Cloning and characterization of geranylgeranyl diphosphate synthetase from Pinus massoniana and its correlation with resin productivity
Bowen Chen•Yufei Xiao•Junji Li•Hailong Liu•Hu Chen•Jie Jia•Nan Chao•Ying Gai•Xiangning Jiang
Introduction
Coniferous trees can release large quantities of aromatic resins called rosin,which are important industrial raw materials.Rosin can be separated into turpentine oil and common rosin at a ratio of approximately 1:4(An and Ding 2012;Yang 2014).The main components of turpentine oil are monoterpenes and sesquiterpenes;the resin acid fraction that makes up the rosin is a mixture of isomeric diterpenoid compounds(Wangetal.2002;AnandDing2012).Globally,about20pinespeciesproducehigh-lipidrosin,andinChina,Pinus massonianaL.is the main widely cultivated rosin tree species,accounting for over 90% of total annual turpentine production(Yang 2007;Luo et al.2013).
Terpenoids are composed of two or more isoprene monomers or their derivatives;their synthetic route can be summarized as precursor synthesis followed by cyclization condensation of isoprenoids.Early in 1992,the sesquiterpene synthase gene was fi rst cloned in tobacco(Facchini and Chappell 1992),and during the next two decades more than 200 monoterpene and sesquiterpene synthase genes were cloned in 40 plant species(Degenhardt et al.2009).In conifers alone,70 terpene synthases,including monoterpene,sesquiterpene and diterpene synthases,have been reported,mainly inAbies grandis,Picea abiesandPinus taeda(Bohlmann et al.1999;Martin et al.2004,Ro et al.2005).Up to now,there has been no relevant report of rosin inP.massoniana.
Generally,the product of each terpene synthase is unique,which makes it possible to signi fi cantly regulate a speci fi c target product by applying genetic engineering to its synthetic enzyme.Earlier studies have shown that when an amorphadiene synthase gene is transferred into tobacco,which lacks an endogenous sesquiterpene synthase,the transformants produced more amorphadiene than in the wild-type donor(Wallaart et al.2001),and similarly,astaxanthin can be detected when beta-carotene ketolase encoded by the algaHaematococcus pluvialisis transferred into tobacco(Mann et al.2000).Additionally,three different lemon monoterpene synthase genes were transferred into wild-type tobaccos,causing the levels of β-pinene,limonene and terpinene in the transformants to increase signi fi cantly(Lucker et al.2004).
The cyclization condensation stage of terpene synthesis forms a divergent path structure;a common precursor can be used to synthesize hundreds of terpenoids.By regulating only one or a few synthases,the level of the target component can be changed effectively,with limited effects on overall terpenoid levels.Rosin is a mixture of terpene components,so this kind of regulatory model cannot be applied to resin synthesis.Additionally,pine resin composition analysis has shown that diterpene compounds are essential for rosin yield and quality,suggesting that of the three key enzymes GPPS,farnesyl pyrophosphate synthetase(FPPS)and geranylgeranyl pyrophosphate synthetase(GGPPS),GGPPS may be a potential regulatory node.It is therefore important to analyze correlations between GGPPS activity and resin productivity.
GGPP is a key precursor of various isoprenoids that have diverse functions in plant primary and secondary metabolism.The synthesis of GGPP from GPP and two molecules of IPP is catalyzed by GGPPS.GGPPS plays a key role in the synthesis of diterpenoids,carotenoids,chlorophyll,abscisic acid and other important active substances and is an important node in regulating carbon fl ow in the isoprenoid pathway(Aharoni et al.2003).Therefore,the regulation of the precursor could have a direct impact on the subsequent synthesis of terpene components.Using methyl jasmonate-induced GGPPS gene expression inTaxus canadensis,Croteau(Croteau 2002)found that both the gene expression level ofGGPPSand the yield of Taxol increased signi fi cantly.When anFPSgene from yeast was inserted into tobacco,the sterol and carotenoid content of the transgenic plant was fourfold higher(Masferrer et al.2002).Induction of the expression of a taxadiene synthase transgene inArabidopsis thalianausing a glucocorticoidmediated system consistently resulted inmore ef fi cient recruitment of GGPP for the production of taxadiene,which reached levels 30-fold higher than in plants constitutively expressing the transgene(Besumbes et al.2004).Currently,GGPPShas been isolated in a variety of plants,but it has not been reported inP.massoniana.
In previous work,high-yield varieties ofP.massonianahave been bred mostly at the provenance and family level,but with little assistance at the molecular level.The potential resin yield is mainly affected by basic resin productivity,which is controlled by genetic factors(Lu and Wang 1992).Other investigations in slash pine,Masson’s pine,maritime pine and loblolly pine have also found that genetic factors strongly affect resin yield(Zhuang et al.2007;Tadesse et al.2002;Lombardero et al.2000;Roberds et al.2003).Additionally,another study found that pine resin productivity was positively correlated with tree height,diameter and wood basic density traits but showed a negative genetic correlation to tracheid length(Lian et al.2002)and no genetic correlation to collateral and branching angle(Blinka 2007).It remains unclear whether there is any correlation between pine resin and gene expression levels in the terpene metabolic pathway.The synthesis of diterpenoid compounds plays an important role in the yield and quality of resin,and to some extent,the expression level ofGGPPSprobably parallels resin productivity.
