶提取物能够促进肝脏脂肪氧化,减少脂肪和葡萄糖生成;增加肌肉组织对葡萄糖吸收和促进脂肪氧化,提高线粒体的生物合成,促进有氧呼吸和代谢作用;提高脂肪组织胰岛素敏感性,降低脂质合成和分解代谢,减少异位脂肪沉积,减少肥胖的发生[19-21]。此外,茶叶还可以调控胰脏减少胰岛素分泌,降低胰岛素水平、降低脂质合成[22](图1)。
不同茶类调节肝脏、胰脏、骨骼肌和脂肪组织的脂质代谢机制各不相同。绿茶含有丰富的儿茶素、茶氨酸和咖啡碱等物质,可以促进肝脏和骨骼肌脂肪酸的β氧化和生热作用[23-26],可提高血清脂联素合成[27-29],还能降低脂肪组织的脂质合成、吸收和沉积[30-31]。红茶的大分子多酚可以通过抑制胰脏分脂肪酶来减少脂质吸收[18,32]。乌龙茶降脂机制和红茶相似,乌龙茶含有聚酯型儿茶素,能够促进去甲肾上腺素分泌和抑制胰脂肪酶活性,从而提高脂质分解和减少脂肪吸收[18,33]。
1.3 茶叶抑制脂质消化吸收
茶叶通过影响脂质乳化、消化和胶束增溶作用干扰肠道吸收脂质(图1)。通过研究体外细胞和高血脂、动脉粥样硬化的大鼠[34-35],以及进行大鼠肠系膜淋巴管插管实验[34, 36-38],发现绿茶及其活性成分儿茶素,尤其是表没食子儿茶素没食子酸酯((-)-Epigallocatechin gallate, EGCG),可以影响脂质乳化、消化和胶束增溶作用,阻碍肠道脂质吸收和肝脏脂肪生成[20,39]。茶多酚可以通过氢键和疏水键与消化蛋白酶和蛋白质进行结合,降低消化系统对营养物质的吸收作用[22]。红茶中茶多酚和EGCG能够干扰脂肪乳化,抑制哺乳动物磷脂酶和胰脂肪酶的活性,降低脂质吸收[31, 40-41]。
以秀丽线虫为模型,通过喂食GFP-OP50(带有绿色荧光的大肠杆菌)观察普洱茶对线虫饮食的影响,结果发现饮用普洱茶的实验组,其咽泵运动频率和肠道荧光强度都明显降低[16]。一种特殊的发酵绿茶可以改变大鼠肠道厚壁菌/拟杆菌和拟杆菌/普氏菌的比率,降低肥胖发生和脂肪肝形成[42]。
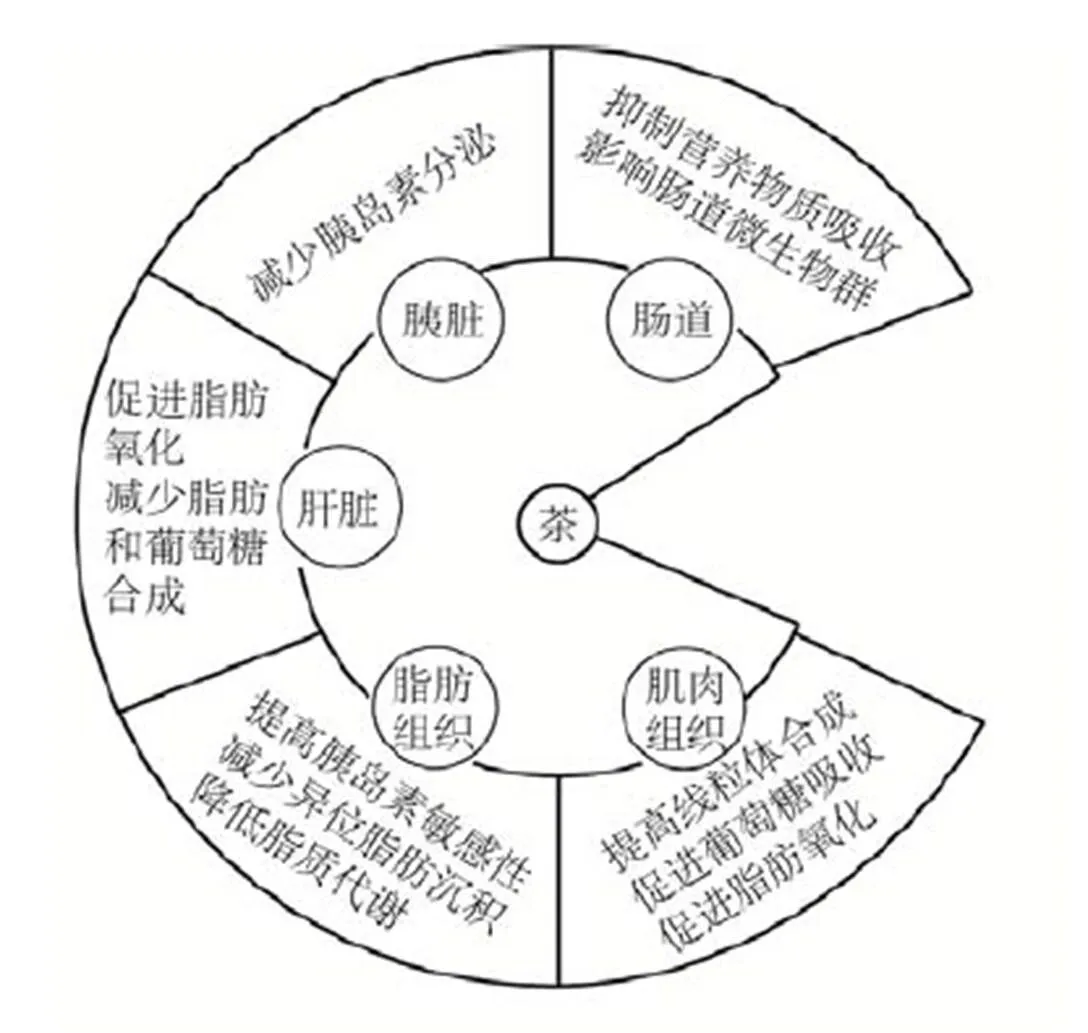
图1 茶叶在组织和器官中的作用
综上所述,未发酵茶、发酵茶和微生物发酵茶均能降低脂肪含量。但不同类型的茶或者来自不同地方同一类型的茶,其降脂减肥机制和作用效果各不相同;同一种茶的不同成分作用效果也各不相同。增强脂质氧化、提高能量消耗或减少食物摄入、抑制脂肪和营养物质吸收是茶叶降脂减肥作用的关键。
2 茶叶调节脂肪相关因子SREBP研究现状
在生物生命活动中,脂肪酸合成、转运和氧化,糖酵解和糖异生等过程都能影响脂质积累[43]。近期研究发现,茶叶对脂质代谢SREBP途径具有显著影响,茶叶活性成分通过调节SREBP及其上游元件(AMPK、PPARγ、FXR与LXR)和下游元件(ACC、FAS、SCD、HMGCR、GPAT与FAT-5/6/7)来抑制脂肪生成,降低脂肪酸不饱和度,减少生物自身脂肪积累[44-46](图2)。
2.1 脂质代谢因子SREBP概述
SREBP属于碱性螺旋-环-螺旋亮氨酸拉链转录因子家族,是真核生物调节脂质合成的关键核转录因子。未活化的SREBP前体先在内质网上合成,然后经过第1位点蛋白酶(Site 1 protease, S1P)和第2位点蛋白酶(Site 2 protease, S2P)的两次裂解活化,成为成熟核型SREBP(n-SREBP),最后转移到细胞核内,与靶基因(脂肪酸、胆固醇代谢基因和SREBP)启动子/增强子的固醇调节元件(Sterol response element, SRE)或E-box结合激活转录[47]。SREBP有3种亚型:包括同一基因编码、通过选择性剪切形成的两个亚型SREBP-1c和SREBP-1a,以及由另1个独立基因编码的SREBP-2亚型[48-49]。
