Nutrient changes in potting mix and Eucalyptus nitens leaf tissue under macadamia biochar amendments
A.Wrobel-Tobiszewska•M.Boersma•J.Sargison•P.Adams•B.Singh•S.Franks•C.J.Birch•D.C.Close
Introduction
Biochar admixture to the soil is a potential innovative solution that may provide both economic and environmental bene fi ts to agricultural and forestry industries while simultaneously sequestering atmospheric carbon in the soil(Klein et al.2007;Milne et al.2007;McHenry 2009).Bene fi cial effects of biochar on changes in desirable soil characteristics and increases in plant growth have been reported in both laboratory and fi eld studies(Chan et al.2007;Lehmann and Joseph 2009).Yet,positive outcomes are inconsistent,and the scienti fi c literature reports a contrasting range of biochar application rates and plant responses.While biochar studies are growing in number,much of the research in this area focuses on agricultural crops and information on the in fl uence of biochar on forestry species remains sparse(Stavi 2013;Thomas and Gale 2015).
Forestry is an important industry sector in Tasmania and production is based on both management of native forests and plantations.Large scale plantations of radiata pine(Pinus radiataD.Don)andEucalyptus(E.globulusandE.nitensH.Deane and Maiden)play an increasingly important role in meeting national and international demand for wood products.Propagating healthy seedlings for planting in the fi eld is an important part of plantation establishment as it in fl uences potential yield,and is a signi fi cant production cost.
In this study,we investigated the in fl uence of a macadamia shell biochar on nutrient availability and nutrient uptake byEucalyptus nitensseedlings in a pot experiment.The objectives of the study were to(1)examine the effects of macadamia shell biochar applied at 8 rates(2,5,10,20,50,80 and 100 t ha-1)combined with two fertiliser rates[100 and 50%the regional forestry nursery rate[200 g Diammonium phosphate per seedling)]on soil nutrient availability,and(2)examine how any observed changes in soil chemistry in fl uence plant nutrient acquisition as measured by concentration changes in foliage analyses.
Materials and methods
Experimental design
A pot trial was established on 09 May 2011 and completed on 02 Feb 2012 in the Horticultural Research Centre,University of Tasmania,Australia.The experimental treatments were a factorial combination of eight biochar rates:0(B0),2(B2),5(B5),10(B10),20(B20),50(B50),80(B80)and 100 t ha-1(B100)and two fertiliser rates:100%(F100)and 50%(F50)of the standard nursery rate consistent with commercial practice in Tasmania.Experimental units were arranged in a randomised complete block with three replicates of four sample plants in each experimental unit.The pots were located on benches to ensure proper air circulation and water was provided through an automatic irrigation system to match evapotranspiration de fi cits(approximately 1.5–3 mm day-1in winter and 5 mm day-1in summer).
Plant husbandry
Eucalyptus nitensseedlings were raised from seed in 4 L(L)pots in a glasshouse.In each pot,9 seeds(from a single open pollinated stand harvested in 2008,Victoria,Australia)were planted at a depth of 5–10 mm,and later thinned to 1 seedling per pot.Seedlings were treated with Previcur drench(Propamocarb 600 g L-1,Bayer®)at sowing and with the insecticides Azamax(40 mL per 10 L)and Eco Oil(40 mL per 10 L)on 11 Aug[94 days after planting(DAPL)],30 Sep(144 DAPL)and 25 Oct(169 DAPL)2011.Forty-two days following germination,seedlings were thinned to the three strongest,and after another ten days to the strongest seedling per pot.During the 9 month measurement period,four destructive sampling harvests were performed at 135,177,219 and 269 DAPL.One seedling from each replicate was destructively sampled for analysis of the potting mix and leaf tissue at each harvest date.
Potting mix and amendments
The potting mix(PM)was an industry standard mix and consisted of pine bark(72%),washed sand(18%)and peat moss(10%)mimicing local forest nursery practice.The fertiliser was comprised of Osmocote Exact®3–4 month(Everris),Osmocote Plus®8–9 month(Everris),dolomite lime,ferrous sulphate,Micromax®(Everris)and fi ne ground rock gypsum.At the full fertiliser rate(F100),this translated to 377 mg K,451 mg Mg,1365 mg Ca and 1 mg Na applied per kg of Potting Mix(PM)(Wrobel-Tobiszewska 2014).Amounts of Ca,Mg and Na introduced under F50 and F100 did not change as both dolomite lime and rock gypsum were applied at the same rate in both treatments.The biochar was sourced from Golden Macadamias Pty Ltd.,Alkmaar province,South Africa in 2008 and consisted of pyrolysed macadamia nut shells heated at the highest temperature treatment of 450–480 °C for 180 min.The choice of biochar was dictated by availability.Biochar rates were calculated on a volumetric basis and equivalent to 222 g biochar(dry weight)added per 4 L pot at 100 t ha-1rate.As biochar properties determine the interaction with soil and plants,chemical characteristics were measured and are presented in Table 1.
