Accumulation of heavy metals in stemwood of forest tree plantations fertilized with different sewage sludge doses
Marius Praspaliauskas•Nerijus Pedisius•Audrius Gradeckas
Introduction
The cultivation of tree plantations for use as energy crops has become particularly important because of the scarcity and rapidly increasing expense of fossil fuels(e.g.,oil,coal,natural gas),and the continued growth of greenhouse gasemissions.Fuelsgenerated from biomasshave increased the potential uses of short rotation trees as an alternative for energy production.Therefore,the development of forest tree plantations has become a public policy objective for countries bound by international conventions for environmental protection.
Recently,the growing demand for wood fuel and increasing quantities of sewage sludge have led to the development of integrated solutions.One such solution is the cultivation of forest tree plantations that are fertilized with sewage sludge.However,the use of sewage sludge as a fertilizer remains controversial because sewage contains high levels of accumulated pollutants,including heavy metals(HMs),which can pollute groundwater and soil and may also be absorbed by plants and move up the food chain.Studies have shown that the concentrations of HM are distributed within the stemwood of trees in the following sequences: Zn>Cu>Cd>Ni>Pb>Cr(Laureysens et al.2004)and Zn>Cu>Cd>Ni(Pulford et al.2002).More detailed HM distributions in different parts of trees are presented in a study by Chang et al.(2014).When energy tree plantations are fertilized with sewage sludge,the HM distributions within different parts of the tree are nonhomogeneous.Long-term observations and studies by Orlandi et al.(2002)and Lettens et al.(2011)have shown that within the stemwood ofLarix deciduaand the leaves ofPopulus trichocarpa×P.deltoides,HMs tend to accumulate at a constant rate.
The establishment of common HM accumulation patterns in trees that grow in polluted soil is complicated because HM absorption and transportation to different parts of the tree is a sophisticated biochemical process that involvesvarioustransportation systems(Rascio and Navari-Izzo 2011).Excess concentrations of HMs in the soil can cause plants to develop a protection mechanism that prevents the absorption of HMs,which suggests that the migration and accumulation of each metal within the tree is an individual process that occurs up to a certain biological saturation limit(Chandra et al.2016).Decreases of HMs are caused by various factors,and the most in fl uentialinclude leaching underacidic conditionsand absorption by the roots,stemwood,bark,and leaves(Unterbrunner et al.2007;Bramryd 2013).The bioavailability of metals to trees and the subsequent accumulation of metals in tree tissue can vary widely according to the source of metal contamination and site conditions(Pulford and Watson 2003).Despite the high sorption capacity of the organic materials found in large concentrations in sewage sludge,excess heavy metals can in fl uence plant growth.Further,pH parameters in the soil can in fl uence heavy metal absorption from sewage sludge.For example,the mobility and accumulation of Cu and Zn in plants increase as the pH decreases.Additionally,pH governs the phytotoxic symptoms that determine plant growth(Planquart et al.1999).In conditions of high pH,the solubility of chemical compounds is lower,which increases the diffi culty of inactive element absorption by plants.The accumulation of Cu,Zn and Ni by trees planted on slag(Salt et al.1996)was less than 1% of the metal contained in the top 10 cm of the waste,thereby rendering phytoextraction unfeasible at this site.The low uptake of metals was attributed to the high pH of the waste and the chemical form of the slag’s metal contaminants.At a less-contaminated site,Watson(2002)reported a decrease at the end of the growing season in the concentrations of Cd,Cu and Zn in the leaves and bark,although a slight increase was observed in the wood.Studies have reported that wood accumulates only a certain quantity of HMs and showed that the levels remain stable,regardless of the HM concentrations in the soil(Hossain et al.2012;Reimann et al.2007).Even at a constant soil concentration,HM accumulation in trees does not follow a stable sequence.One analysis of different birch(Betula pubescens)parts showed that at lower soil concentrations,the HM levels in the wood did not differ substantially from the levels observed in the present study(Reimann et al.2007).