In this study we report for the fi rst time the cloning and characterization of aP.massoniana GGPPShomolog(PinGGPPS).We found a correlation between resin productivity and the level of gene expression,which would provide support for breeding strains ofP.massonianahighly productive for resin.
Materials and methods
Plant material
Seeds of Gupeng provenance were collected from Xincheng County of Guangxi Gupeng Town,planted in the experimental nursery of Guangxi Forestry Research Institute for 6 month,which were used for gene cloning and to test gene expression in provenance offspring.Seeds of the superior groupwerecollectedfromprimaryseedorchardsofanational improved variety of Masson pine seed base in Guangxi Nanning Forestry Science Research Institute.Likewise,they were sown in the experimental nursery of Guangxi Forestry Research Institute and grown for 6 months to use for gene expression of superior group offspring.
The experimental nursery of Guangxi Forestry Research Institute is located in north latitude 22°93′,longitude 108°35′,at an altitude of 110 m,with mean annual precipitation of 350 mm and mean temperature of 21.8°C,in latosolic red soil.Adult pine trees were from Guangxi State-owned Daguishan Forest Farm,Liupai breakout,which was afforested in 1992.The woodland is located in north latitude 24°48′,longitude 111°88′,at an altitude of 272 m,with mean annual precipitation of 1535 mm and mean temperature of 19.9°C,in latosolic red soil.
Cloning of the GGPPS gene
Total RNA was isolated from 6-month-old conifer seedlings using an RNA Extraction Kit(Takara,Japan)according to the manual.First-strand cDNA was synthesized from total RNA using a RevertAid First Strand cDNA Synthesis Kit(Thermo Fisher Scienti fi c,Waltham,MA,USA)following the manufacturer’s protocol.The cDNA products were stored at-80°C until use.
Basedonhomologoussequenceanalysis,theGGPPSgene sequence was obtained from NCBI,and primers were designedaccordingtotheconservedregionusingVectorNTI(v.10.0).The primer sequences were sense primer GGPPSF1 5′-ATGGCTTACAGTGGTAGAC-3′and anti-sense primer GGPPS-R1 5′-TCAGTTTTGTCGAATGCAA-3′.Utilizing the cDNA as a template,PCR was conductedusing the following program:predenaturation at 95°C for 5 min;28 cycles of the standard thermal ampli fi cation cycling with denaturation at 94 °C for 30 s,annealing at 60 °C for 30 s and extension at 72°C for 1 min;continuing extension at 72 °C for 10 min;and fi nal hold at 8 °C for 30 min.
PCR products were validated using 0.8%agarose gel electrophoresis,and then the DNA fragment was inserted into the pMD-18T vector at 16°C overnight.The recombinant was inserted intoE.coliDH5α competent cells,followed by overnight cultivation at 37°C.After overnight growth,three randomly selectedE.colitransformants were con fi rmed by PCR ampli fi cation using GGPPS-F1/GGPPSR1 primers,and the positive clones were sequenced by Shenzhen Genomics Biotechnology Co.
Sequence analysis of GGPPS
First,sequence homology analysis of theGGPPSgene was conducted using the NCBI blastn online tool,and open reading frame(ORF)analysis was performed using ORF Finder software and the DNA sequence was translated.In addition,theconservedregionsandtheactivesiteoftheGGPPSprotein were analyzed using blastp,while the transmembrane region andsignalpeptideofthePinGGPPSsequencewerepredicted using the TMHMM and SignalP tools provided by the Technical University of Denmark.The secondary and three-dimensional(3D)structures ofPinGGPPSencoding protein were predicted using the PSIPRED service in ExPASy and SWISS-MODEL software,respectively.A phylogenetic tree for the PinGGPPS and GGPPS sequences from other species was constructed using the neighbor-joining method and Clustal W in Molecular Evolutionary Genetics Analysis(MEGA)software(v.5.0)(Tamura et al.2011).
RT-qPCR conditions
Quantitative RT-PCR primers forGGPPSwere designed using Vector NTI (qRT-PCR-F: 5′-AGGCACTGGAAAGGG-3′;qRT-PCR-R:5′-AATGCACAGAACAGG-3′).The housekeeping geneactinwas selected as the most stably expressed reference gene inP.massonianaand its primer sequences are as follows:P-actin-F 5′-CTGGAATC CATGAGACTACTTACAA-3′;P-actin-R 5′-AACCGCC ACTGAGCACAATA-3′.
Plant tissues fromP.massonianawere collected and immediately stored in liquid nitrogen until use.Total RNA was isolated using an RNAprep Pure Plant Kit(Polysaccharides and Polyphenolics-rich)(Tiangen,Beijing),and then cDNA was synthesized using an RNA LA PCR Kit(Takara).
