Patterns of biomass,carbon,and nitrogen storage distribution dynamics after the invasion of pine forests by Bursaphelenchus xylophilus(Nematoda:Aphelenchoididae)in the three Gorges Reservoir Region
Ruihe Gao•Youqing Luo•Zhuang Wang•Hanjun Yu•Juan Shi
Introduction
Forests play a dominant role in carbon(C)and nitrogen(N)storage in the terrestrial ecosystem(Houghton;Li et al.;Noh et al.).However,the understanding of C and N allocation among forest ecosystem compartments remains incomplete due to its complex site-speci fi c characteristics,inconsistent measurement methodologies and de fi nitions,and large spatial and temporal uncertainties(Dixon et al.;Litton et al.;Noh et al.).In China,insect epidemics result in signi fi cant tree mortality across millions of hectares,with substantial effects on C and N storage.
Pine wilt disease(PWD),which is caused by the pine wood nematodeBursaphelenchus xylophilus(Steiner and Buhrer)Nickle(Nematoda:Aphelenchoididae),was fi rst introduced to China from North America in 1982 and is now wide spread in central and southeastern China(Wan et al.;Shi et al.).B.xylophilusis spread via the pine sawyer beetle(Monochamus alternatusHope),and no geographic or physiographic barriers appears to limit its distribution in affected areas.What’s worse,nearly all of the native pine species are highly susceptible to the invasion ofB.xylophiluswithin the current PWD distribution areas in China.As one of the mainB.xylophilushosts,the Masson pine(Pinus massonianaLamb.)is a native species in China and can grow well in the arid,sandy soils and the dry climate areas(Zhao et al.).In China,it is dif fi cult to completely eradicate PWD because pines will be die within 2–3 months after being infected byB.xylophilus.Therefore,theB.xylophilusinvasion has caused huge economic losses in timber industry,important ecological destruction in the forest ecosystem,as well as a huge loss of biomass,C storage and N storage(Shi et al.;Yu et al.;Gao et al.).
Insect-induced forest disturbances have been widely investigated and well documented across many ecosystems(Swank et al.;Schultz and Baldwin;Fujihara;Cohen and Carlton;Frost and Hunter;Kurz et al.;Gandhi and Herms;Kim et al.;Pfeifer et al.;Jeong et al.).For example,Swank et al.()have shown that a certain number of insect herbivores can signi fi cantly in fl uence soil and surface water NO3-.Due to the death and decomposition of infected trees,insect disturbances can cause reduced autotrophic respiration and increased heterotrophic respiration(Kurz et al.;Edburg et al.;Pfeifer et al.;Jeong et al.).Kim et al.()found that after PWD infection,soil fertility was generally higher at undamaged sites than at damaged plots.Additionally,many research studies have proven that forest insects and diseases can markedly affect C and N cycles by reducing leaf area and killing healthy trees(Morehouse et al.;Lorenz and Lal).
However,few studies have speci fi cally addressed the impacts of insect disturbance on the forest ecosystem compartments of biomass,C,and N storage and allocation,particularly among individual trees at the stand level before and following disturbance.Outbreaks of spruce budworm in eastern North America have been shown to signi fi cantly increase forest net primary production(Hicke et al.).Kurz et al.()found that mountain pine beetles can cause forests to serve as net sources of C to the atmosphere for several years in British Columbia.Pfeifer et al.()observed that the C stocks in a lodgepole pine forest of the western United States increased continuously following a bark beetle outbreak,recovering to pre-outbreak levels in 25 years or less.Morehouse et al.()found that the C:N mass ratio of ponderosa pine needle fall in bark beetle infected plots was lower than in uninfected plots throughout the growing season.
However,no studies have been conducted regarding the effects of PWD on pine ecosystem biomass,C,and N storage and allocation.Thus,accurate information must be gathered regarding total ecosystem C and N storage and allocation in the aboveground and belowground biomass of pine stands after PWD outbreaks.Such knowledge is crucial for predicting the responses of regional and global C and N cycling in the future.
The primary objective of this study was to investigate the immediate response and subsequent trajectories of biomass,C,and N storage in the stand-level major ecosystem compartments of Masson pine forest stands following a PWD epidemic.We measured tree characteristics in different Masson pine stands to compare the changes in the number of stems and mean diameter at breast height(DBH)in the overstory layer.We then estimated the biomasses of the ecosystem compartments and assessed the differences of C and N storage to determine the changes to total ecosystem C and N pool sizes as a function of increasing PWD infection in the Three Gorges Reservoir Region.
Materials and methods
Study site
The study took place in the Yiling District(latitude 30°32′–31°28′N,longitude 110°51′–111°39′E),an eastern part of the Three Gorges reservoir region.With an eastern midsubtropical monsoon climate,the Yiling District has a mean annual precipitation and mean annual temperature of 997–1370 mm and 16.6 °C,respectively.P.massonianais the primary coniferous tree species in this area.Since the fi rst occurrence in 2006,B.xylophilushas spread rapidly in Yiling District(Fig.).
According to the vegetation survey data,which provided by the Yiling District Forest Pest and Disease Control and Quarantine Station,most of the Masson pine forests in the study areas originated in the late 1970s(Gao et al.).Before the PWD-induced damage,Masson pine was the most prevalent species (Importance Value(IV)=67.23%),followed by relative lower IV tree species,such asQuercus aliena,Quercus variabilis,Rhus chinensis,andCeltis bungeana(Gao et al.).In this study,the stands were divided into fi ve types based on duration of the PWD infection,which allowed us to compare the impact of PWD on the basic ecosystem characteristics,biomass C and N storage and allocation along a space-for-time chronosequence(Hu et al.).
In our study,ST0 was an uninfected control stand,while ST1,ST3,ST5,and ST7 had all been infected by PWD for 1,3,5,and 7 years,respectively,and contained 2.67,4.33,6.67,and 8.50 Masson pine stumps(stumps indicate infected trees that were removed)per 100 m2(Table),respectively.In addition,each stand type has three stands.Within each stand,three permanent 15 m×15 m Masson pine plots were randomly established that were at least 50 m apart and 50 m from the stand edge,each with same bedrock type and same-facing slopes and aspects.