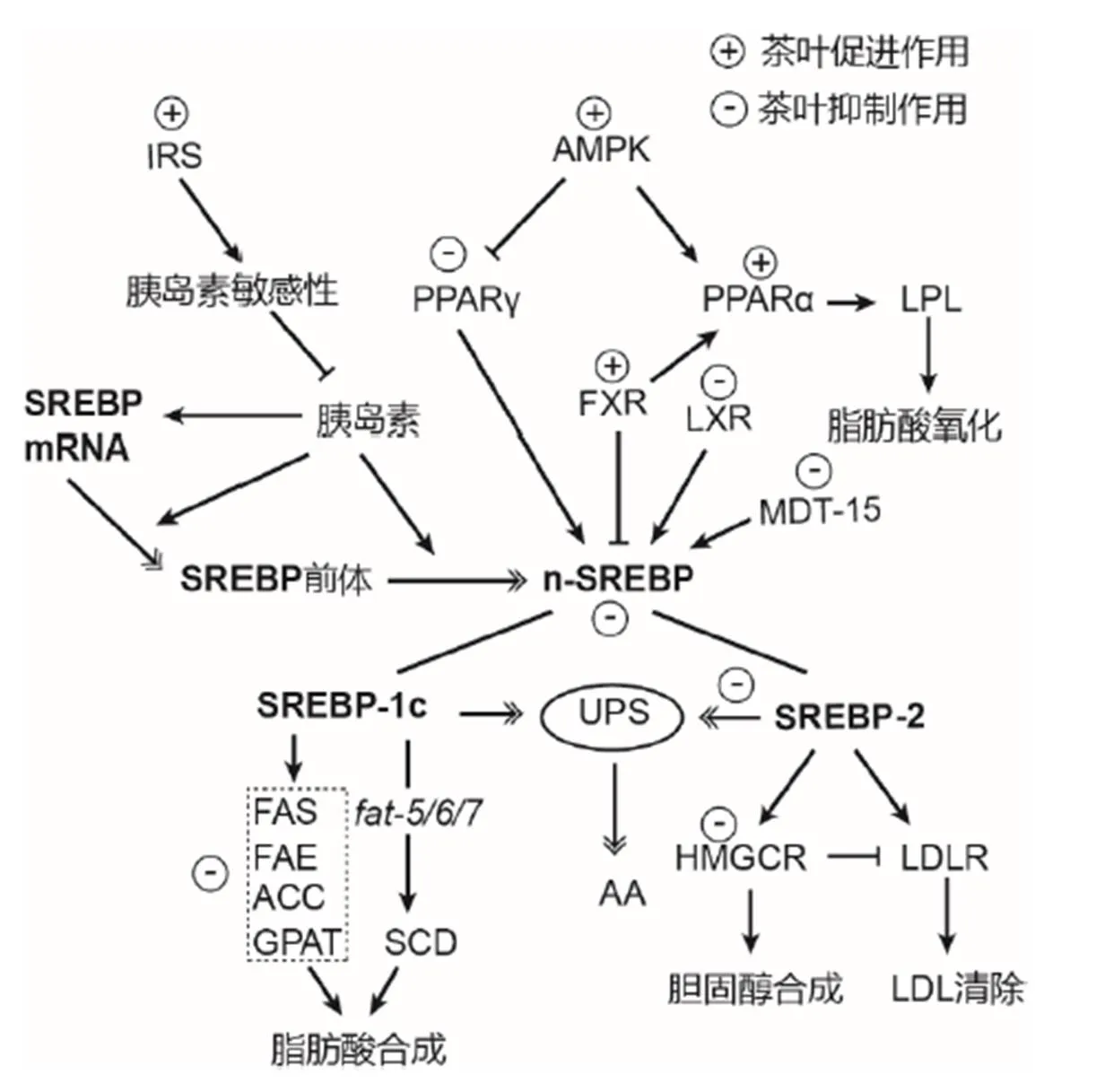
注:→:激活、促进或正调控;:抑制或负调控;:SREBP合成和降解。UPS:泛素-蛋白酶体系统;AA:氨基酸。
无脊椎动物与哺乳动物中脂肪的合成、分化都需要SREBP参与,SREBP与脂质代谢转录调节因子的辅因子MDT-15相互作用,调节脂肪酸与胆固醇生成和转运[50-51]。SREBP-1c主要调控脂肪酸合成途径的基因表达,SREBP-2主要调控胆固醇合成途径的基因表达以及低密度脂蛋白受体基因表达,SREBP-1a则是全身性低水平行使以上两种亚型的功能[15-16, 52]。SREBPRNAi或SREBP功能缺失的生物会出现生长延迟、脂肪含量降低、肠道脂滴减少和脂肪合成基因(和)表达量下降等现象[53-54]。
2.2 茶叶对SREBP的调节机制
2.2.1 茶叶对SREBP-1c下游因子的调节机制
SREBP-1c是乙酰辅酶A羧化酶(Acetyl-CoA carboxylase, ACC)、脂肪酸合成酶(Fatty acid synthetase, FAS)、SCD、脂肪酸延伸酶(Fatty acids extends enzyme, FAE)和甘油脂酰转移酶(Glycerol phosphate acyltransferase, GPAT)的上游因子[15,55]。茶叶提取物能够影响SREBP-1c表达、降低甘油三酯含量、刺激脂质氧化分解,且呈剂量依赖性趋势[15,55-56]。在分化的脂肪前体细胞中,0.5%白茶提取物能够降低SREBP-1c的mRNA与蛋白表达水平,抑制脂肪合成[56-57]。茶叶提取物特别是EGCG通过下调脂肪生成基因的表达来抑制脂肪生成[3,58-59]。EGCG能够下调H4IIE大鼠肝癌细胞中FAS和SCD的mRNA表达水平[60]。相比对照组,含有1.0% EGCG的高脂饮食的实验组C57BL/6J小鼠血浆中甘油三酯的含量明显降低,脂肪酸合成限速酶基因(ACC, SCD和FAS等)表达显著下调[61]。
此外,正常培养条件下茶叶提取物下调秀丽隐杆线虫SBP-1(同源哺乳动物SREBP)的表达,从而抑制其靶目标基因SCD活性,导致脂肪储存下降[16]。在高脂饮食条件下,茶叶活性成分能够降低C57BL/6J小鼠脂肪酶mRNA表达,抑制脂肪和胆汁酸积累,影响粪便总胆固醇的含量[15]。在高葡萄糖环境中,绿茶和红茶都会增加脂肪酸合成酶(SREBP-1c, FAS, ACC)和氧化酶(PPAR-α, CPT-1, ACO)的表达,促进肝脏脂肪合成和分解,提高脂质代谢,抑制脂肪分化,抑制脂肪组织吸收脂肪酸,降低脂肪和葡萄糖积累,减少葡萄糖的毒害作用和提高机体对葡萄糖的耐受性[50,62]。高果糖饮食增加大鼠肝脏SREBP-1c、FAS和SCD1的mRNA表达,通过长期饮茶处理,这些基因的表达显著下调[20,63-64]。
2.2.2 茶叶对SREBP-2下游因子的调节机制
低密度脂蛋白受体(Low density lipoprotein receptor, LDLR)和羟甲基戊二酸单酰辅酶A还原酶(3-hydroxy-3-methyl glutaryl coenzyme A reductase, HMGCR)是SREBP-2下游因子,主要参与调控胆固醇的合成和转运[15,55]。LDLR是清除低密度脂蛋白的跨膜受体,HMGCR是胆固醇生物合成的第一步限速酶。普洱茶通过竞争性抑制HMGCR减少细胞胆固醇合成,从而反馈刺激LDLR数量和活性增加,促进血清胆固醇清除[15,65-67]。绿茶及其活性成分茶多酚可以作为一种蛋白酶体抑制剂,抑制HepG2和HeLa活化的SREBP-2泛素化-蛋白质降解,促进LDLR表达[68-69]。高脂饮食的实验组C57BL/6J小鼠饮用普洱茶70 d后,HMGCR的mRNA表达水平显著下调,且呈剂量依赖性趋势[15]。