Real-time fl uorescent quantitative PCRs were conducted using a Roche Light Cycler 480 System.Each reaction contained 1 μL of cDNA template and 9 μL of the reaction mixture,which were used in 2×SG Fast qPCR Master Mix(BBI).Cycling conditions were as follows:95°C for 3 min;40 cycles at 95 °C for 7 s,57 °C for 10 s,and 72°C for 15 s.Each reaction was repeated for at least three technical and biological replicates.
Analysis of PinGGPPS gene expression pattern
Samples were collected from four different tissues from one 6-month-oldP.massonianaseedling from Gupeng provenance for analysis of thePinGGPPSgene expression pattern.Four pine needles each from the upper,middle and lower part of the plant were bulked as the respective needle samples,and the root samples were root tissues of plant.After removing needles and roots,the 1/3 upper stems were considered as immature stem samples and the 1/3 lower stems as the semiligni fi ed stem samples.All the samples were collected and stored immediately in liquid nitrogen until use.All total RNAs were extracted using an RNAprep Pure Plant Kit(Polysaccharides and Polyphenolics-rich)(Tiangen,Beijing),and then cDNA was synthesized using an RNA LA PCR Kit(Takara).Subsequently,real-time fl uorescent quantitative PCRs were conducted.Setting three repetitions,all data were treated by SPSS 19.0 software(SPSS Chicago,IL,USA),and were carried out with one-way ANOVA,as well as Duncan’s multiple range test.
Correlation analysis of GGPPS gene expression levels and different resin productivity in adult plants
Pine needles were collected from 20 adult trees on a clear morning in mid-September.Five bundles of needles were collected from four directions(east,west,north,south)in the upper,middle and lower part of the trees,then all samples were immediately placed into liquid nitrogen until use.All total RNAs were extracted using an RNAprep Pure Plant Kit(Polysaccharides and Polyphenolics-rich)(Tiangen,Beijing),and then cDNA was synthesized using an RNA LA PCR Kit(Takara).Subsequently,real-time fl uorescent quantitative PCRs were conducted to analyze theGGPPSgene expression levels and resin productivity for each plant.
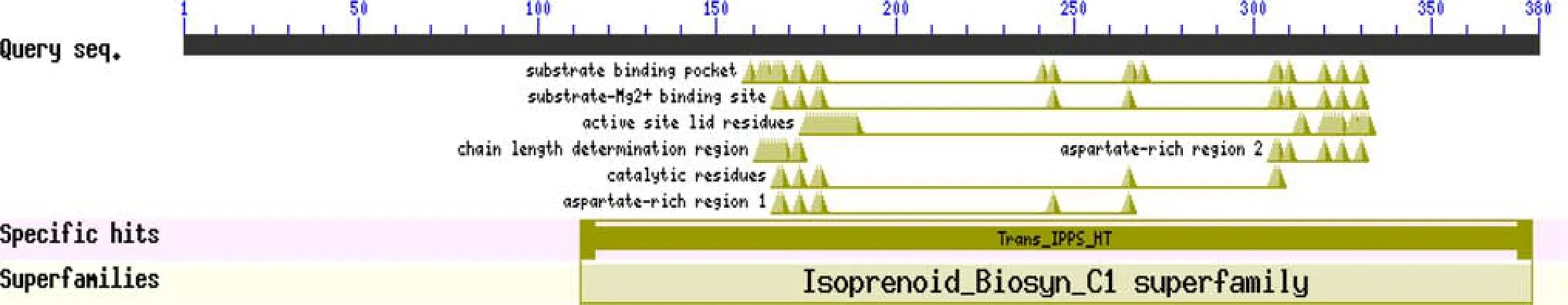
Fig.1 Protein domains of PinGGPPS.The PinGGPPS domain was analyzed using the blastn online tool of the NCBI(https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSea rch&LINK_LOC=blasthome),identifying a domain belonging to the IPP synthase superfamily,namely domain Trans IPPS)
GGPPS gene expression analysis of seedlings of fi lial generation with different resin productivity
Twenty 6-month-old seedlings from superior samples with high-yield resin productivity were randomly selected as the superior group,while the provenance control consisted of 20 6-month-old seedlings from provenance seeds.All pine needles were collected from the above trees on a clear morning in mid-May.Four bundles of needles were collected in the upper,middle and lower part of each tree,then immediately placed into liquid nitrogen until use.Subsequently,total RNA and qPCR were carried out using these samples.Three repetitions were carried out,and the data were statistically compared with Independent-Samples T Test using SPSS19.0 software(SPSS Chicago,IL,USA).
Results
Cloning and sequence analysis of PinGGPPS
Using RT-PCR with speci fi c primers based on analysis ofGGPPShomologsinotherplants,weobtainedaGGPPSgene sequence fromP.massoniana,which we namedPinGGPPSand submitted to an online database for domain analysis.The 1143-bp full-length cDNA ofPinGGPPScontained an ORF that encoded a 380-amino-acid protein.Online analysis showed thatPinGGPPSincluded a domain belonging to the IPP synthase superfamily,namely,domain Trans IPPS(Fig.1).ThededucedaminoacidsequenceofPinGGPPSwas compared with other species,and two Asp-rich regions at residues 183–189 and 324–328 were found with typical sequences DDXXXXD and DDXXD,called FARM(the fi rst aspartate-rich motif)and SARM(the second aspartate-rich motif),which are binding sites for allylic substrates and IPP,respectively(Hemmi et al.2003).All these characteristics correspond to previously reported GGPPS proteins.