Fig.1 The current pine wilt disease distribution areas in Yiling District.(Data obtained from the Station of Pest and Disease Control and Quarantine of Yiling District,Hubei province,P.R.China)
Field sampling and measurements
Basic ecosystem ? characteristics
All tree species with diameter at breast height(DBH)>2.5 cm were surveyed in each 15 m×15 m plot,including individual species name,tree height,DBH,and crown width.In the meantime,allP.massonianatrees in the plots have been surveyed forB.xylophilusinfection using Zhao’s PWD rating system(Zhao et al.),which based on the external symptoms and internal changes of theB.xylophilusinfected pine trees.
Overstory biomass estimation
The destructive sampling method of Masson pine trees employed in the present study was similar to the one used in previously reported studies(Peichl and;Li et al.;Noh et al.;Zhao et al.llometric equation for Masson pine tree component biomass was developed using one independent variable(DBH)(Peichl and;Li et al.;Noh et al.;Zhao et al.
Y=axb+εwherexis the stem diameter at breast height(cm),Yis the biomass(kg)of a given tree component,aandbare equation parameters,and ε is the error term.This equation explained signi fi cant levels of variability for all components across the study sites and was thus used to calculate the Masson pine tree component biomasses.
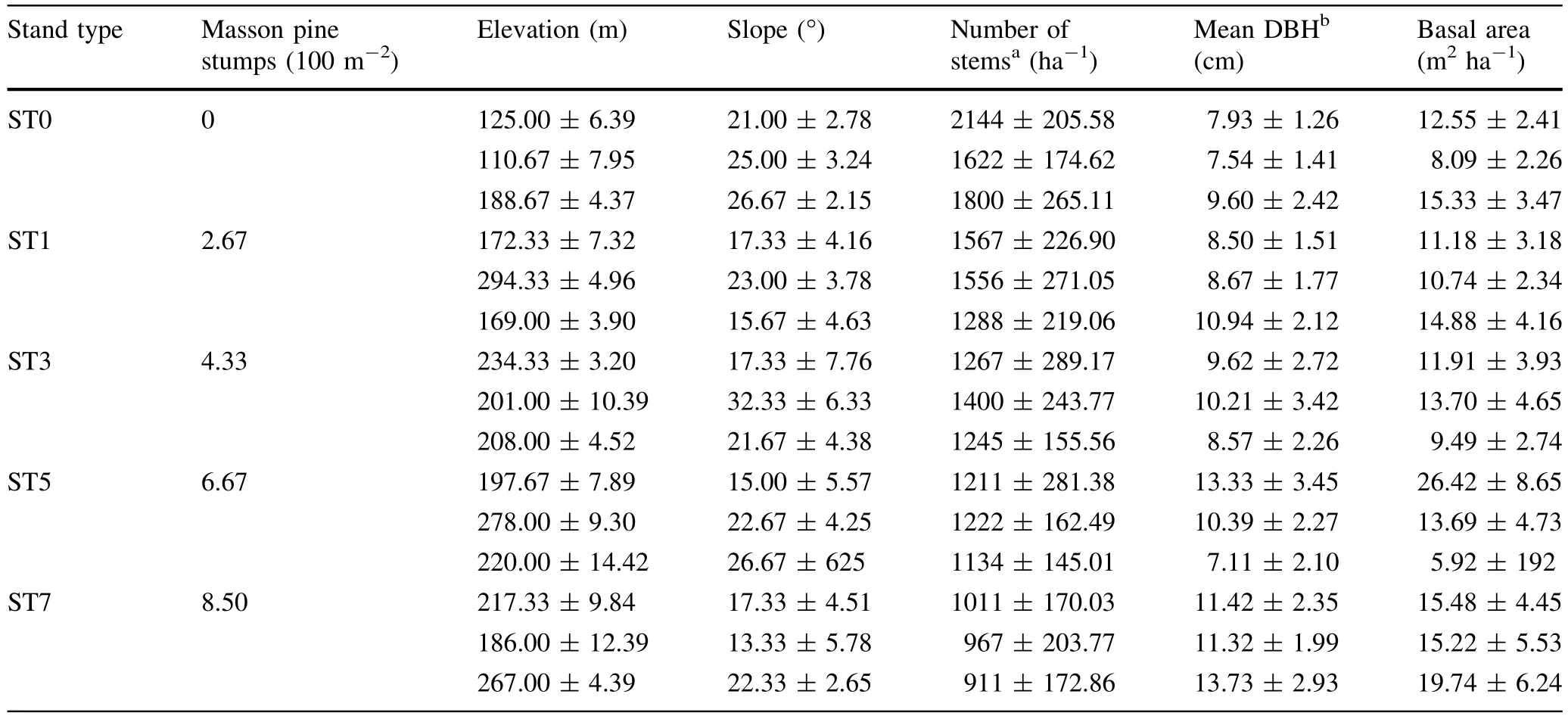
Table 1 Characteristics of Masson pine stands infected by pine wood nematode
To estimate the biomasses of the other tree species,including aboveground and belowground components,we used the allometric regression equations for other tree species(mainlyCinnamomum camphora,Quercus aliena,andQuercus variabilis)in the Three Gorges Reservoir Region developed by Zeng et al.().
Understory biomass estimation
We employed destructive sampling techniques to determine understory biomass.Five subplots were randomly selected in each 15 m×15 m plot.Sampling for the shrub layer was conducted in fi ve 2 m×2 m subplots,and herb layer sampling was conducted in fi ve 1 m×1 m subplots.The samples were collected and oven-dried at 75°C to reach constant weight and then ground into powder using a mill.This powder was used for C and N concentration analysis.
Forest fl oor biomass estimation
Samples of the forest fl oor layer(excepting living plants)were sampled from fi ve random subplots(1 m×1 m)by collecting the total organic material.All collected materials were sorted into two compartment:an undecomposed layer and semi-full decomposed layer(Tao et al.).The samples were oven-dried at 75°C to reach constant weight and then ground in a ring grinder to produce a fi ne powder with a particle size of approximately1 μm.This powder was used for C and N concentration analysis.