2.2.3 茶叶对SREBP上游因子的调节机制
腺苷酸活化蛋白激酶(Adenosine 5′-monophosphate (AMP)-activated protein kinase, AMPK)、肝脏X活化受体(Liver X-activated receptor, LXR)、FXR(法尼酯衍生物X受体)和过氧化氢酶体增殖激活受体(Peroxisome proliferator-activated receptor, PPAR)是SREBP的上游因子,茶叶可以通过调控这些基因来间接调节SREBP转录和蛋白剪切,从而调控脂肪酸和胆固醇的生物合成。
AMPK是调节生物能量代谢的关键因子,作为能量调节开关,AMPK在脂质代谢中发挥着重要作用[70-72]。绿茶EGCG可以通过瘦素和脂联素等肽类激素介导的AMP非依赖通路或AMPK上游激酶,如肝激酶B1(Liver kinase B1, LKB1),激活AMPK磷酸化[27]。磷酸化AMPK增加其下游分子PPARγ的磷酸化水平,降低SREBP活性,进而下调ACC和HMGCR表达,导致脂肪合成和转运下降,脂肪沉积减少[70,73-76];同时,AMPK磷酸化可以进一步促进其下游分子PPARα和脂蛋白脂酶(Lipoprotein lipase, LPL)活性,增强脂肪分解代谢[48-49]。绿茶多酚可以通过抑制细胞外调节蛋白激酶(Eextracellular regulated protein kinases, ERK-1/2)活性降低PPARγ磷酸化,增加PPARγ表达,促进脂联素合成[29],而脂联素能够降低高脂饮食小鼠体重和血糖水平、提高胰岛素敏感性、刺激AMPK磷酸化[27]。
EGCG能显著激活FXR,从而抑制SREBP-1c和促进PPARα表达来降低甘油三酯合成[77]。绿茶茶多酚上调PPARα基因表达促进脂肪氧化,同时下调LXR和PPARγ基因表达降低SREBP-1c转录,从而抑制动脉粥样硬化变病[69]。
此外,胰岛素能够提高SREBP mRNA和前体蛋白水平,以及促进SREBP蛋白的剪切加工[47]。绿茶及其活性物质茶多酚上调胰岛素受体底物(Insulin receptor substrate, IRS)水平,提高哺乳动物胰岛素敏感性,抑制胰脏释放胰岛素,从而下调SREBP mRNA和蛋白水平,减少脂质合成[31,78-79]。茶叶还能够下调转录调节因子辅因子MDT-15,抑制固醇调节元件结合蛋白SREBP、核激素受体NHR-49和核转录因子SKN-1(同源哺乳动物Nrf2)与其结合,从而降低脂肪酸合成[51,53]。
2.2.4 茶叶通过调节SREBP改变脂肪酸组成成分
软脂酸(C16:1)和油酸(C18:1n-9)是主要单不饱和脂肪酸,也是甘油三酯、磷脂质、胆固醇酯的生物合成重要前体物质[80-81]。茶叶提取物能够增加硬脂酸(C18:0)含量,显著降低不饱和脂肪酸油酸和亚麻酸(C18:2)的储存水平[82]。
在饱和脂肪酸(C16:0和C18:0)转化为不饱和脂肪酸(C16:1n-7和C18:1n-9)的过程中,SCD能够促进C9和C10之间双键生成,催化饱和脂肪酸去饱和化[83]。茶叶活性成分可以降低小鼠SCD-1 mRNA表达,增加饱和脂肪酸含量[16]。普洱茶下调C57BL/6J小鼠和秀丽隐杆线虫的SREBP/SBP-1及其相关作用元件如MDT-15的表达,抑制脂质合成过程中关键酶SCD的活性,降低硬脂酸去饱和化,从而导致脂质储存下降[15-16,84]。普洱茶还能够下调编码SCD蛋白的基因(),降低SCD含量,减少脂质积累[85-86]。此外,茶提取物也会下调和来降低油酸/硬脂酸的比率[87-89]。