To identify whether the protein PinGGPPS contains transmembrane and signal peptides,we used the tools TMHMM and SignalP,but no transmembrane or signal peptide sequence was detected in PinGGPPS,indicating that this protein is not a membrane protein or a secreted protein,and must be located in the cell matrix,which coincides with its predicted function.
Secondary structure elements of the deduced PinGGPPS were predicted using PSIPRED on the ExPaSy website(http://www.expasy.org/),and 16 α-helices and no β-sheets were found(Fig.2).The 3D structure of PinGGPPS modeled using SWISS-MODEL Workspace con fi rmed this result(Fig.3).Using the large subunit of heterotetrameric GPPS from mint(Mentha piperita)as the modeling reference template(Chang et al.2010),the result showed a coverage rate of 77%,from residue 86 to 380,with 66.67%sequence identity to mint GPPS,suggesting that PinGGPPS may have the same catalytic function.
Analysis of PinGGPPS expression patterns
To clarify the patterns ofPinGGPPSexpression in the plant,we investigated transcript levels in roots,needles,immature stem,and semiligni fi ed stems of 6-month-old seedlings ofP.massonianausing qPCR,withactinas the reference gene.The results showed the highest transcript level ofPinGGPPSin the needles,and the level differed
slightly between immature stem and semiligni fi ed stems,although the level in each was signi fi cantly lower than in the needles.The lowest level was found in roots(Fig.4).Meanwhile,a one-way ANOVA demonstrated that there were signi fi cant differences among the tissues,we also found that the differences in the expression levels in these four tissues were highly signi fi cant differences in a Duncan’s multiple range test(Fig.4).

Fig.2 Secondary structure of PinGGPPS.Secondary structure elements of the deduced PinGGPPS were predicted using PSIPRED on the ExPaSy website,showing 16 α-helices,but no β-sheets
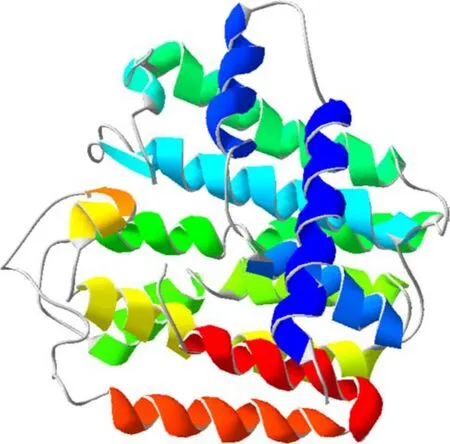
Fig.3 Predicted three-dimensional structure of PinGGPPS.The 3D structure of PinGGPPS modeled using SWISS-MODEL Workspace,also showing 16 α-helices,but no β-sheets
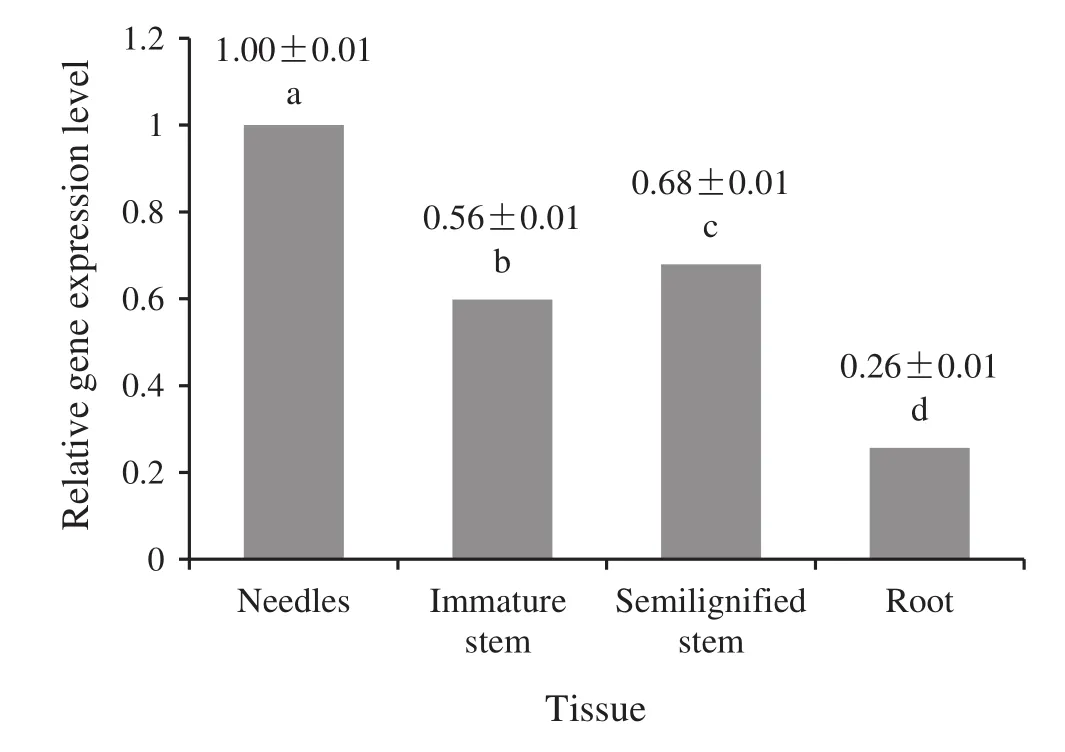
Fig.4 PinGGPPS expression in root,leaf(needle),immature stem and semiligni fi ed stem tissue from 6-month-old seedlings of Pinus massoniana revelaed by qRT-PCR.Values are mean±SE calculated from three repetitions;different letters represented a highly signi fi cant difference at 0.01 level
Because pine needles are the main site of resin synthesis and also the center of plant photosynthesis,the high expression level ofPinGGPPSobserved in pine needles is consistent with their physiological function.Physiological processes in the stem mainly involve internode elongation and ligni fi cation,which have less correlation with the physiological function of PinGGPPS,which agrees with the signi fi cantly reduced expression seen in stem tissue.Furthermore,the physiological structure and function of roots differ remarkably from those of stems and needles,and the low level ofPinGGPPSin roots is consistent with our expectations.