The C and N concentrations of the different ecosystem compartment(Masson pine components,understory,and forest fl oor)were determined using an Elemental Analyzer(Elementar Analysensysteme Gmbh,Germany)(Noh et al.;Zhao et al.).
Mineral soil sampling and measurement
Soil C and N storage were estimated by multiplying the C and N concentrations by soil bulk density in each stand.Using a 100 cm3stainless steel cylinder,three replicate soil samples were extracted from four depths(0–10 cm,10–20 cm,20–30 cm,and 30–40 cm in depth)in each study plot.Prior to measurement,rocks,litter,and other large particles were removed manually from the samples,which were then air-dried,ground,and passed through a 2-mm sieve.The bulk density for each soil depth was analyzed by weighing the whole sample and drying subsamples at 105°C.The potassium dichromate oxidation method and Kjeldahl nitrogen method were used to analyze soil organic carbon(%)and soil total nitrogen(%),respectively.
Data analysis
The allometric equations for Masson pine tree component biomass were calculated using simple linear regression analysis:Y=a(DBH)b+ε.The correction factors were determined using the standard error of the estimated,which was converted from base 10 to e-base to obtain the correct value(Noh et al.).One-way analysis of variance(ANOVA)followed by the least signi fi cant difference(LSD)test was performed to detect signi fi cant differences(p<0.05)between stand means and variation within these stands.All statistical analyses were performed using SPSS 18.0 for Windows(SPSS Inc.,Chicago,IL,USA).
Results
Basic ecosystem characteristics
The analyses of the DBH classes of healthy and infected trees are shown in Fig..The results indicate that the rapid spread of PWD kills all sizes of Masson pine.In addition,the majority of the infected Masson pine trees were in the second DBH class(7.51–12.50 cm),comprising 37.96% of the total Masson pine mortality.As DBH increased,the extent of damage tended to decrease.These results suggest that PWD tends to infect young and middle-aged Masson pine trees,leaving larger stems comparatively less infected.

Fig.2 The distribution of diameter classes of healthy and infected Masson pine trees
The stem number and mean DBH in the overstory layer of the Masson pine stands are compared in Figs.and.As expected,uninfected stands had a greater density of total trees and Masson pine trees than infected stands.With the increase of PWD damage degrees,the numbers of Masson pine decreased sharply.The density of this species signi fi cantly differed between ST0 and ST3,ST5,and ST7.Conversely,the number of other species had a little change.Infestation withB.xylophilusincreased the mean DBHs of bothP.massoniaanaand other species,which ranged from 10.67 to 21.95 cm and 3.45 to 4.52 cm,respectively.
Biomass of ecosystem compartments
C storage

Fig.3 The number of stems for all tree species measured in the fi ve Masson pine stands.Different letters indicate a signi fi cant difference among different stand types(p<0.05).Error bars standard deviation(SD)

Fig.4 The distribution of DBH(cm)for all tree species measured in the fi ve Masson pine stands.Different letters indicate a signi fi cant difference among different stand types(p<0.05).Error bars standard deviation(SD)
N storage
Mineral soil
In general,the C and N concentration of the mineral soil decreased signi fi cantly soil depth as the soil depth increased.The mean total C storage of the mineral soil from 0 to 40 cm in ST0 to ST7 was 86.43,80.51,77.35,69.52,and 74.89 Mg C ha-1,respectively(Fig.).More than 70% of soil C was stored within 0–20 cm for each stand type.In addition,little variation was observed in the total mineral-soil nitrogen content as PWD damage degrees increased.The soil nitrogen content within 0–20 cm was much greater in comparison to that in deeper soil(Fig.).

Table 2 Biomass of different ecosystem compartments of Masson pine stands infected by pine wood nematode
Ecosystem C and N pools
The contribution of the forest fl oor to total ecosystem C storage ranged from 4.83 to 9.09%.Tree root C storage represented 5.63%in ST0 and decreased to 4.59%in ST7,with a mean contribution of 5.21%across all stand types.Understory vegetation was the smallest contributor to total ecosystem C storage.
In general,the N storage of the different ecosystem compartments followed a pattern similar to that of biomass and C storage.The N pools of the aboveground trees and tree roots decreased with the extent of infection(Table),and mineral soil and aboveground trees were the two largest contributors to total ecosystem N storage.The contribution of N storage in tree root biomass to total ecosystem N ranged from 1.22%in ST0 to 0.91%in ST7.The contribution of forest fl oor N storage to total ecosystem N ranged from 3.46 to 7.03%,with a mean contribution of 5.35%across all stand types.
Discussion
Pine forest does not become reestablished following PWD-induced mortality but is instead replaced throughout its range by other tree species(Fuj;Fujihara et al.;Yu et al.;Hu et al.is study investigated the immediate response and subsequent trajectories of biomass,C,and N storage in the stand-level major ecosystem compartments of Masson pine forest stands in the Three Gorges Reservoir Region following a PWD epidemic.