3 结论
综上所述,茶叶的降脂作用机制主要通过调节脂质代谢关键转录因子SREBP来实现降脂减肥作用。
茶多酚、咖啡碱和茶氨酸是茶叶降脂减肥的主要活性成分,能够有效抑制脂质积累和降低脂肪含量。茶叶及其活性成分降脂减肥的机制如下:(1)抑制脂肪前体细胞增殖;(2)诱导脂肪前体细胞和成熟脂肪细胞凋亡;(3)抑制脂肪前体细胞分化和成熟脂肪细胞生成;(4)抑制肠胃消化酶活性,干扰脂质乳化、水解和胶束增溶作用;(5)干扰肠道乳糜微粒的装配和分泌,阻碍脂质吸收和运输;(6)抑制胰脏分泌胰脂肪酶,减少脂质吸收;(7)增加粪便排泄物脂质含量和全氮含量;(8)改变肠道微生物菌群,影响营养物质吸收;(9)促进脂联素合成,提高胰岛素敏感性,降低胰岛素分泌水平;(10)下调肝脏脂肪和糖原合成基因与相关转录因子,提高肝脏脂肪酸的β氧化基因mRNA水平;(11)促进骨骼肌脂肪酸的氧化和葡萄糖的吸收;(12)提高脂肪组织的脂肪酸氧化和脂质分解基因表达,抑制脂肪组织葡萄糖吸收和脂肪合成相关因子表达;(13)调节脂质合成基因表达,改变脂肪酸组分。
SREBP的mRNA表达、蛋白剪切、加工和降解均与脂质代谢密切相关。在SREBP脂质代谢途径中,茶叶及其活性成分即可直接抑制SREBP,也可通过上调AMPK和FXR或下调PPARγ、LXR和MDT-15表达来间接抑制SREBP。茶叶提取物通过提高AMPK磷酸化、激活FXR、下调LXR和PPARγ来下调SREBP mRNA表达或抑制SREBP蛋白活性。同时,茶叶提取物也可以通过抑制ERK-1/2提高PPARγ表达,促进脂联素合成,继而刺激AMPK磷酸化,抑制PPARγ表达,从而降低SREBP活性,因此ERK-1/2和AMPK途径相互抑制又相互依赖。
茶叶活性物质还能够通过促进脂联素合成或者上调IRS来提高胰岛素敏感性,降低胰岛素水平,抑制SREBP表达。茶叶活性物质还能够通过下调SREBP的转录调节因子辅因子来降低SREBP水平。
脂肪酸合成酶(SREBP-1c、ACC、SCD和FAS等)和氧化酶(PPAR-α、CPT-1、ACO)基因是调节脂肪酸合成和脂质积累的重要因子。正常情况下,茶叶提取物下调脂肪酸合成酶基因,上调氧化酶基因,提高脂质分解,减少脂肪酸合成,降低脂肪含量。在高糖条件下,为了减少高糖对生物体的毒害作用,茶叶提取物能够同时提高脂质合成和氧化基因表达,促进糖异生作用,增加脂肪酸氧化分解,降低脂质,提高葡萄糖耐受性。当SREBP-1c活性降低则会进一步降低脂肪酸合成酶基因FAS、ACC、SCD、CPAT、HMGCR、FAT-5/6/7和LDLR的表达,导致甘油三酯含量下降、脂质积累降低。据研究,茶叶对SREBP-2下游因子LDLR和HMGCR具有调控作用。茶叶提取物能够抑制活化的SREBP-2降解,提高LDLR表达,清除多余的胆固醇;相反,抑制未活化的SREBP-2表达,可以下调HMGCR mRNA水平,减少胆固醇合成。此外,茶叶还能够竞争性抑制HMGCR表达,反馈调节LDLR活性,进而调节胆固醇的合成和转运。
肥胖发生不仅取决于脂肪含量,还取决于脂肪酸的组成成分,特别是多不饱和脂肪酸(Polyunsaturated Fatty Acids, PUFAs)的组分,如ω-3 PUFAs可以通过抑制肠道吸收甘油三酯和肝脏合成内源甘油三酯等途径降低血浆甘油三酯水平。茶叶提取物通过抑制SREBP表达,下调ACC、FAS、FAE和SCD表达,从而分别抑制乙酰辅酶A转化、饱和脂肪酸合成、延伸和去饱和化,继而影响脂肪酸组成成分。