Several models investigating the factors affecting the HM uptake in trees have been described in McLaughlin(2001)and Pinto et al.(2014).Most of these models have been applied to Cd,Zn,Cu and Pb.The results of such models are often relevant only for a particular tree species growing in certain environmental conditions.Therefore,it is dif fi cult to determine the distribution of HM within separate parts of forest plantations because of a lack of controlling mechanisms(Evangelou Michael et al.2012).The studies of Reimann et al.(2007)and Hossain et al.(2012)indicate that HM accumulation mainly occurs in the leaves and to a lesser extent in the roots and stemwood.Because energetic trees are mostly cut during winter,HM accumulation within stemwood is extremely important.
A number of by-products are formed by incinerating wood fuel,and some of these forms are undesirable as bottom ash.The incinerated stemwood of trees grown on soil fertilized with sewage sludge could contain more HM than trees from nonfertilized plots.Consequently,the ashes of trees fertilized with sludge cannot be used to fertilize agricultural land,which might affect the potential of sludge as a forest fertilizer.In certain cases,biomass combustion causes a greater release of HM into the atmosphere(as fl y ash)compared with that remaining as bottom ash(Maciejewska et al.2006:Berra et al.2010;Hazrat et al.2013).
The levels of HMs that accumulate in trees is dependent on whether the sample is collected from a young or a mature tree(Turner and Dickinson 1993).Mature trees show a number of differences compared with young saplings(3–4 years),such as their wood carbon content,the biochemical plant cell resistance,the number of tissues involved in photosynthesis.An analysis of the heavy metal concentrations in various trees(maple,ash,pine,birch and cottonwood)grown on sludge-amended mine spoils(Pulford and Watson 2003)showed that in the fi rst-year tree samples,the metal accumulation patterns were high,whereas in the 3rd-year harvest samples,signi fi cant increases in the concentrations were not observed,although this result does not necessarily indicate a decline in metal assimilation over time.Rather,the concentrations were attributed to dilution by higher biomass production and metal accumulation in the litter layer.
Because of these differences,conclusions generated for mature trees based on studies of young trees are problematic(Turner 1994;Labrecque et al.1995).However,most studies investigating HM accumulation from sewage sludge are limited to short-rotation(up to 5 years)tree plantations.HM accumulation has been described in leaves for 7-year rotations ofPopulus trichocarpa×P.deltoides(Lettens et al.2011)and in fi ne roots for 3-year rotations ofSalixsp.,Picea abies(Dimitriou et al.2006)and inPopulus tremula(Brunner et al.2008).Also the accumulation of HMs has been described in stemwood ofPopulus trichocarpa×P.deltoides‘Beaupré’andPopulustrichocarpa‘Trichobel’(Moffat et al.2001);and 2-year rotations ofPopulus euramericana‘Robusta’in leaves and stems(Smilde 1981).Less commonly,studies have been performed with fast-growing trees(hybrids of thePopulusgenus,birches,and grey alders),and the investigation period is short compared with the entire growth period of a tree(18–20 years).Trees that are not usually selected for studies of metal tolerance can generally survive in metalcontaminated soil,albeit with a reduced growth rate(Dickinson et al.1992).Even in contaminated soils,most of these seedlings can survive for at least 3 years despite impaired growth,suggesting a low level of innate tolerance;however,facultative tolerance,such as that observed with a proliferation of fi ne roots in uncontaminated soil zones,is important(Pulford and Watson 2003).
However,when considering trees for use as wood fuel,studies of mature trees are more reasonable than those conducted on young saplings.In addition,tree leaves and roots are less relevant when trees are to be used as energy resources.In plantations,trees are usually felled in winter when they reach maturity or at their largest mass increase.Therefore,the main objectives of this study are to evaluate the accumulation of Cd,Cr,Cu,Ni,Pb and Zn in the stemwood of trees cultured for 20 years in a peat quarry fertilized with sewage sludge and to determine whether such wood can be used as fuel in combustion processes without causing environmental problems,i.e.,environment pollution through ash reuse.