Basic ecosystem characteristics

nematode od wo pine by infected ds stan pine Masson of ents artm mp ass co t biom differen pools of ble 3 Carbon Ta 7 ST g C ha-1)(%)(M)(%5 ST g C ha-1)(M 3 ST g C ha-1)(%)(M 1 ST g C ha-1)(%)(M)(%0 ST g C ha-1)(M ents artm mp Co 6.581 2.0 7.592.65 9.96 2.65 12.61 2.43.35 11.8.13 4.36 0.61 3.01 2.65 0±±1±1.7±0±1.9±0±2±0±0.5±1 30.4 4.90 35.30±8.39 3.176±46.8 12.05±58.91±7.49 1.64 9.14 68.05±3.91 1.21 5.126±11.5 15.27±26.83±a 2.498a a.3 2.87a 5a 4a 3..7060 a 4.76a 2a.16.89a 4.20a 1b 1a.51b.23b 4c 1.01c 3.02a 8±±0±0.6±0.2±1±0.7±0±0±0.3±0.2±0±0±1.1 11.4 1.85 13.33±3.17 1.209±17.6 4.55 22.24±2.83 0.62 3.45 25.69±1.48 0.46 1.93 4.36 5.77.13±10.75±37 9.08.40 8 10.4.39.90.7722 3.65.6 17.4±1.98±0 19 13.55.12 0.86 2.98 3.76.64±0±±2±1±0.46±2.43±0±0±0.68 7±325.35.0 389.18 3.47.65±50.87±12.52±636.01 1.30 7.31.83±702.62 0.90 3.53.49±10.17±15 25.6 a 3.463a a a.5 4.00a 1a 4a 5.25.39a 6.64a 5a 7a.93c 7..4281 a 5a.26a.33ab 4c.13 c 7.19a 4±±0±0.9±0.3±1±0.7±0.1±0±0±0.0±0±0±1±1.4 12.4 2.04 14.48±3.50 1.321±19.3 4.91 24.21±2.29 0.51 2.790±27.0 1.00 0.34 1.34 4.00 5.78 9.78 38.13±8.60 9.95 6±1.35 5 2.3.87.12 13 3.44 16.5.19.50.1 19.91.31 0.25 1.19.13±1±7±±0 0±7±±2±0±2.69 4±±0±0±0.60±1.31 5±3±365.98 42.1 10.2 3.89.27±56.2 14 70.4 5.49 1.19 6.67.1 771.93 0.89 2.82 9.37 10.6 20.0 3.46a.54aa 4.00.93a.35a 5.27a a 6.66.88a.2aa 7.70.37a.13a.24a.10b.48ba 7.33.52±±0 3±±0±0±1.38a 3±±0±0±1.08a 1±±0±0±0±0.53a±0±0 0±142.41 16.9 4.13 1.56.62±225.71 28.3 2.21 0.48 2.68.0 310.78 0.36 1.14 3.77 4.28 8.05 40.2 11.76 8.53 13 3.03 17.70 42.27 5.25 77.81 20 2915 0.18 39.40±±1.7 6.58.98±±1.14±1.3±0.30±1.6±0.3±0.1±0.19±0.6±0.4 45.29±114.28 61.56±.45±15.01±774.95 1.07 6.02.03±831.46 0.77 2.24 8.44 6.28.72±14 4.77a 2a 5.49a 7.18a 2a 9.10a 9a 8.44a 3a 5a 8a 8.38a.98±±0.7±1.23a±0.46a±1.9±0.56a±0.12a±0.6±0.1±0.08a±0.08a±0.2±0.1±0.07a 152.67.65±184.58 1.74 24.96±6.27.22±312.01 0.43 2.44.67±330.59 0.31 0.91 3.42 2.55 5.97.55±40 4.2111 14.27 16.5 3.88.46.85 21 5.66 27.5.15.48 26.8.46.33.23.00 0.88 3±±2±1±2±0±2.62±0±0±0±0.22±1.44±2±4±7±1±8±2±396.71 46.1 11.5 4.38.0 62 15.3 77.4 5.00 1.08 6.08 83.4 1.40 1.00 2.40 8.15 5.97 14.1 6.41a 2a 7.43a 4a 9.83a 5a.38a 12 7a 8a 12.07a 1a 5a 0a 5a 0a.64a 11 17.74±±1.0 3.02.76±±1.7±0.66a±2.5±0.9±0.22a±1.1±0.2±0.1±0.11a±0.1±0.4±0.4 205.19 1.97 27.92±6.92.83±342.25 0.49 2.73.57±370.63 0.45 1.08 3.67 2.69 6.35.00±45 wood d bark un ro d full an Stem Stem stem Tree ch Bran le Need eground Abov ots Ro Total Aboveg Belowground Total Total Shurb HerbtalTo Undecomposed Semi decomposed tal To pine ry Masson p.Other sp erstory f l oor Ov Understo Forest Total level 0.05 p<t at differen wercase letters are signif i cantly different lo by ed a row follow within C stocks of values Mean).n(SD d deviatio ithin-stan±w the stand mean are presented as Data

de nemato wood pine by infected stands pine Masson of ts en ass compartm different biom pools of en ble 4 Nitrog Ta 7 ST g N ha-1)(%)(M 5 ST g N ha-1)(%)(M 3 ST g N ha-1)(%)(M 1 ST g N ha-1)(%)(M 0 ST g N ha-1)(%)(M ents artm mp Co 2.382 3.003 6 5.201 6.21 3.294 4.05 4.508 85 0.73 6.91 6.46.99±±0.6±0.9±1.2±1.0±0.7±0.6±1.2±1.9 103.03.03±144.54 6.18.75±244.59.34±29.86±122.82.69±15.02±453.19 2.86 6.05.88±13.06±35.94±48.01a±0.00a.02a.01a.01a.03a.01a.04a.02a.00a.02a.03a.00b.00a.01b.00b.04c.04c.04a 0.07±0 0.02±0 0.09±0 0.03±0 0.04±0 0.15±0 0.03±0 0.18±0 0.08±0 0.02±0 0.10±0 0.27±0 0.02±0 0.02±0 0.04±0 0.09±0 0.22±0 0.31±0 0.61 3.41.90 4.31.35.83 7.49 8.93 3.53 4.34 4.9 12.47.30 1.07 7.12 8.07 7±±0±1±1±1.45±0.81±0±0±0.77.22±3±3±3±4±8±0±1±1±123.45 15.7 5.17 7.05.9 275.10 33.0 10.7 2.32.0 13 46.0 2.22 2.22 4.44.1 13 36.2 49.3 2a±0.0 1a 3a 1a 1a 4a 1a 5a 2a 0a 2a 8a 0a 0a 0a.0 1ab 4c 5c 8a 0.07±0.0 0.02±0.0 0.10±0.0 0.03±0.0 0.04±0.0 0.17±0.0 0.03±0.0 0.20±0.0 0.07±0.0 0.01±0.0 0.08±0.0 0.28±0.0 0.01±0.0 0.01±0 0.03±0.0 0.08±0.0 0.22±0.0 0.30±0.0 0.60 3.617 4.585 6 7.992 9.51 4.358 5.36 14.74 650 1.83 0.67 1.56 15.16±±0.9 4.30.46±±1.4±1.9±1.5±0.9±0.8±0.8±0.7 196.45 8.80 34.71±6.28.99±40.93±102.36 13.29±.28±541.83 2.45 4.28 13.06±.38±28.44±41±0.02a 4a 3a 0.09±0.01a 0.02±0.03a 0.11±0.01a 0.04±0.01a 0.05±0.0 0.20±0.01a 0.04±0.05a 0.23±0.02a 0.06±0.01a 0.01±0.0 0.07±0.08a 0.31±0.00a 0.01±0.00a 0.01±0.00a 0.02±0.01a 0.07±0.00b 0.16±0.01b 0.23±0.07a 0.56 5.55 6.99 21.29 0.03.39 14 3.07 31.07 5.73 0.96 1.35 0.43.61±±1.44 3±±2.14 1±±2.36 0±±0.68±0.34±0.58±0.35 185.32.9 237.98.9 10.82±427.69.51±50.1 112.40.49±13.00±641.56 2.38 