Potting mix,biochar and plant material analyses
Leaves from the whole plant were collected at each destructive harvest and immediately dried in a forced air drying oven at 60°C for 96 h.PM samples of approximately 500–600 g were dried in a forced air drying oven at 40°C for approximately 96 h and any undissolved fertiliser granules were removed prior to analysis.All plantand soil samples were stored for 3–6 weeks in a cool,dry place in sealed bags prior to analysis.Chemical analyses of all plant and PM samples were conducted at the CSBP Soil and Plant Analysis Laboratory,Western Australia,using accredited sample preparation procedures.Biochar chemical composition was analyzed similarly to the plant and soil material as described above.To gain information on the surface and structural characteristics of the biochar,Scanning Electron Microscopy(SEM)accompanied by Energy Dispersive Spectrometry(EDS)was also performed on the macadamia nut shell biochar.The analyses were performed in the Central Science Laboratory(CSL)at the University of Tasmania.The samples for analysis were fi rst sputter coated with carbon.The equipment used to carry out analyses was Hitachi®SU-70 fi eld emission scanning electron microscope set for 15 kV accelerating voltage.
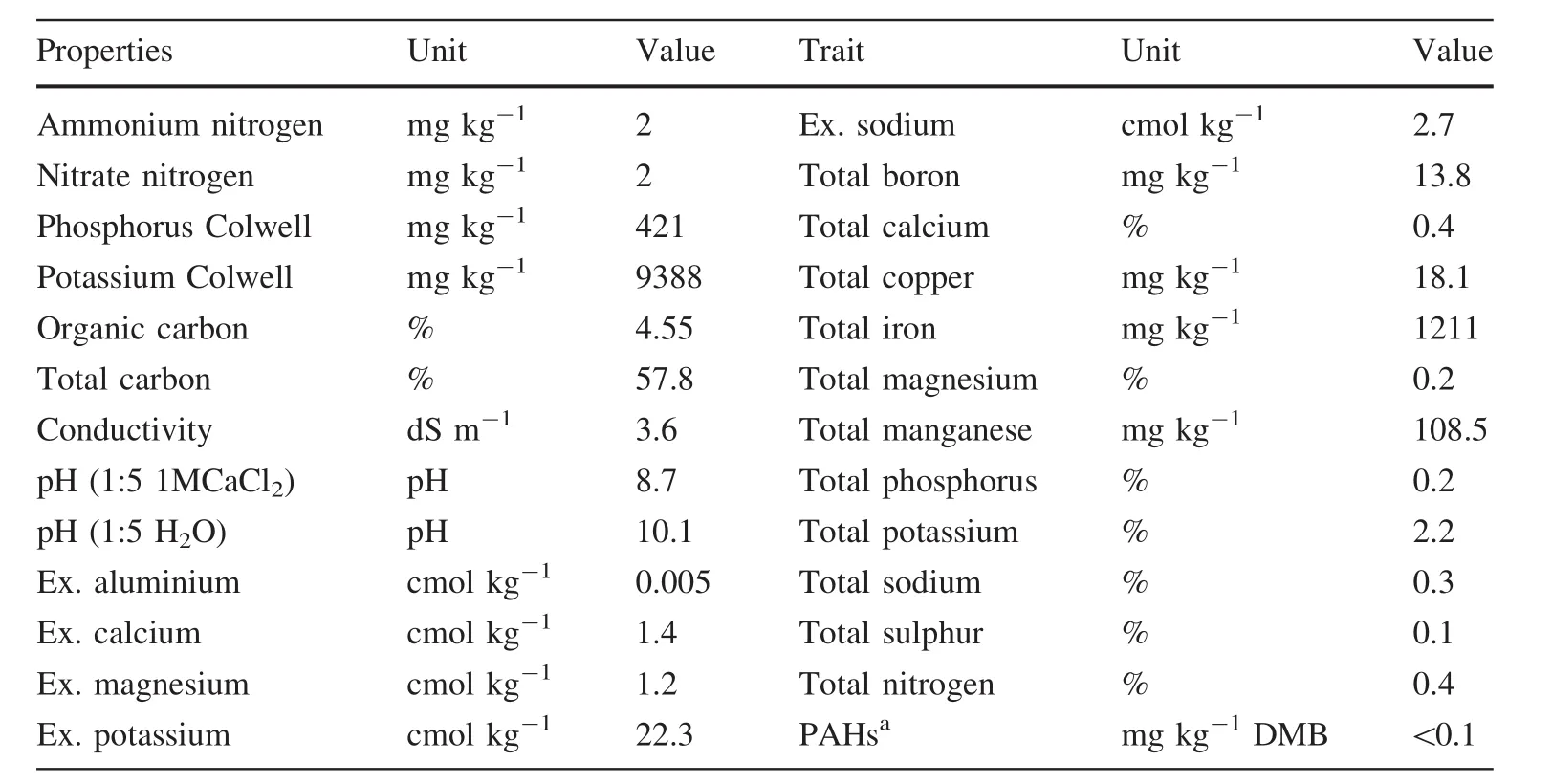
Table 1 Chemical and physical characteristics of macadamia nut shell biochar
Statistical analyses
IBM SPSS Statistics ver.19 was used to analyze data by ANOVA with range testing conducted using an LSD(least signi fi cant difference)value ofp=0.05,with biochar,fertiliser and time as fi xed factors.A rate of decay analysis(performed on soil and plant tissue data)was calculated on the basis of estimating the parameters ‘a’(initial quantity)and ‘c’(decay rate)using a fi tted exponential decay model.Analysis of variance(ANOVA)was performed on the resulting decay rate parameters(c).The decay results are presented only where statistically signi fi cant.The relationship between nutrient concentration in the PM and leaf tissue was analyzed using Spearman’s Rank Correlation test.