治疗前4组患者血液CD3+比例、CD4+比例、CD8+比例、CD4+/CD8+比值比较,差异均无统计学意义(P >0.05);治疗后4组患者CD3+比例、CD4+比例、CD8+比例、CD4+/CD8+比值均提高,B组及D组CD4+比例及CD4+/CD8+比值高于A组及C组,但两组的CD8+比例较低,差异具有统计学意义(P<0.05)。见表3。
Analysis of resin productivity and PinGGPPS gene expression level in different plants
To analyze the correlation betweenPinGGPPSgene expression level and resin productivity,pine resin yield and thePinGGPPSgene expression levels in pine needles were measured in 20 adult plants for 28 days in succession.The average gene expression level in these 20 adult plants was 0.88±0.45(Fig.5),and the average resin productivity was 776.71±64.38 g(Fig.6).Simultaneously,one-way ANOVA results showed that the resin productivities among the tested plants differed signi fi cantly.Additionally,except for two plants(nos.14 and 19),others differed signi fi cantly at 0.01 level in Duncan’s multiple range test(Fig.6).The gene expression levels among the tested plants also differed signi fi cantly,and Duncans multiple range test also showed that the level in most plants differed remarkably at 0.01 levels(Fig.5).
Gene expression levels of eight plants(sample nos.10,9,16,13,14,8,4,11)were higher than average,and their corresponding resin productivities were also higher than average,accounting for 72.7% of the plants with high resin yield(11 plants:nos.10,13,16,11,20,8,9,14,19,15,4).In addition,PinGGPPSgene expression levels in the nine plants with lower than average resin yield were also below average,accounting for 75% of all plants with lower expression(12 plants).Overall,the consistency rate of these two sets of data was slightly more than 70%.
We found that thePinGGPPSgene expression levels present a substantially linear distribution when plotted against their corresponding resin yield(Fig.7).All data were entered into SPSS(v.19.0)for analyses of the correlation between these two groups;the correlation coef ficient was 0.733,withp<0.01,showing a moderate positive correlation.
PinGGPPS gene expression in F1 seedlings with different resin productivities
The superior group was compared with the provenance group,as shown in Fig.8,and average expression level ofPinGPPSwas 1.108 and 1.029,respectively,indicating that expression in the superior group was slightly higher than in the provenance group,but the difference was not signi fi cant.
In the superior group,the number of plants with moderate expression levels(mean±30%)was in the majority,about 55%;fewer plants showed higher(>30%above the mean)and lower(>30%below the mean)expression levels.However,in the provenance group,the number of plants in these three categories was fairly evenly distributed(35,40 and 25%,respectively),illustrating thatPinGPPSgene expression and resin productivities in the superior group are stable.In comparison with the superior group,the range in the high and low expression levels observed in provenance offspring was much greater,and the distribution of offspring showed no obvious trend,indicating that the provenance group was presumably a mixed population with low selective breeding,and therefore represents the natural range of resin productivities.

Fig.5 PinGGPPS gene expression in different plants.Pine needles from 20 adult plants were analyzed for GGPPS gene expression using real-time fl uorescent quantitative PCR.Different letters represent a highly signi fi cant difference at 0.01 level
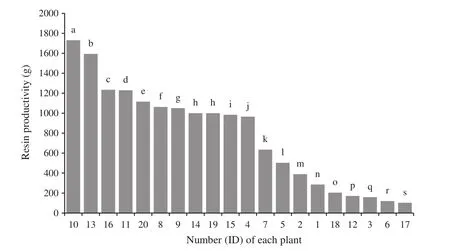
Fig.6 Resin productivity in different plants.Resin was collected continuously from 20 adult plants for 28 days and weighed.Different letters represent a highly signi fi cant difference at 0.01 level
Discussion
The condensation reaction catalyzed by GGPPS is a node for the synthesis of the common diterpene precursor from terpene precursors and is thus an important target for the regulation of diterpene biosynthesis.GGPPSgenes have also been identi fi ed in fungi,archaea,eubacteria,protozoa,and mammals(Soderberg et al.2001;Artz et al.2010;Ling et al.2007).