茶叶可通过多条途径和多个作用位点调控脂质代谢,不同茶叶对脂质代谢调控作用机制不同,其效果也不同。据统计,研究茶叶降脂减肥机制以绿茶为主,而红茶、乌龙茶、黑茶和普洱茶研究仍然较少。自然环境的不可控性、制茶工艺的多样性、茶叶活性成分的不稳定性、脂质代谢机制的复杂性限制了我们对茶叶降脂减肥机制的研究。因此茶叶降脂减肥机制还需要从多方面深入研究探索。
[1] MOKDAD A H, FORD E S, BOWMAN B A, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors [J]. Jama, 2003, 289(1): 76-79.
[2] FINUCANE M M, STEVENS G A, COWAN M J, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants [J]. The Lancet, 2011, 377(9765): 557-567.
[3] MOON H-S, LEE H-G, CHOI Y-J, et al. Proposed mechanisms of (−)-epigallocatechin-3-gallate for anti-obesity [J]. Chemico-biological Interactions, 2007, 167(2): 85-98.
[4] FUJITA H, YAMAGAMI T. Efficacy and safety of Chinese black tea (Pu-erh) extract in healthy and hypercholesterolemic subjects [J]. Annals of Nutrition and Metabolism, 2008, 53(1): 33-42.
[5] HOU Y, SHAO W, XIAO R, et al. Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model [J]. Experimental Gerontology, 2009, 44(6): 434-439.
[6] FUJITA H, YAMAGAMI T. Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia [J]. Nutrition Research, 2008, 28(7): 450-456.
[7] CAO Z H, GU D H, LIN Q Y, et al. Effect of Pu-erh tea on body fat and lipid profiles in rats with diet-induced obesity [J]. Phytotherapy Research, 2011, 25(2): 234-238.
[8] WOLFRAM S, WANG Y, THIELECKE F. Anti-obesity effects of green tea: from bedside to bench [J]. Molecular Nutrition & Food Research, 2006, 50(2): 176-187.
[9] HURSEL R, VIECHTBAUER W, WESTERTERP-PLANTENGA M. The effects of green tea on weight loss and weight maintenance: a meta-analysis [J]. International Journal of Obesity, 2009, 33(9): 956-961.