3.94.24±13.83±18.07±32 3a.01 a 4a 1a 1a 7a 2a 0a 6a 0a 0a 0a 1a 1a 5a±0.0.0.06a.01a.0.0.02a.0.0.00a.0.0 0.09±0 0.03±0 0.12±0.0 0.04±0.0 0.06±0 0.22±0 0.04±0 0.25±0 0.06±0.0 0.01±0 0.07±0 0.32±0 0.01±0.0 0.01±0.0 0.02±0 0.07±0 0.10±0 0.16±0.0 0.51 6.743 4.8 8.574 3.73 15.8.02 17.87 4.827 5.89 17.01 992 0.35 3.02 2.82 18.64±±1 5.44 24.08±±2.7 8.16 11.17±43.40±±2 7.66 51.06±11.21±±1.0 2.42 13.64±64.70±±0.4 1.49±0.9 3.10±0.6 4.59 12.80±17.92±30.72±4a±0.0 1a 5a 2a 8a 2a 0a 2a 3a 0a 0a 0a 0a 1a 1a 0.10±0.0 0.03±0.0 0.14±0.0 0.05±0.02a 0.06±0.0 0.24±0.0 0.04±0.1 0.29±0.0 0.06±0.01a 0.01±0.0 0.08±0.1 0.36±0.0 0.01±0.0 0.02±0.00a 0.03±0.0 0.07±0.0 0.10±0.0 0.17±0.07a 0.56 wood ll bark und d fu an Stem Stem stem Tree Branch Needleeground Abov ots egro Ro Total Abov Belowground Total Total Shurb Herb Total Undecomposed Semi decomposed Total pine ry Masson p.Other sp erstory f l oor Ov dersto Un Forest Total level 0.05 p<t at differen if i cantly t lowercase letters are sign differen by ed llow a row fo in with N stocks of values Mean).ithin-stand deviation(SD±w d mean e stan th are presented as Data

Fig.5 Soil carbon storage at different soil depths in the Masson pine forest.Different letters indicate a signi fi cant difference about different stand types in the same soil depth(p<0.05).Error bars standard deviation(SD)
In the study plots,all diameter classes of Masson pine were infected byB.xylophilus,but the greatest rates of infection occurred in classes of relatively lower diameter.This may be due to the smaller Masson pine trees may provide better food source and oviposition sites for the pine sawyer beetle(Zai and Chen 1992;Chai et al.1996).In addition,these results were supported by a study conducted by Shi et al.(2007),who found that small-diameter Masson pine trees were more susceptible to attack by the pine wood nematode in both inland and island environments,as well as in pure and mixed forests.
At sites whereB.xylophilushas caused serious damage,Masson pine mortality has been signi fi cant.What’s worse,the rapid spread of PWD may cause Masson pine functional extinction across its range(Gao et al.).In our study,PWD disturbance caused remarkable changes in overstory vegetation density and mean DBH ofP.massoniaana.This result was mainly due to the selective cutting of PWD-induced mortality of Masson pine trees,which opens short-term ecological niches for other tree species(Fujihara;Hu et al.).
Biomass

Fig.6 Soil nitrogen storage at different soil depths in the Masson pine forest.Different letters indicate a signi fi cant difference about different stand types in the same soil depth(p<0.05).Error bars standard deviation(SD)
The change of total biomass indicating that tree biomass decreased with the increase of damage degrees.The pattern of Masson pine biomass distribution among the different Masson pine components followed the order of stem wood>roots>branches>bark>needles.This pattern is similar to that reported by Ding and Wang(2001),who found the same order of biomass distribution in Masson pine components across different stand ages.Although the infestation ofB.xylophilushas no signi fi cant effect on the biomasses of Masson pine components in the short term,the contribution of Masson pine biomass tends to decrease as the extent of infection increases.Conversely,the biomass of other tree species increased over the extent of PWD infection.These results indicate that the invasion ofB.xylophiluschanges the allocation of overstory biomass in the short term and highlight the importance of other tree species in the succession from Masson pine to broad-leaved forest after infection by PWD.
Many studies have reported that both forest fl oor biomass and understory biomass are highly susceptible to disturbances and variations in stand treatment(Yanai et al.;Johnson et al.;Li et al.).We hypothesized that the thinning Masson pine canopy would transmit morelight to the understory layer after Masson pine mortality,thus enabling rapid increase of understory layer biomass.Our estimates of understory and forest fl oor biomass show an initial decline immediately after PWD infection and recovery over the following several years.Such high variation may be due to the selective cutting of damaged trees,which affectslight,nutrientavailability,and decomposition rate,key factors in the development of understory vegetation and the forest fl oor(Yanai et al.;Peichl and Arain;Li et al.).