Phylogenetic analysis indicates that GGPPS protein sequences of different species form distinct clusters;i.e.,GGPPSs from animals,plants,bacteria,fungi and archaea belong to different branches.Furthermore,GGPPSs from bacteria and archaea are more closely related to those from plants,and those from fungi and animals are closely related to each other.Currently,GGPPSs from different sources are divided into three types:I(archaea),II(plants and eubacteria)and III(fungi,insects and mammals)(Hemmi et al.2003;Ling et al.2007;Sagami et al.1994).Although the fi nal product of GGPPS in all organisms is GGPP,kinetic studies show that type I and II GGPPSs preferentially catalyze the condensation of DMAPP or GPP with IPP to form GGPP,while type III GGPPSs prefer to use FPP as their main substrate.
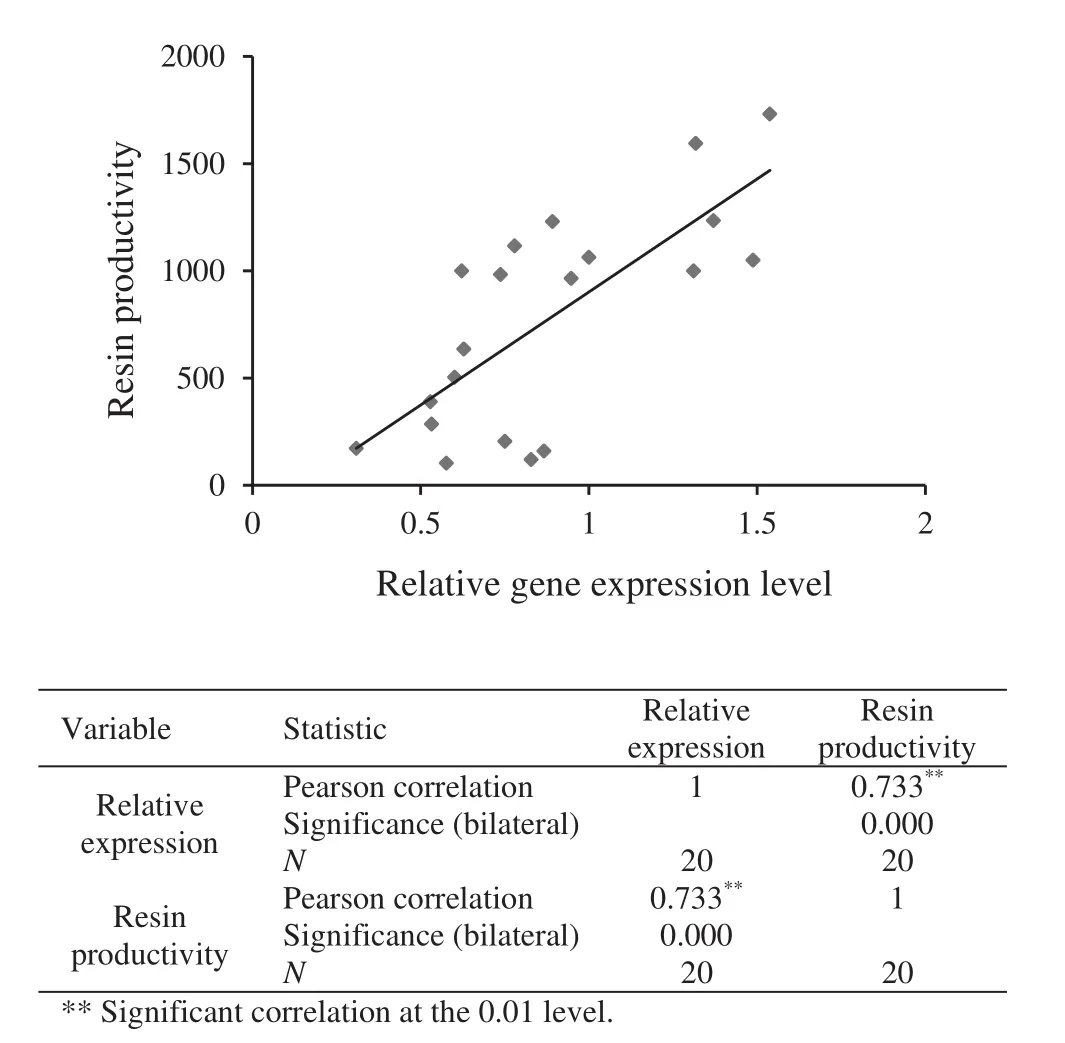
Fig.7 Correlation analysis of PinGGPPS gene expression levels and resin productivity.Gene expression levels and resin productivities of the above 20 adult plants were analyzed using SPSS,as was the corresponding correlation analysis
Previous research has found that the enzymes involved in the synthesis of GGPP,monoterpenes,diterpenes and tetraterpenes are mainly located in plastids(Tholl,2006).In the plant cytoplasm,FPPS catalyzes the synthesis of FPP from IPP,and sesquiterpenes,triterpenoids and other terpenoids are then successively synthesized,but in plastids,GGPPS catalyzes the synthesis of GGPP from GPP and DMAPP.GGPP then takes part in the production of monoterpenes,diterpenes,chlorophyll,and carotene.GGPPS is the primary enzyme responsible for catalyzing the formation of GGPP in plastids,providing raw materials for the synthesis of diterpene,chlorophyll and other active substances.In addition,plastids serve as factories for the synthesis of pine resin,and investigation into ultrastructural changes during the secretion of pine resin found that with the development of resin ducts,the more the number of plastids increased,the more pine resin synthesis increased(Li et al.2008).