Results
Characterisation of macadamia biochar
The macadamia biochar was high in potassium,silica and sodium while the pH was in the upper range reported in the literature(pH 6.2–9.9)(Chan et al.2008;Chan and Xu 2009;Ma and Matsunaka 2013).Detailed chemical characteristics are presented in Table 1.The SEM-EDS analysis showed a range of pore sizes on the surface of the biochar(Fig.1).In addition,a large number of KCl particles were detected by SEM(Table S1,supplementary material)congruent with the relatively high levels of potassium recorded in the chemical analysis.
Chemistry of potting mix as in fl uenced by biochar and fertiliser treatments
PM pH decreased in response to high biochar rates(B50–B100)(p≤0.001),but this effect was transient,with pH decreasing in time until 219 DAPL(Fig.2).
Biochar added at high rates decreased PM water content(data not presented).At day 135 of the experiment,biochar added at high rates to the potting mix(B50–B100)increased nitrate-N availability while that of ammonium-N decreased.At this time PM available nitrate-N concentrations were highest at biochar rates of B50–B100(p≤ 0.05)with no differences observed below these rates(Fig.3a,c).Biochar application diminished PM ammonium-N concentrations at 135 DAPL(all biochar treatments)and 216 DAPL(B50–B100)(p=0.004).At 177 DAPL the effect of biochar application on nitrate-and ammonium-N was different resulting in control treatment being statistically similar to majority of biochar treatments.No effect of biochar was detected at 269 DAPL(Fig.3b,d)and the interaction between fertiliser and biochar was not signi fi cant.

Fig.1 Electron microscopy(SEM)image of macadamia biochar.Performed in Central Science Laboratory,University of Tasmania in 2013(Hitachi SU-70 FESEM).Samples carbon coated and images taken at a beam energy of 15 kV.Pore size diameter(μm)(arrows)were measured in situ
When compared to the control treatment,biochar increased Colwell P(p=0.009)when added to the PM at 80 and 100 t ha-1at the lower fertilisation rate(F50)(Fig.4).In contrast at the full fertiliser dose,Colwell P was suppressed when biochar was added at 2,5 and 20 t ha-1.Colwell P was signi fi cantly greater under full fertiliser treatments during the whole experiment regardless of biochar dose.
Extractable K increased with higher rates of biochar application,and when applied at B50 to B100,concentrations were greater in the potting mix at all four harvests(p≤0.001)(Fig.5).Over time,extractable K decreased between 135 and 269 DAPL but were similar between the two middle harvests(177 and 219 DAPL).
The highest exchangeable calcium in the PM was found where no biochar was applied,and the availability of exchangeable Ca decreased by at least 4 cmol kg-1in response to the application of biochar across all rates.This response was uniform across all biochar application rates(p≤0.001)(Fig.6a).When the treatment response was pooled,Ca increased slightly between 135 and 177 DAPL,and declined after this(Fig.6b)(p=0.018).
As with exchangeable calcium,exchangeable magnesium declined following biochar application(p<0.05),although there was no clear trend among biochar treatments(data not shown).Concentrations of both Ca and Mg decreased in F100 treatment when pooled across biochar treatments(p≤0.05)(data not shown).
The effect of biochar on exchangeable sodium was similar to exchangeable K,resulting in higher concentrations under B80–B100 at(p≤ 0.001)(Fig.6),but did not persist beyond 177 DAPL.Pooled across all four harvests the availability of exchangeable sodium fell byca.0.1 cmol kg-1in response to low biochar application rates(B2,B5)in comparison to control treatment(Fig.7a).