[10] KOVACS E M, LEJEUNE M P, NIJS I, et al. Effects of green tea on weight maintenance after body-weight loss [J]. British Journal of Nutrition, 2004, 91(3): 431-437.
[11] NAGAO T, HASE T, TOKIMITSU I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans [J]. Obesity, 2007, 15(6): 1473-1483.
[12] RAINS T M, AGARWAL S, MAKI K C. Antiobesity effects of green tea catechins: a mechanistic review [J]. The Journal of Nutritional Biochemistry, 2011, 22(1): 1-7.
[13] KHAN N, MUKHTAR H. Tea polyphenols for health promotion [J]. Life Sciences, 2007, 81(7): 519-533.
[14] ASHIDA H, FURUYASHIKI T, NAGAYASU H, et al. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors [J]. Biofactors, 2004, 22(1/2/3/4): 135-140.
[15] SHIMAMURA Y, MIYUKI Y, SAKAKIBARA H, et al. Pu-erh tea suppresses diet-induced body fat accumulation in C57BL/6J mice by down-regulating SREBP-1c and related molecules [J]. Bioscience, Biotechnology, and Biochemistry, 2013, 77(7): 1455-1460.
[16] DING Y, ZOU X, JIANG X, et al. Pu-erh tea down-regulates sterol regulatory element-binding protein and stearyol-CoA desaturase to reduce fat storage in Caenorhaditis elegans [J]. PloS One, 2015, 10(2): e0113815. Doi:10.1371/journal.pone.0113815.
[17] PENG Y, XIONG Z, LI J, et al. Water extract of the fungi from Fuzhuan brick tea improves the beneficial function on inhibiting fat deposition [J]. International Journal of Food Sciences and Nutrition, 2014, 65(5): 610-614.
[18] HEBER D, ZHANG Y, YANG J, et al. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets [J]. The Journal of Nutrition, 2014, 144(9): 1385-1393.
[19] EGAWA T, HAMADA T, MA X, et al. Caffeine activates preferentially α1-isoform of 5′AMP‐activated protein kinase in rat skeletal muscle [J]. Acta Physiologica, 2011, 201(2): 227-238.
[20] SHRESTHA S, EHLERS S J, LEE J-Y, et al. Dietary green tea extract lowers plasma and hepatic triglycerides and decreases the expression of sterol regulatory element-binding protein-1c mRNA and its responsive genes in fructose-fed, ovariectomized rats [J]. The Journal of Nutrition, 2009, 139(4): 640-645.
[21] COLLINS Q F, LIU H-Y, PI J, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase [J]. Journal of Biological Chemistry, 2007, 282(41): 30143-30149.
[22] YANG C S, ZHANG J, ZHANG L, et al. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea [J]. Molecular Nutrition & Food Research, 2016, 60(1): 160-174.
[23] LEE L S, CHOI J H, SUNG M J, et al. Green tea changes serum and liver metabolomic profiles in mice with high-fat diet-induced obesity [J]. Molecular Nutrition & Food Research, 2015, 59(4): 784-794.
[24] MURASE T, HARAMIZU S, SHIMOTOYODOME A, et al. Reduction of diet-induced obesity by a combination of tea-catechin intake and regular swimming [J]. International Journal of Obesity, 2006, 30(3): 561-568.
[25] SHINICHI MEGURO T H, TADASHI HASE. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract [J]. PloS One, 2015, 10(3): e0120142. Doi:10.1371/journal.pone.0120142.
[26] CUNHA C A, LIRA F S, ROSA NETO J C, et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet [J]. Mediators of Inflammation, 2013 (6778): 635470. Doi:org/10.1155/2013/635470.
[27] SANTAMARINA A B, OLIVEIRA J L, SILVA F P, et al. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet [J]. PloS One, 2015, 10(11): e0141227.Doi:10.1371/journal.pone.0141227.
[28] YANG X, YIN L, LI T, et al. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ [J]. International Journal of Clinical and Experimental Medicine, 2014, 7(12): 4906-4914.
[29] TIAN C, YE X, ZHANG R, et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARγ-adiponectin pathway [J]. PloS One, 2013, 8(1): e53796.Doi:10.1371/journal.pone.0053796.
[30] JANSSENS P L, HURSEL R, WESTERTERP-PLANTENGA M S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults [J]. The Journal of Nutrition, 2015, 145(5): 864-870.
[31] HUANG J, WANG Y, XIE Z, et al. The anti-obesity effects of green tea in human intervention and basic molecular studies [J]. European Journal of Clinical Nutrition, 2014, 68(10): 1075-1087.
[32] PAN MH, LAI CS, WANG H, et al. Black tea in chemo-prevention of cancer and other human diseases [J]. Food Science and Human Wellness, 2013, 2(1): 12-21.
[33] HUNG M W WL. Chemistry and health beneficial effects of oolong tea and theasinensins [J]. Food Science and Human Wellness, 2015, 4(4): 133-146.
[34] KOO S I, NOH S K. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect [J]. The Journal of Nutritional Biochemistry, 2007, 18(3): 179-183.
[35] SHISHIKURA Y, KHOKHAR S, MURRAY B S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system [J]. Journal of Agricultural and Food Chemistry, 2006, 54(5): 1906-1913.
[36] WANG S, NOH S K, KOO S I. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats [J]. The Journal of Nutrition, 2006, 136(11): 2791-2796.