Materials and methods
Plantation establishment and initial evaluation
The plantation was established by the Institute of Forestry of the Lithuanian Research Centre for Agriculture and Forestry in a high-moor peatland as part of a project conducted between 1991 and 1996(Gradeckas et al.1998).In 1993,an abandoned peat quarry plot with an area of 0.2 ha was selected for the cultivation of forest trees.A drained plot that presented a cut-away upper-type high-moor peatland with a 1 m deep peat layer was chosen.The groundwater level was more than 1 m in depth.Sewage sludge was spread over the entirety of the surface of the plot in three layers with different thicknesses:6,12 and 24 cm.Each investigated plot was fertilized with different amountsofsewagesludge:minimal(180 Mg ha-1),moderate(360 Mg ha-1),maximal(720 Mg ha-1)of dry matter.A nonfertilized plot was used as the control.The plots were ploughed,and the sewage sludge was ploughed up to peat.The plots were only fertilized once,in 1993.
The soil from the plots included in the experiment was initially analyzed using the following instruments and methods:the pHKClwas measured with a potentiometer;the total nitrogen was measured with the Korn fi eld method;the available phosphorus was measured with the Kirsanov method;and the available potassium extraction was performed with a fl ame photometer.
Five tree species were chosen for the tests.In every plot,30 trees were planted in rows(1×3 m)at a planting density of 3.3×103ha-1,for a total of 600 trees.The height of the saplings was 0.5–1.0 m.
In 2013, fi ve samples of the mature trees(grey alder[Alnus incana],silver birch[Betula pendula],common aspen×American aspen [Populustremula×Populus tremuloides],European spruce[Picea abies]and box elder[Acer negundo])and plantation soil were collected.In total,200 stemwood samples were collected from the stems(10 samples per tree species)at 1.3 m from the ground(Fig.1)using a Pressler drill.
Five soil samples were randomly collected between the trees from the top 6–24 cm of the soil surface in each separate plot(for a total of 20 samples).
Laboratory analysis
In the laboratory,the stemwood and soil samples were dried for 18 h in a low temperature electrical furnace at 105°C.The dried samples were cooled in a desiccator to room temperature.The stemwood samples were mechanically chopped into 3–4 mm chips,and the soil samples were quantitatively ground to the size of a 0.5 mm sieve.
The samples(approximately 0.2–0.4 g)were fl ooded with 2 mL of concentrated fl uoric acid and 6 mL of concentrated nitric acid.The samples were placed in a mineraliser and mineralised for 1 h 10 min(at 800 W,6 MPa,pRate:50 kPa/s),with 10 min allocated for heating,45 min for mineralisation(in accordance with established parameters)and 15 min for cooling.After the fi rst mineralisation,the samples were fl ooded with 20 mL boric acid(H3BO3)and again placed into a mineraliser for 1 h 10 min(at 800 W,6 MPa,pRate:30 kPa/s).
After the mineralisation,the solution was poured into 50 mL fl asks and diluted to 50 mL using deionised water.The solutions prepared from the different stemwoods and soil samples were analyzed using an inductively coupled plasma optical emission spectrometer(ICP-OES).
Soil were mixed for 5 min in 1 M KCl solution at a ratio of 1:5 to measure soil pHKCl.The suspension was allowed to sit for 2 h to stabilize the ionic balance,and then the hydrogen ion concentration was measured with a potentiometer.

Fig.1 Forest plantation after 20 years.Sampling locations in left image are encircled in blue
Statistics and calculations
The results of the chemical analyses were analysed using Statistica 7 software(StatSoft,Tulsa,OK,USA).The mean values and standard deviations(SDs)were calculated for each species at each fertilization plot.