Effects of biochar and fertiliser on foliage chemistry
Seedling growth washigherunderfullfertilisation(p≤0.05),but was not in fl uenced by biochar(data not presented).Higher concentrations of nitrate-N in the potting mix did not result in higher leaf tissue N.
Biochar added at rates above 50 t ha-1increased leaf P concentration.In both F50 and F100,there was a relatively consistent trend of higher leaf P as the rate of biochar increased.Fertiliser and biochar rates interacted,with P tissue concentrations lower under F50 up to 20 t ha-1biochar,and greater at 80 t ha-1biochar(p=0.018)(Fig.8a).However,importantly,leaf P increased from 0.125%at B0(averaged across fertiliser rates)to around 0.2%in the B50,B80 and B100 rates i.e.60%increase.The impact of biochar on leaf P concentration declined over time from 0.375%to 0.07%at 219 DAPL,with no further decline by 269 DAPL(p≤0.001)(Fig.8b).

Fig.2 pH dynamics of the potting mix in Eucalyptus nitens pot experiment in response to:a biochar application at 0–100 t ha-1(mean across sampling times and fertiliser treatments)and;b time,the response measured at 135,177,219 and 269 days after planting(mean across biochar and fertiliser treatments).Error bars indicate the LSD(p=0.05;a 0.11,b 0.16)

Fig.3 Effects of biochar treatment(0–100 t ha-1)on potting mix nitrate nitrogen(a,c)and ammonium nitrogen(b,d)concentration of an Eucalyptus nitens pot experiment(mean across sampling times and fertiliser treatments)(a,b)and time 135,177,219 and 269 days after planting(c,d)(mean across biochar and fertiliser treatments).Error bars indicate LSD(p≤0.05;a 7.27,b 2.00,c 5.14,d 1.41 mg kg-1)
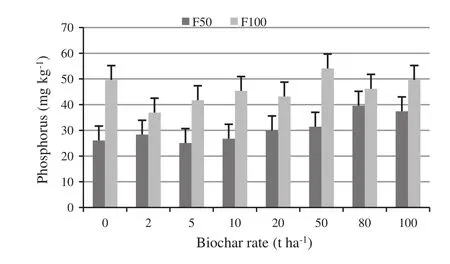
Fig.4 Phosphorus concentration (mg kg-1)in potting mix in response to 8 biochar application rates(0–100 t ha-1)in factorial combination with two fertilisation rates,pooled through time.Error bars indicate the LSD(p≤0.05;5.60 mg kg-1)
Biochar added above 50 t ha-1increased potassium in the leaf material and was in fl uenced by an interaction between biochar,fertiliser and time(p=0.003).Similar to leaf P,leaf K exhibited a mixed response to combinations of biochar and fertiliser application rates(p<0.001)(Fig.9a),with leaf K content at F50 being either lower or similar to F100 as the biochar rate increased to B20,equal at B50,and higher at B80.In addition to this at B100,a higher leaf potassium content was observed at F50 in comparison to F100.The response continued until 177 DAPL,although at this later date,leaf phosphorus at B50 was also higher at the half fertilisation rate.The analysis of biochar’s in fl uence over time showed K concentrations decreased until 219 DAPL(p≤0.001;Fig.9b).
Calcium concentration in leaf material decreased in response to biochar application(p≤0.001;Fig.10a).Over time,leaf Ca was approximately 0.45%with the exception of the third harvest(219 DAPL)at which time the mean concentration fell by 0.1%(p=0.004)(data not shown).Leaf Mg decreased(p≤0.001)under high biochar treatments(B80 and B100)in comparison to the control treatment(Fig.10b).From the fi rst to third harvest(135–219 DAPL)the concentration ofmagnesium decreased,after which the response was an intermediate increase(data not shown).