Fig.8 PinGPPS gene expression levels in offspring with different resin productivities.Twenty 6-month-old seedlings from superior samples with high-yield resin productivity were randomly selected as the superior group,while the provenance control consisted of 20 6-month-old seedlings from provenance seeds.All pine needles from the above plants were analyzed by qPCR
In the present work,we analyzed the correlation betweenPinGGPPSgene expression level and resin productivity and found that gene expression levels corresponded to resin productivity,but that consistency was very low when the results were examined at the level of individual plants.We speculate that this is because resin productivity data were collected continuously over an extended period,while gene expression data were collected one time.If we were to collect data over a long period,the results might correspond more closely.In addition,the correspondence between gene expression and resin productivity will be more obvious for samples with greater differences from the mean,but smaller differences might be more easily masked by the environment and other factors.Our results show a correlation coef fi cient of 0.733(p<0.01),indicating that thePinGGPPSgene expression level is signi fi cantly correlated with resin productivity.
We analyzedPinGGPPSgene expression patterns in seedlings of different fi lial generations.Compared with the provenance group,the overall expression level in the superior group was slightly higher,but the difference was not signi fi cant.PinGGPPSgene expression and resin productivity in the superior group were stable,and most plants had moderate expression levels,because the superior group consisted of the offspring of plants with high resin yield after many generations of screening.In contrast,expression levels in the provenance group were not clearly distributed in relation to resin productivity,and its resin productivity re fl ected the natural range,perhaps because the offspring were from a mixed population with low selective breeding.
In this study,we characterized the genePinGGPPSinP.massonianafor the fi rst time,and we attempted to establish a correlation between its expression level and resin productivity at a molecular biological level.Detection of gene expression has many advantages,including the lack of an age requirement,so that seedlings can be tested,allowing a short life cycle as well as high throughput.Resin productivities can be measured using the target genePinGGPPSas an index,rather than using traditional direct measurement methods.Combining molecular biology techniques and traditionalbreeding methods enables breeding requirements to be achieved easily and would provide support for breeding high resin productivity strains ofP.massoniana,underlining the importance of theory and production practice.
AcknowledgementsThis research was supported by the Guangxi Natural Science Foundation No.2014GXNSFBA118106.
Aharoni A,Giri AP,Deuerlein S,Bouwmeester HJ(2003)Terpenoid metabolism in wild-type and transgenicArabidopsisplants.Plant Cell 15(12):2866–2884
An N,Ding GJ(2012)Study on chemical constituents of oleoresin fromPinus msssonianain Guangxi.J Cent S Univ For Technol 3:59–62
Artz JD,Wernimont AK,Dunford JE,Hui R(2010)Molecular characterization of a novel geranylgeranyl pyrophosphate synthase fromPlasmodiumParasites. J Biol Chem 286(5):3315–3322
Besumbes O,Sauret-Greto S,Phillipa MA,Boronat A(2004)Metabolic engineering of isoprenoid biosynthesis inArabidopsisfor the production of taxadiene,the fi rst committed precursor of Taxol.Biotechnol Bioeng 88(2):168–175
Blinka KW(2007)Resin fl ow in clonalLoblolly Pine.North Carolina State University,Raleigh
Bohlmann J,Phillips M,Ramachandiran V,Croteau R(1999)cDNA cloning,characterization,and functional expression of four new monoterpene synthase members of the Tpsd gene family from Grand Fir (Abiesgrandis). Arch Biochem Biophys 368(2):232–243
Chang TH,Hsieh FL,Ko TP,Wang AH(2010)Structure of a heterotetrameric geranyl pyrophosphate synthase from Mint(Mentha piperita)reveals intersubunit regulation.Plant Cell 22(2):454–467
Croteau R(2002)Biosynthesis and catabolism of monoterpenoids.Chem Rev 87(5):929–954
Degenhardt J,Kollner TG,Gershenzon J(2009)Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants.Phytochemistry 70(15–16):1621–1637
Facchini PJ,Chappell J(1992)Gene family for anelicitor-induced sesquiterpenecyclase in tobacco.Proc Natl Acad Sci USA 89(22):11088–11092