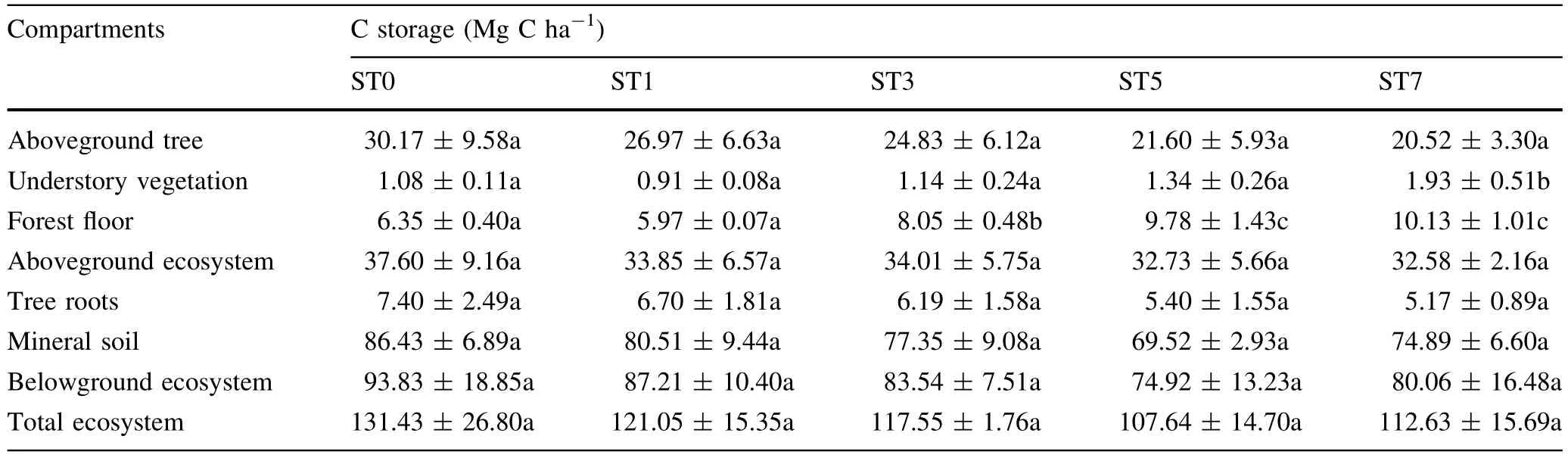
Table 5 Carbon pools of ecosystem compartments of Masson pine stands infected by pine wood nematode
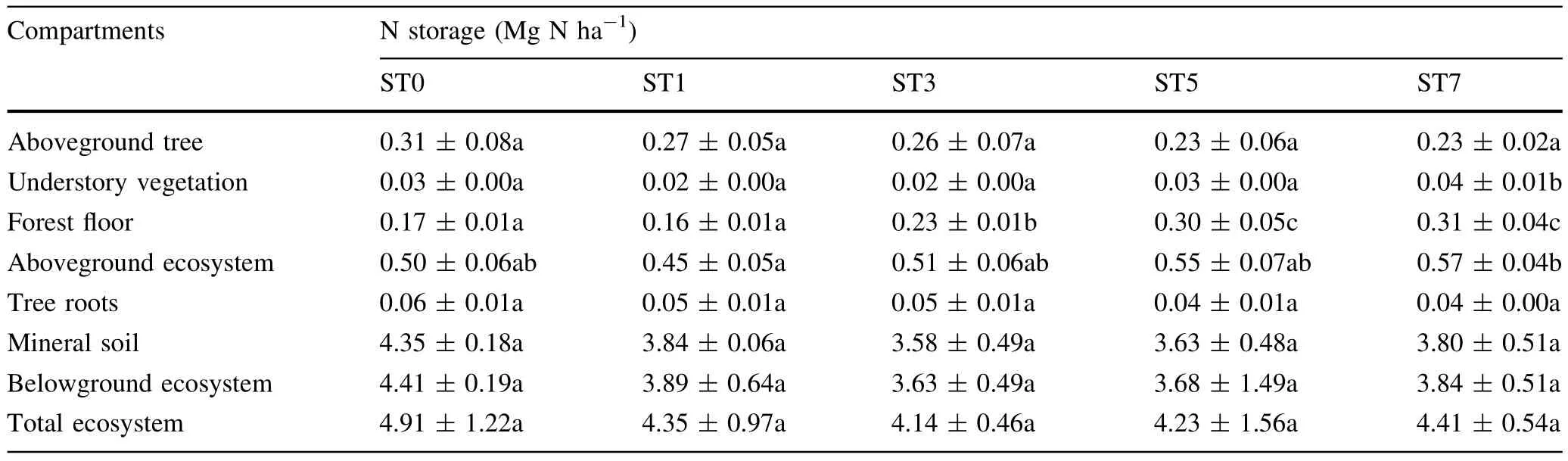
Table 6 Nitrogen pools of ecosystem compartments of Masson pine stands infected by pine wood nematode
C and N storage
In our study,the C and N storage of the different ecosystem compartments followed similar patterns to those of biomass as the extent of infection increases.We found that PWD infestation changed the biomass C and N of the different ecosystem compartments in Masson pine forests.The estimated overstory C and N storage decreased steadily with the extent of infection.Additionally,the C and N storage of other tree species increased with the extent of infection increases,likely due to the selective cutting of Masson pine trees infected byB.xylophilus,which opened canopy gaps and facilitated signi fi cant changes in forest composition and community structure(Fujihara;Spiegel and Leege).
We observed several relationships between C and N storage in the different ecosystem compartments and the extent of infection,but no clear pattern occurred for the pine forest in total.Interestingly,the contributions of Masson pine C and N to the total different compartments biomass C and N pools varied from 77.41 to 58.91%and from 51.06 to 29.34%,respectively,indicating that C and N storage experienced signi fi cant changes following the PWD epidemic.
Generally,stand development is strongly related to C and N storage over the entire life cycle of a forest ecosystem,and C and N cycles are signi fi cantly coupled(Vesterdal et al.;Noh et al.).However,relatively little research is available concerning the impact of PWD on total C and N storage and its allocation in the aboveground and belowground vegetation,forest fl oor,and soil layers of the Masson pine forest ecosystem.Therefore,it is important to understand the shifts in stand-level C and N allocation that maintain C and N balance in Masson pine stands.