[37] WANG S, NOH S K, KOO S I. Green tea catechins inhibit pancreatic phospholipase A2and intestinal absorption of lipids in ovariectomized rats [J]. The Journal of Nutritional Biochemistry, 2006, 17(7): 492-498.
[38] NOH S K, KIM J, SEO Y, et al. Green tea (GT) extract lowers the lymphatic absorption of benzo [a] pyrene (BaP) in rats [J]. The FASEB Journal, 2008, 22(s1): 315.5-315.
[39] WOLFRAM S. Effects of green tea and EGCG on cardiovascular and metabolic health [J]. Journal of the American College of Nutrition, 2007, 26(4): 373-388.
[40] IKEDA I, YAMAHIRA T, KATO M, et al. Black tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats [J]. Journal of Agricultural and Food Chemistry, 2010, 58(15): 8591-8595.
[41] GROVE K A, SAE-TAN S, KENNETT M J, et al. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice [J]. Obesity, 2012, 20(11): 2311-2313.
[42] SEO D-B, JEONG H W, CHO D, et al. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice [J]. Journal of Medicinal Food, 2015, 18(5): 549-556.
[43] SALWAY J G. Metabolism at a glance [M]. Wiley-Blackwell, 2016: 50-82.
[44] ASHRAFI K. Obesity and the regulation of fat metabolism [M]. WormBook, 2007: 1-20. Doi:10.1895/wormbook.1.7.1.
[45] KENNEDY L M, PHAM S C, GRISHOK A. Nonautonomous regulation of neuronal migration by insulin signaling, DAF-16/FOXO, and PAK-1 [J]. Cell Reports, 2013, 4(5): 996-1009.
[46] SRINIVASAN S. Regulation of body fat in Caenorhabditis elegans [J]. Annual Review of Physiology, 2015, 77(1): 400-408.
[47] 张进. SREBP小分子调节剂的发现及其作用机制研究 [D]. 上海:华东师范大学, 2014.
[48] WATSON R T, KANZAKI M, PESSIN J E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes [J]. Endocrine reviews, 2004, 25(2): 177-204.
[49] AZZOUT-MARNICHE D, BÉCARD D, GUICHARD C, et al. Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes [J]. Biochemical Journal, 2000, 350(2): 389-393.
[50] LEE D, JEONG D-E, SON H G, et al. SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat [J]. Genes & Development, 2015, 29(23): 2490-2503.
[51] TAUBERT S, VAN GILST M R, HANSEN M, et al. A mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and-independent pathways in C elegans [J]. Genes & Development, 2006, 20(9): 1137-1149.
[52] VENABLES M C, HULSTON C J, COX H R, et al. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans [J]. The American Journal of Clinical Nutrition, 2008, 87(3): 778-784.
[53] YANG F, VOUGHT B W, SATTERLEE J S, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis [J]. Nature, 2006, 442(7103): 700-704.
[54] ASHRAFI K. Mapping out starvation responses [J]. Cell Metabolism, 2006, 3(4): 235-236.
[55] FERRE P, FOUFELLE F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective [J]. Hormone Research in Paediatrics, 2007, 68(2): 72-82.
[56] [56] S HLE J, KNOTT A, HOLTZMANN U, et al. White tea extract induces lipolytic activity and inhibits adipogenesis in human subcutaneous (pre)-adipocytes [J]. Nutrition & Metabolism, 2009, 6(1): 20.Doi:10.1186/1743-7075-6-20.
[57] GREGOIRE F M. Adipocyte differentiation: from fibroblast to endocrine cell [J]. Experimental Biology and Medicine, 2001, 226(11): 997-1002.
[58] LIN J K, LIN-SHIAU S Y. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols [J]. Molecular Nutrition & Food Research, 2006, 50(2): 211-217.
[59] KAO Y H, CHANG H H, LEE M J, et al. Tea, obesity, and diabetes [J]. Molecular Nutrition & Food Research, 2006, 50(2): 188-210.
[60] WOLFRAM S, RAEDERSTORFF D, PRELLER M, et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents [J]. The Journal of Nutrition, 2006, 136(10): 2512-2518.
[61] WOLFRAM S, RAEDERSTORFF D, WANG Y, et al. TEAVIGOTM (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass [J]. Annals of Nutrition and Metabolism, 2005, 49(1): 54-63.
[62] CHEN N, BEZZINA R, HINCH E, et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet [J]. Nutrition Research, 2009, 29(11): 784-793.
[63] BASCIANO H, FEDERICO L, ADELI K. Fructose, insulin resistance, and metabolic dyslipidemia [J]. Nutrition & Metabolism, 2005, 2(1): 5.Doi:10.1186/1743-7075-2-5.
[64] RUTLEDGE A C, ADELI K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms [J]. Nutrition Reviews, 2007, 65(suppl 1): S13-S23.
[65] 吕海鹏, 谷记平, 林智, 等. 普洱茶的化学成分及生物活性研究进展[J]. 茶叶科学, 2007, 27(1): 8-18.
[66] YANG D-J, HWANG L S. Study on the conversion of three natural statins from lactone forms to their corresponding hydroxy acid forms and their determination in Pu-Erh tea [J]. Journal of Chromatography A, 2006, 1119(1): 277-284.