Fig.5 Colwell potassium concentration(mg kg-1)of the potting mix in response to a 8 biochar application rates from 0 to 100 t ha-1(mean across sampling times and fertiliser treatments)and;b time,the response measured at 135,177,219 and 269 days after planting(mean across biochar and fertiliser treatments).Error bars indicate the LSD(p≤0.05;a 32.18,b 22.76 mg kg-1)

Fig.6 Exchangeable calcium concentration(cmol kg-1)in potting mix in response to a 8 biochar application rates(0–100 t ha-1)and fertilisation at two concentrations(50 and 100% of the commercial rate)(mean across sampling times)and;b time,the response measured at 135,177,219 and 269 days after planting(mean across biochar and fertiliser treatments).Error bars indicate the LSD(p≤0.05;a 0.36,b 0.26 cmol kg-1)

Fig.7 Exchangeable sodium concentration(cmol kg-1)in potting mix in response to a 8 biochar application rates applied from 0 to 100 t ha-1(average data from 135,177,219 and 269 days after planting(DAPL)and;b rate of decay under 8 biochar application rates(0–100 t ha-1).Error bars indicate the LSD (p ≤ 0.05;0.02 cmol kg-1)
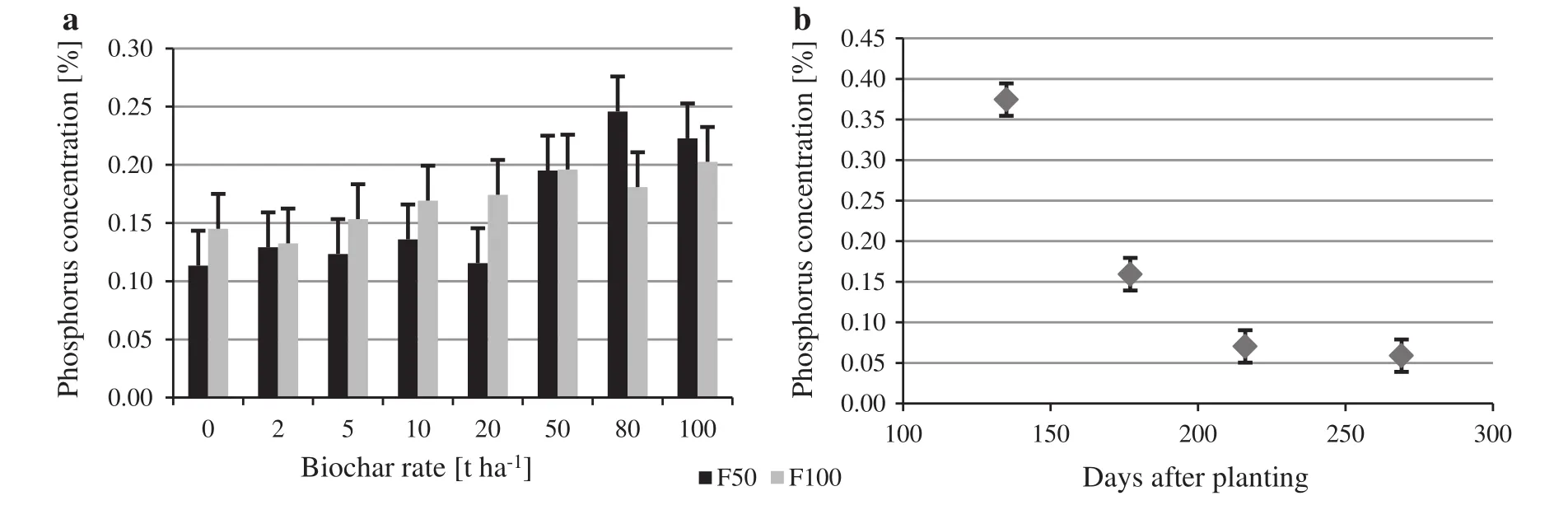
Fig.8 Mean phosphorus concentration(%)in leaf tissue of Eucalyptus nitens seedlings in the pot trial in response to:a fertilisation at two levels(50 and 100% of the commercial rate)in factorial combination with 8 biochar application rates(0–100 t ha-1)with means pooled from four harvests:135,177,219 and 269 days after planting;b mean phosphorus concentrations across biochar and fertiliser treatments at 135,177,216 and 269 days after planting.Error bars indicate the LSD(p≤0.05;a 0.03,b 0.02%)

Fig.9 Mean potassium concentration(%)in leaf tissue of Eucalyptus nitens seedlings in the pot trial in response to:a fertilisation at two levels(50 and 100% of the commercial rate)in partial factorial combination with 8 biochar application rates(0–100 t ha-1)(meansacross sampling times);b days after planting,135,177,219 and 269(mean across biochar and fertiliser treatments).Error bars indicate the LSD(p≤0.05)

Fig.10 a Calcium and b magnesium concentrations(%)in leaf tissue of Eucalyptus nitens seedlings in the pot experiment in response to 8 biochar application rates(0–100 t ha-1)(mean across sampling times and fertiliser treatments).Error bars indicate the LSD(a 0.04,b 0.02%;p≤0.05)
Sodium concentration in the leaf material increased at biochar application rates from B50 to B100(p≤0.001)(Fig.11a)but decreased to an average 0.02%over time to 219 DAPL.Leaf Na was higher at F50 than at the full fertiliser rate at 135 DAPL,but this effect did not persist in later samplings(Fig.11b).