Hemmi H,Noike M,Nakayama T,Nishino T(2003)An alternative mechanism of product chain-length determination in type III geranylgeranyl diphosphate synthase. Eur J Biochem 270:2186–2194
Li AM,Wang YR,Wu H(2008)Initiation and development of resin ducts in major organs ofPinus massoniana.Sci Silvae Sin 44(9):36–40
Lian HM,He BX,Zeng LH,Qin J(2002)Study on the comprehensive election of half-sib families of Masons pine.For Sci Technol Guangdong Prov 18(1):1–6
Ling Y,Li ZH,Miranda K,Moreno SN(2007)The farnesyldiphosphate/geranylgeranyl-diphosphate synthase ofToxoplasma gondiiis a bifunctional enzyme and a molecular target of bisphosphonates.J Biol Chem 282(42):30804–30816
Lombardero MJ,Ayres MP,Lorio PL,Ruel JJ(2000)Environmental effects on constitutive and inducible resin defences ofPinus taeda.Ecol Lett 3(4):329–339
Lu PX,Wang Y(1992)Variation of oleoresin yield among high-gum yielding slash pine families and their realized genetic gain.For Res 5(6):700–705
Lucker J,Schwab W,van Hautum B,Verhoeven HA(2004)Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from lemon.Plant Physiol 134(1):510–519
Luo LP,Shu WB,Nie HQ,Yan ZY(2013)Comparison on growth volume and resin-producing capacity of tugong provenance between pure forest and mixed forest ofPinus massoniana.Guangxi For Sci 42(1):66–70
Mann V,Harker M,Pecker I,Hirschberg J(2000)Metabolic engineering of astaxanthin production in tobacco fl owers.Nat Biotechnol 18(8):888–892
Martin DM,Fa ldt J,Bohlmann J(2004)Functional characterization of nine Norway Spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily.Plant Physiol 135(4):1908–1927
Masferrer A,ArróM,Manzano D,Ferrer A(2002)Overexpression ofArabidopsis thalianafarnesyl diphosphate synthase(FPS1S)in transgenicArabidopsisinduces a cell death/senescence-like response and reduced cytokinin levels.Plant J 30(2):123–132
Ro DK,Arimura G,Lau SY,Bohlmann J(2005)Loblolly pine abietadienol/abietadienal oxidase PtAO(CYP720B1)is a multifunctional,multisubstrate cytochrome P450 monooxygenase.Proc Natl Acad Sci 102(22):8060–8065
Roberds JH,Strom BL,Hain FP,Lott LH(2003)Estimates of genetic parameters for oleoresin and growth traits in juvenile loblolly pine.Can J For Res Rev 33:2469–2476
Sagami H,Morita Y,Ogura K(1994)Puri fi cation and properties of geranylgeranyl-diphosphate synthase from bovine brain.J Biol Chem 269(32):20561–20566
Soderberg T,Chen A,Poulter CD(2001)Geranylgeranylglyceryl phosphate synthase.Characterization of the recombinant enzyme from Methanobacterium thermoautotrophicum.Biochemistry 40(49):14847–14854
Tadesse W,Nanos N,Auñon F,Gil L(2002)Evaluation of high resin yielders of Pinus pinaster Ait.For Genet 8(4):271–278
Tamura K,Peterson D,Peterson N,Kumar S(2011)Mega5:molecular,evolutionary,genetics,analysis,using maximum,likelihood,evolutionary,distance,and maximum,parsimony,methods.Mol Biol Evol 28(10):2731–2739
Tholl D(2006)Terpene synthases and the regulation,diversity and biological roles of terpene metabolism.Curr Opin Plant Biol 9(3):297–304
Wallaart TE,Bouwmeester HJ,Hille J,Maijers NC(2001)Amorpha-4,11-diene synthase:cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin.Planta 212(3):460–465
Wang YS,Zeng LH,Luo M,Qin J(2002)Analysis of tapping resin trial in natural stands of masson’s pine.For Sci Technol 18(2):1–4
Yang ZQ(2007)Status and countermeasures about developing pine resin in Guangxi.Guangxi For Sci 36(3):143–146
Yang ZQ(2014)Comparative study on the resin yield and rosin components ofPinus massonianasuperior provenances among different ages.Sci Silvae Sin 50(6):147–151
Zhuang WY,Zhang YY,Zou YX(2007)Selection for high-resin yield of Slash Pine and analysis of factors concerned.Acta Agric Univ Jiangxiensis 29(1):55–60
 Journal of Forestry Research2018年2期
Journal of Forestry Research2018年2期
- Journal of Forestry Research的其它文章
- Measurement of lumber moisture content based on PCA and GSSVM
- Characterization of mean stem density, fi bre length and lignin from two Acacia species and their hybrid
- Theoretical modeling of the effects of temperature and moisture content on the acoustic velocity of Pinus resinosa wood
- The properties of fl ax fi ber reinforced wood fl our/high density polyethylene composites
- Risks involved in fecal DNA-based genotyping of microsatellite loci in the Amur tiger Panthera tigris altaica:a pilot study
- Genecological zones and selection criteria for natural forest populations for conservation:the case of Boswellia papyrifera in Ethiopia