Previous studies have reported that stand development is closely associated with C storage in both the forest fl oor layer and understory layer(Covington;Taylor et al.;Noh et al.).The distribution patterns of C and N storage in the understory and forest fl oor layers were similar to that of biomass,which declined initially after PWD infection and recovered almostentirely after 3–7 years.These results may be explained by the changes in species quantity in the understory layer,and of litter input and slow decomposition in forest fl oor layer(Zhao et al.),after the selective cutting of Masson pine trees infected byB.xylophilus.
Due to the interaction among productivity,C sequestration,and N availability,soil C and N storage exhibit variation in managed forests(Jandl et al.),but these differences were not statistically signi fi cant among different stands.These results were supported by a study conducted by Jeong et al.(),who found that soil C storage was relatively unaffected by increasing PWD damage intensity.In addition,over 70% of the total soil C and N was stored within 0–20 cm in depth,where the soil could be easily disturbed.Therefore,investigating the spatial distribution of soil C and N storage and protecting the top soil from disturbances are vital to C and N allocation and sequestration(Gao et al.;Zhao et al.).Additionally,the invasion ofB.xylophilusdecreased total soil C and N storage in the present study.This result is similar to those obtained by Jenkins et al.(),who found that the quality of soil N and soil organic matter declined at sites experiencing adelgid infestation.
In addition,it should be noted that the present study examined sites at 1–7 years after the detection of PWD,which may have been too soon for the Masson pine ecosystem compartments to adequately respond to the weathering processes,light conditions,and changes in forest composition associated with severely Masson pine decline(Kim et al.;Spiegel and Leege).Therefore,we hope to continue to work with these forest ecosystem sites to determine their long-term changes in biomass,C,and N storage distribution dynamics following the PWD outbreak.
Conclusion
The PWD-induced Masson pine mortality demonstrated that biomass,C,and N storage in Masson pine forest stands are driven by the invasion ofB.xylophilus.We found that all diameter classes of Masson pine can be infected byB.xylophilus,and the greatest rates of infection occurred in classes of relatively lower diameter.The invasion ofB.xylophiluschanges the allocation of biomass,C,and N storage in overstory layer,which transformed from Masson pine tree to other tree species.We also observed that the biomass,C,and N storage of the understory and forest fl oor initially declined after PWD infection before recovering over the following several years.Little variation was observed in the total mineral soil C and N storage as PWD damage degrees increased.Additionally,understanding biomass,C,and N storage distribution dynamics and their subsequent trajectories is vital to understand the shifts in stand-level C and N allocation in PWD-damaged forest stands,as well as for predicting the responses of regional and global C and N cycling.
AcknowledgementsAuthors gratefully acknowledge the support from the Hubei Academy of Forestry and Station of Pest and Disease Control and Quarantine of Yiling District,Yichang city,and they are Jingyuan Chen,Chenghao Hong,Dewen Song,and Honggao Liu.
Chai X,He Z,Li C,Tang L,Cheng S(1996)Studies on oviposition habit of the Japanese pine sawyerMonochamus alternatusHope.J Beijing For Univ 19:69–73
Cohen AN,Carlton JT(1998)Accelerating invasion rate in a highly invaded estuary.Science 279:555–558
Covington WW(1981)Changes in forest fl oor organic matter and nutrient content following clear cutting in northern hardwoods.Ecology 62:41–48
Ding G,Wang P(2001)Study on change laws of biomass and productivity of masson pine forest plantation II.Biomass and productivity of stand at different ages.For Res 15:54–60
Dixon RK,Solomon A,Brown S,Houghton R,Trexier M,Wisniewski J(1994)Carbon pools and fl ux of global forest ecosystems.Science 263:185–190
Edburg SL,Hicke JA,Lawrence DM,Thornton PE(2011)Simulating coupled carbon and nitrogen dynamics following mountain pine beetle outbreaks in the western United States.J Geophys Res 116:G04033.doi:
Frost CJ,Hunter MD(2004)Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms.Ecology 85:3335–3347
Fujihara M(1996)Development of secondary pine forests after pine wilt disease in western Japan.J Veg Sci 7:729–738
Fujihara M,Hada Y,Toyohara G(2002)Changes in the stand structure of a pine forest after rapid growth ofQuercus serrataThunb.For Ecol Manag 170:55–65
Gandhi KJ,Herms DA(2010)Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America.Biol Invasions 12:389–405
Gao P,Wang B,Geng GG,Zhang GC(2013)Spatial distribution of soil organic carbon and total nitrogen based on GIS and geostatistics in a small watershed in a hilly area of northern China.PLoS ONE 8:e83592
Gao RH,Shi J,Huang RF,Wang Z,Luo YQ(2015a)Effects of pine wilt disease invasion on soil properties and Masson pine forest communities in the Three Gorges reservoir region,China.Ecol Evol 5:1702–1716
Gao RH,Song DW,Huang RF,Shi J,Luo YQ,Chen JY(2015b)Characteristics of typical Masson pine community and soil properties at the early invasive stage of pine wood nematode in the Three Gorges Reservoir Region of central China.J Beijing For Univ 37:84–91
Hicke JA,Asner GP,Randerson JT,Tucker C,Los S,Birdsey R,Jenkins JC,Field C(2002)Trends in North American net primary productivity derived from satellite observations,1982–1998.Global Biogeochem Cycles 16:2-1–2-14