Fig.11 Sodium concentrations(%)in leaf tissue of Eucalyptus nitens seedlings in response to a 8 biochar application rates(0–100 t ha-1)in the pot experiment(mean across sampling times and fertiliser treatments),b fertilisation (50 and 100% of a commercial dose)in time(135,177,216 and 269 days after planting)in pot experiment(means across biochar rates).Error bars indicate the LSD(a 0.008,b 0.006%;p≤0.05)
Relationships between nutrient availability and foliage chemistry
There were mostly positive correlations between nutrient availability in the potting mix and their concentration in leaf material(Table 2)(p≤0.05),particularly early in the experiment.In the majority of cases the rho value decreased over time and the regressions equations were not signi fi cant,albeit,for potassium,the correlation remained signi fi cant over the whole experiment.Magnesium in the PM was the only analyte which did not correlate with its concentration in plants during any time of the experiment(data not presented).Ammonium-N and nitrate-N in potting mix positively correlated(p≤0.05)when pooled over time,but not when analysed at each of the sampling dates.The correlation for most nutrients was the strongest at the beginning of the experiment and weakened over time(Table 2).
Discussion
Nitrogen
The increased availability of nitrate-N and decreased availability of ammonium-N at 135 DAPL suggest biochar application might have stimulated nitri fi cation.This may have occurred due to the adsorption of nitri fi cation inhibitors present in the potting mix to the chars surface,allowing nitri fi cation to proceed at a greater rate(Lehmann and Joseph 2009).Alternatively the lower concentrations of ammonium-N were not related to nitri fi cation rates,but instead due to the adsorption ofto biochar particle surfaces(Lehmann et al.2003;Steiner et al.2008),where the presence of acidic functional groups are typically in abundance at pyrolysis temperatures of 450–500°C(Spokas et al.2010).However,Jones et al.(2011)suggested thatwas more sorbed to soil than to biochar,whichtogether with increased PM pH under high biochar treatments(Wrobel-Tobiszewska 2014)and non-limiting water availability during the experiment supports the hypothesis of increased ammonium-N nitri fi cation.Higher levels of PM nitrate-N in response to biochar application did not result in higher plant uptake,and N(%)in the leaf tissue was only dependent on fertiliser level.This is most likely connected with the very low concentration of both nitrate and ammonium N content of macadamia biochar.However,there was a positive correlation(p≤0.05)between the total available N in PM(nitrate-N and ammonium-N)and N content in plant material(Table 2)as well as positive correlation between the level of ammonium-N and nitrate-N in PM(Table 2).These correlations are most likely explained as a response to fertiliser application,which quantitatively made a much greater contribution to nitrate-N and ammonium-N than biochar.The comparative increase in nitrogen availability after biochar application was much smaller,and this small increase in PM-N in response to biochar application may have been harder to detect as an increase in leaf N.

Table 2 Summary of Spearman’s Rank Correlations between potting mix(ammonium N,nitrate-N,total N,P,K,Ca,Na)and leaf chemistry(N,P,K,Ca,Na)of E.nitens,at four harvest times:135,177,219 and 269 DAPL(days after planting)pooled across 8 biochar treatments(0,2,5,10,20,50,80 and 100 t ha-1)and 2 fertilisation concentrations(50 and 100% of the standard commercial application)
Phosphorus
When comparing biochar treatments within each fertilisation level,the application of biochar at high rates(B80–B100)initially increased PM phosphorus concentrations.The total quantity of phosphorus contained in the macadamia biochar was 0.6(B2)to 31.8(B100)mg P per kg PM,compared to fertiliser ranging from 43(F50)to 86(F100)mg P per kg PM.This suggests that the increased concentration of available P in PM under F50×B80 and F50×B100 treatments could be explained by soluble P release from biochar and fertiliser together(Lehmann and Joseph 2009).Despite more phosphorus being available in the potting mix at the full fertiliser rate,leaf phosphorus concentration was more responsive to biochar application than it was to increased fertilisation.It would be expected that higher concentrations of P available in the PM solution at full fertilisation would result in increased leaf P concentrations,yet the presence of biochar had greater effect.As there were no differences in plant growth,this could not be due to growth dilution,and would suggest that biochar by some mechanism increased leaf P assimilation at both rates of fertilisation.
Potassium
Adding high rates of biochar increased the potting mix Colwell potassium concentration at all four harvests,possibly due to K supplementation from the biochar.In the leaf material,K concentrations were positively correlated(Table 2)with that in the PM,and was also increased with greater biochar application rates (Table 2).Similar increases in available K in soil as a result of biochar application have been reported by Lehmann et al.(2003),Chan et al.(2007)and Major et al.(2010)and attributed to the release of K into the soil from the char.The total quantity of potassium introduced to PM with biochar,was 3.64(B2)to 182.25(B100)mg K per kg PM compared to fertiliser;189(F50)to 377(F100)mg K per kg PM.The K dose applied with biochar was not high considering the range of 0.9–58 g K per kg reported for other chars(Lehmann and Joseph 2009)but compared to K released from the fertiliser at half rate,it doubled plant available K when applied at the highest biochar rate in the F50 treatment,and increased it by 50%with the F100 treatment.SEM-EDS analysis revealed large amounts of KCL salt particles present on the char’s surface,suggesting that increased K concentrations were mainly due to solubilisation of K from the char’s surface.The effect of lowered K concentration in the leaf material under F100 in comparison to F50,when high biochar doses were applied was also observed for leaf P.It suggests that there must have been a factor decreasing both leaf P and K when high biochar doses were combined with fertilisation at a high level.