[67] 陈智雄, 齐桂年, 邹瑶, 等. 黑茶调节脂质代谢的物质基础及机理研究进展[J]. 茶叶科学, 2013, 33(3): 242-252.
[68] KUHN D J, BURNS A C, KAZI A, et al. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor [J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2004, 1682(1): 1-10.
[69] KAUL D, SIKAND K, SHUKLA A. Effect of green tea polyphenols on the genes with atherosclerotic potential [J]. Phytotherapy Research, 2004, 18(2): 177-179.
[70] HARDIE D G. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis [J]. Current Opinion in Cell Biology, 2015, 33: 1-7.
[71] XIAO B, SANDERS M J, UNDERWOOD E, et al. Structure of mammalian AMPK and its regulation by ADP [J]. Nature, 2011, 472(7342): 230-233.
[72] ZHOU J, FARAH B L, SINHA R A, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance [J]. PloS One, 2014, 9(1): e87161.Doi:10.1371/journal.pone.0087161.
[73] WAY T-D, LIN H-Y, KUO D-H, et al. Pu-erh tea attenuates hyperlipogenesis and induces hepatoma cells growth arrest through activating AMP-activated protein kinase (AMPK) in human HepG2 cells [J]. Journal of Agricultural and Food Chemistry, 2009, 57(12): 5257-5264.
[74] HUANG H-C, LIN J-K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet [J]. Food & Function, 2012, 3(2): 170-177.
[75] LONG Y C, ZIERATH J R. AMP-activated protein kinase signaling in metabolic regulation [J]. The Journal of Clinical Investigation, 2006, 116(7): 1776-1783.
[76] HARDIE D G, ROSS F A, HAWLEY S A. AMPK: a nutrient and energy sensor that maintains energy homeostasis [J]. Nature Reviews Molecular Cell Biology, 2012, 13(4): 251-262.
[77] 傅冬和, 刘仲华, 黄建安, 等. 茯砖茶降脂功能成分研究[J]. 茶叶科学, 2012, 32(3): 217-223.
[78] WANG S, MOUSTAID-MOUSSA N, CHEN L, et al. Novel insights of dietary polyphenols and obesity [J]. The Journal of Nutritional Biochemistry, 2014, 25(1): 1-18.
[79] CAO H, HININGER-FAVIER I, KELLY M A, et al. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet [J]. Journal of Agricultural and Food Chemistry, 2007, 55(15): 6372-6378.
[80] WATTS J L. Genetic dissection of polyunsaturated fatty acid synthesis in caenorhabditis elegans [C]. Proceedings of the National Academy of Sciences, 2002, 99(9): 5854-5859.
[81] KNIAZEVA M, CRAWFORD Q T, SEIBER M, et al. Monomethyl branched-chain fatty acids play an essential role in caenorhabditis elegans development [J]. PLoS Biology, 2004, 2(9): e257.
[82] HODSON L, FIELDING B A. Stearoyl-CoA desaturase: rogue or innocent bystander? [J]. Progress in Lipid Research, 2013, 52(1): 15-42.
[83] NTAMBI J M, MIYAZAKI M. Regulation of stearoyl-CoA desaturases and role in metabolism [J]. Progress in Lipid Research, 2004, 43(2): 91-104.
[84] JEON T-I, OSBORNE T F. SREBPs: metabolic integrators in physiology and metabolism [J]. Trends in Endocrinology & Metabolism, 2012, 23(2): 65-72.
[85] BROCK T J, WATTS J L. Fatty acid desaturation and the regulation of adiposity in caenorhabditis elegans [J]. Genetics, 2007, 176(2): 865-875.
[86] NTAMBI J M, MIYAZAKI M, STOEHR J P, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity [C]. Proceedings of the National Academy of Sciences, 2002, 99(17): 11482-11486.
[87] BROCK T J, WATTS J L. Genetic regulation of unsaturated fatty acid composition in[J]. PLoS Genetics, 2006, 2(7): e108.Doi:10.1371/journal.pgen.0020108.
[88] VAN GILST M R, HADJIVASSILIOU H, JOLLY A, et al. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in[J]. PLoS Biol, 2005, 3(2): e53.Doi:10.1371/journal.pbio.0030053.
[89] LIANG B, FERGUSON K, KADYK L, et al. The role of nuclear receptor NHR-64 in fat storage regulation in[J]. PloS One, 2010, 5(3): e9869.Doi:10.1371/journal.pone.0009869.
The Mechanism of the Lipid-lowering Effect of Tea by Regulating the SREBP
PAN Lianyun, LU Yan, GONG Yushun*
Hunan Agricultural University, College of Horticulture and Landscape, National Engineering Center of Plant Functional Components Utilization, Key Lab of Tea Science, Hunan Co-Innovation Center for Utilization of Botanical Functional Ingredients, Changsha 410128, China
Tea has a lipid-lowering effect through regulating lipid metabolism in different tissues and inhibiting digestion and absorption of lipid. The lipid metabolism pathway affects the synthesis and decomposition of lipid and fat decreasing through regulating the expression of Sterol Regulatory Element Binding Proteins (SREBPs) and its relative factors.
tea, obesity, lipid metabolism, SREBP
TS272;R972+.6
A
1000-369X(2018)01-102-10
2017-06-29
2017-09-20
潘联云,女,在读硕士研究生,主要从事茶叶加工及功能成分化学研究,jiayoualei@sina.com。*通讯作者: gongyushun@foxmail.com