Houghton RA(2007)Balancing the global carbon budget.Annu Rev Earth Planet Sci 35:313–347
Hu G,Xu X,Wang Y,Lu G,Feeley KJ,Yu M(2012)Regeneration of different plant functional types in a Masson pine forest following pine wilt disease.PLoS ONE 7:e36432
Jandl R,Lindner M,Vesterdal L,Bauwens B,Baritz R,Hagedorn F,Johnson DW,Minkkinen K,Byrne KA(2007)How strongly can forest management in fl uence soil carbon sequestration?Geoderma 137:253–268
Jenkins JC,Aber JD,Canham CD(1999)Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests.Can J For Res 29:630–645
Jeong J,Kim C,Lee KS,Bolan NS,Naidu R(2013)Carbon storage and soil CO2ef fl ux rates at varying degrees of damage from pine wilt disease in red pine stands.Sci Total Environ 465:273–278 Johnson DW,Todd D,Tolbert VR(2003)Changes in ecosystem carbon and nitrogen in a loblolly pine plantation over the fi rst 18 years.Soil Sci Soc Am J 67:1594–1601
Kim C,Jang KS,Kim JB,Byun JK,Lee CH,Jeon KS(2010)Relationship between soil properties and incidence of pine wilt disease at stand level.Landsc Ecol Eng 6:119–124
Kurz WA,Dymond CC,Stinson G,Rampley GJ,Neilson ET,Carroll AL,Ebata T,Safranyik L(2008)Mountain pine beetle and forest carbon feedback to climate change.Nature 452:987–990
Li X,Yi MJ,Son Y,Park PS,Lee KH,Son YM,Kim RH,Jeong MJ(2011)Biomass and carbon storage in an age-sequence of Korean pine(Pinus koraiensis)plantation forests in central Korea.J Plant Biol 54:33–42
Litton CM,Ryan MG,Knight DH(2004)Effects of tree density and stand age on carbon allocation patterns in post fi re lodgepole pine.Ecol Appl 14:460–475
Lorenz K,Lal R(2010)Carbon sequestration in forest ecosystems.Springer,Dordrecht
Morehouse K,Johns T,Kaye J,Kaye M(2008)Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests.For Ecol Manag 255:2698–2708
Noh NJ,Son Y,Lee SK,Seo KW,Heo SJ,Yi MJ,Park PS,Kim RH,Son YM,Lee KH(2010)Carbon and nitrogen storage in an agesequence ofPinus densi fl orastands in Korea.Sci China Life Sci 53:822–830
Noh NJ,Kim C,Bae SW,Lee WK,Yoon TK,Muraoka H,Son Y(2013)Carbon and nitrogen dynamics in aPinus densi fl oraforest with low and high stand densities.J Plant Ecol 6:368–379
Peichl M,Arain MA(2006)Above-and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests.Agric For Meteorol 140:51–63
Peichl M,Arain MA(2007)Allometry and partitioning of above-and belowground tree biomass in an age-sequence of white pine forests.For Ecol Manag 253:68–80
Pfeifer EM,Hicke JA,Meddens AJ(2011)Observations and modeling of aboveground tree carbon stocks and fl uxes following a bark beetle outbreak in the western United States.Global Change Biol 17:339–350
Schultz JC,Baldwin IT(1982)Oak leaf quality declines in response to defoliation by gypsy moth larvae.Science 217:149–151
Shi J,Luo YQ,Song JY,Wu HW,Wang L(2007)Traits of Masson pine affecting attack of pine wood nematode.J Integr Plant Biol 49:1763–1771
Shi J,Luo YQ,Wu HW,Yan XS,Jiang P(2009)Impact of invasion of pine wood nematode on the growth of dominant shrub Pleioblastus amarus inPinus massonianacommunities.For Stud China 11:61–63
Spiegel KS,Leege LM(2013)Impacts of laurel wilt disease on redbay(Persea borbonia(L.)Spreng.)population structure and forest communities in the coastal plain of Georgia,USA.Biol Invasions 15:2467–2487
Swank W,Waide J,Crossley JD,Todd R(1981)Insect defoliation enhances nitrate export from forest ecosystems.Oecologia 51:297–299
Tao LC,Meng XQ,Sun C,Liu QJ(2014)Storage of litter and coarse woody debris of Pinus tabulaeformis plantation in Beijing.J Fujian Coll For 34:26–32
Taylor AR,Wang JR,Chen HY(2007)Carbon storage in a chronosequence of red spruce(Picea rubens)forests in central Nova Scotia,Canada.Can J For Res 37:2260–2269
Vesterdal L,Schmidt IK,Callesen I,Nilsson LO,Gundersen P(2008)Carbon and nitrogen in forest fl oor and mineral soil under six common European tree species.For Ecol Manag 255:35–48
Wan F,Zheng X,Guo J(2005)Biology and management of invasive alien species in agriculture and forestry.Science Publication,Beijing,pp 14–19
Yanai RD,Arthur MA,Siccama TG,Federer CA(2000)Challenges of measuring forest fl oor organic matter dynamics:repeated measures from a chronosequence.For Ecol Manag 138:273–283
Yu M,Xu X,Ding P(2011)Economic loss versus ecological gain:the outbreaks of invaded pinewood nematode in China.Biol Invasions 13:1283–1290
Zai JZ,Chen YL(1992)The distributing characteristic and control methods on pine wood nematode in Xiangshan county.J Zhejiang For Sci Technol 12:22–25
Zeng LX,Wang PC,Xiao XF,Wan R,Huang ZL,Pan L(2008)Allocation of biomass and productivity of main vegetations in Three Gorges reservoir region.Scientia Silvae Sinicae 44:16–22
Zhao BG,Futai K,Sutherland JR,Takeychi Y(2008)Pine wilt disease.Springer,Tokyo,pp 202–203
Zhao JL,Kang FF,Wang LX,Yu XW,Zhao W(2014)Patterns of biomass and carbon distribution across a chronosequence of Chinese pine(Pinus tabulaeformis)forests.PLoS ONE 9:e94966
 Journal of Forestry Research2018年2期
Journal of Forestry Research2018年2期
- Journal of Forestry Research的其它文章
- Effect of species composition on ecosystem services in European boreal forest
- Analysis of SSR loci and development of SSR primers in Eucalyptus
- Optimal and synchronized germination of Robinia pseudoacacia,Acacia dealbata and other woody Fabaceae using a handheld rotary tool:concomitant reduction of physical and physiological seed dormancy
- Genetic effects of historical anthropogenic disturbance on a longlived endangered tropical tree Vatica mangachapoi
- Genetic variation in relation to adaptability of three mangrove species from the Indian Sundarbans assessed with RAPD and ISSR markers
- Cloning and characterization of geranylgeranyl diphosphate synthetase from Pinus massoniana and its correlation with resin productivity