Calcium and magnesium
Application of biochar reduced availability of exchangeable calcium and magnesium in the PM regardless of the quantity of char applied.Availability of exchangeable Ca in the PM declined minimally(1 cmol kg-1over the course of the experiment),suggesting a strong sorption of this ion by macadamia biochar.This is consistent with a high sorption capacity observed in pecan shell biochar(Novak et al.2009).Both leaf Ca and Mg decreased as biochar application rates increased,this phenomena also observed for other crop species after biochar application(Major et al.2010).Both calcium and magnesium ions are strongly adsorbed in comparison to other exchangeable cations(UNSW 2007),as they are divalent(2+)and have small hydrated radii.The correlation between available Ca in the PM and leaf Ca(Table 2)suggested that the general in fl uence biochar had on available Ca was extended to plant uptake.In this instance,it might be postulated that reduced exchangeable calcium in the PM could lead to a de fi ciency in leaf tissue Ca.However,in this experiment,leaf Ca concentration did not fall below typical values reported for eucalyptus seedling reported in the literature(White and Broadley 2003).
Exchangeable Mg can be a good indicator of total plant available Mg(Peverill et al.1999)however,in this experiment there was no correlation between availability in PM and plant leaf tissue concentration.Abundant supply of K in the soil solution,can limit the uptake of Mg(Bear 1965),and in this experiment increased K and Na availability in the potting mix might explain the reduced leaf Mg concentration.Nevertheless,magnesium levels in leaf material were high relative to the reported concentration forE.nitensof 0.09–0.15%(Reuter and Robinson 1997).
Sodium
The SEM-EDS analysis showed a considerable concentration of Na in various compounds on macadamia biochar surfaces(Wrobel-Tobiszewska 2014).As with K,increased Na in the potting mix may be indicative of Na+ion release to the soil solution when introduced with the biochar;in this study ranging from 4.8 to 236.8 mg kg-1.The analysis of exchangeable Na over time(rate of decay parameter)showed that biochar application at high rates(B80–B100)initially increased exchangeable Na,and slowed the rate of decay in the PM.The rate of decay in this analysis most probably re fl ects leaching rates,as there is some evidence PM macro-porosity increased with biochar addition,and sodium is reported to leach earlier than other cations,the result of a weak force of attraction to the surfaces of soil or biochar(Manoa2014).Hencethe decline in PM exchangeable sodium as the experiment progressed may have resulted from increased leaching from PM.Concomitant with this,increased acquisition of Na by the plant should also be taken into account,and evidence that this also occurred is provided by elevated leaf Na concentrations at high biochar application rates.
Conclusions
Biochar raised the concentration of nitrate-N in PM and decreased ammonium-N,and we suggest that biochar most likely enhanced nitri fi cation,yet,the higher levels of nitrate-N in the PM were not high enough to increase growth rates(Wrobel-Tobiszewska et al.2012).The interactions between fertiliser and biochar on nitrate-N and P concentrations in the PM at the end of experiment can be possibly explained by the exhaustion of nutrients in the PM,or may indicate the in fl uence of undetermined factors.Increased leaf P and K under F50 and high biochar treatments implied the existence of a mechanism limiting plant uptake.The analysis of K and Na concentration in macadamia biochar used in the present experiment suggests that elevated levels in both PM and leaf tissue resulted from release of these cations from the biochar surfaces.We propose that both magnesium and calcium cations were sorbed to biochar surfaces.It has been suggested that these cations are primarily sorbed onto biochar surfaces(UNSW 2007)which was supported by reductions in exchangeable Mg and Ca availability when biochar was added.In addition,Mg tended to be lowered by increased K availability and plant uptake,which was the case in this experiment.
Despite chemical changes in PM and leaf tissues,seedlings growth was unaffected by biochar,which indicates that nutrient concentrations were suf fi cient in all treatments.Therefore,we conclude that the lack of biochar effect on growth might have been a result of nutrient-suffi cient environment and a different response could be expected under nutrient and/or water de fi cient conditions.Further studies are required before macadamia biochar can be used for forestry plantations on a commercial scale.
Bear FE(1965)Soils in relation to crop growth.Agricultural College and Experiment Station,Rutgers—the State University,New Brunswick,NJ,USA:Reinhold Publishing Corporation
Chan KY,Xu Z(2009)Biochar:Nutrient properties and their enhancement.In:Lehmann J,Joseph S(eds)Biochar for environmental management.Earthscan,London,pp 67–81
Chan KY,Van Zwieten L,Meszaros I,Downie A,Joseph S(2007)Agronomic values of greenwaste biochar as a soil amendment.Aust J Soil Res 45(8):629
Chan KY,Van Zwieten L,Meszaros I,Downie A,Joseph S(2008)Using poultry litter biochars as a soil amendments.Aust J Soil Res 48:526–530
Jones DL,Murphy DV,Khalid M,Ahmad W,Edwards-Jones G,DeLuca TH(2011)Short-term biochar-induced increase in soil CO2release is both biotically and abiotically mediated.Soil Biol Biochem 43(8):1723–1731
Klein RJT,Huq S,Denton F,Downing TE,Richels RG,Robinson JB,Toth FL(2007)Interrelationships between adaptation and mitigation.In:Climate change 2007:impacts,adaptation and vulnerability.Cambridge University Press(ed)Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change.Cambridge,pp 27–69
Lehmann J,Joseph S(2009)Biochar for environmental management,an introduction.Earthscan,London,pp 1–9
Lehmann J,Da Silva JP,Steiner C,Nehls T,Zech W,Glaser B(2003)Nutrient availability and leaching in an archaeological anthrosol and a Ferrasol of the Central Amazon basin:fertilizer,manure and charcoal amendments.Plant Soil 249:343–357
Ma YL,Matsunaka T(2013)Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity.Soil Sci Plant Nutr 59:628–641
Major J,Rondon M,Molina D,Riha SJ,Lehmann J(2010)Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol.Plant Soil 333(1–2):117–128
Manoa UoHa(2014)Soil-nutrient management for Maui Country.http://www.ctahr.hawaii.edu/mauisoil/c_relationship.aspx
McHenry MP(2009)Agricultural bio-char production,renewable energy generation and farm carbon sequestration in Western Australia:certainty,uncertainty and risk.Agr Ecosyst Environ 129(1–3):1–7
Milne E,Powlson DS,Cerri CE(2007)Soil carbon stocks at regional scales.Agr Ecosyst Environ 122(1):1–2
Novak JM,Busscher WJ,Laird DL,Ahmedna M,Watts DW,Niandou MAS(2009)Impact of biochar amendment on fertility of a southeastern coastal plain soil.Soil Sci 174(2):105–112
Peverill KI,Sparrow LA,Reuter DJ(1999)Soil analysis:an interpretation manual.CSIRO Publishing,Australia,pp 12–34
Reuter DJ,Robinson JB(1997)Plant analysis:an interpretation manual,2nd edn.CSIRO Publishing,Australia
Spokas KA,Baker JM,Reicosky DC(2010)Ethylene:potential key for biochar amendment impacts.Plant Soil 333(1–2):443–452
Stavi I(2013)Biochar use in forestry and tree-based agro-ecosystems for increasing climate change mitigation and adaptation.Int J Sustain Dev World Ecol 20(2):166–181
Steiner C,Glaser B,Teixeira WG,Lehmann J,Blum WEH,Zech W(2008)Nitrogen retention and plant uptake on a highly weathered central Amazoniam ferrasol amended with compost and charcoal.Soil Sci 171:893–899
Thomas SC,Gale N(2015)Biochar and forest restoration:a review and meta-analysis of tree growth responses.New Forest 46:931–946
UNSW(2007)Soil properties:exchangeable cations.Accessed 2013 White PJ,Broadley MR (2003)Calcium in plants.Ann Bot 92:487–511
Wrobel-Tobiszewska A(2014)Biochar as a soil amendment and productivity stimulus forEucalyptus nitensfrestry plantations.University of Tasmania,online University database.Available 2015
Wrobel-Tobiszewska A,Boersma M,Sargison J,Close DC,Krull E,Adams P(2012)Macadamia biochar as a growth stimulus forEucalyptus nitensforestry nurseries.Paper presented at the soil science conference,Hobart
 Journal of Forestry Research2018年2期
Journal of Forestry Research2018年2期
- Journal of Forestry Research的其它文章
- Effect of species composition on ecosystem services in European boreal forest
- Analysis of SSR loci and development of SSR primers in Eucalyptus
- Optimal and synchronized germination of Robinia pseudoacacia,Acacia dealbata and other woody Fabaceae using a handheld rotary tool:concomitant reduction of physical and physiological seed dormancy
- Genetic effects of historical anthropogenic disturbance on a longlived endangered tropical tree Vatica mangachapoi
- Genetic variation in relation to adaptability of three mangrove species from the Indian Sundarbans assessed with RAPD and ISSR markers
- Cloning and characterization of geranylgeranyl diphosphate synthetase from Pinus massoniana and its correlation with resin productivity