A bioconcentration factor or accumulation factor(AF)was calculated(in%)according to the following equation(Wilson and Pyatt 2007):

where AF is the accumulation factor(%);Cwood-2013is the metalconcentrationinthe stemwoodin2013;andCsoil-1993is the metal concentration in the soil in 1993 after fertilization.
The signi fi cance of differences between the metal distribution in the stemwood and soil was evaluated using the Pearson correlation coef fi cient(r);p<0.05 was considered suf fi cient for signi fi cance.For demonstrating a relationship between two variables,the relationship in most cases was described as a linear function or as a seconddegree polynomial function for the accumulated Cu and Zn concentrations in the stems of silver birch.
Results
Sewage sludge and soil parameters in 1993
Based on granulometric composition,sewage sludge,composed mainly of sand with a high concentration(16.4–30.2%)of organic matter,was applied to the experimental plantation.Sludge moisture content was 55%.Mean pH indices of sludge and nonfertilized soil were 6.6 and 3.4,respectively.The mean pH index of fertilized soil was approximately the same as that of sludge;thus,the sludge strongly reduced the acidity of the soil.The soil contained a large concentration of humus(12.6–17.5%),substantial quantities of total nitrogen(533–792 mg kg-1),‘available phosphorus’(1470–1930 mg kg-1)and a small concentration of available potassium(153–298 mg kg-1).
Leaching of HM during the 20-year period
Figure 2 shows the initial concentration of HM in the soil as determined in 1993 after the plantation was fertilized with sewage sludge(Gradeckas et al.1998)and in 2013 after 20 years of growth.
As shown Fig.2,the HM concentration in the nonfertilized soil in 1993 was low:Cr~48.9 mg kg-1,Cu~8.6 mg kg-1,Ni~13.5 mg kg-1,Pb~10.3 mg kg-1and Zn~75.8 mg kg-1.Cd was not initially measured.The application of sludge signi fi cantly increased the HM concentrations in the soil,and the increments of HM concentrationsinthesoilwerethelargestat180 Mg ha-1input.The Cu concentration increment was the largest and reached up to 14-fold.Increases in the HM element concentrations occurred in the following sequence:Cu>Pb>Zn>Ni and Cr.
As expected,as the intensity of fertilization increased above 180 Mg ha-1,the rate of HM concentration increments in the soil gradually decreased and eventually approached the HM concentration level of the sludge in the upper ploughed layer of the soil.Thus,the Cu concentration increased by only 3.8 and 1.9 times when the fertilization input increased from 180 to 360 Mg ha-1and 720 Mg ha-1,respectively.OtherHM concentrations increased to a lesser degree.In the fertilization range of 360 to 720 Mg ha-1,the increment of HMs in the soil increased by only 1.1–1.2 times.

Fig.2 Cr,Cu,Ni,Pb and Zn concentrations in the soil in 1993 and relationship to sewage sludge input(I)rates in 2013.The Cd concentration was measured only in 2013
Fertilization also changed the sequence of HM elements in terms of quantity from Cu>Ni>Cr>(Pb,Zn)at a fertilization level of 180 Mg ha-1to Cu>Zn>(Cr,Pb)>Ni at a level of 720 Mg ha-1.
After 20 years,the HM concentrations in the soil signi fi cantly decreased.However,the analysis shows that these reductions are linked to the metal type.If the relative averaged values of the HM concentration decreases in all fertilized plots are excluded,then the values for Ni and Zn;Cr and Pb;and Cu reach up to 30,55 and 70%,respectively.In most cases,especially between the nonfertilized plots and the plots fertilized to 360 Mg ha-1,the HM concentrations in the soil increased along with the fertilization intensity,and the values followed a logistic curve.However,with increases in the fertilization intensity from 360 to 720 Mg ha-1,the HM concentrations increased at a declining rate(Fig.2).These differences were not dependent on the absolute concentration values but rather on the metal type.The biggest change in the concentration of Cu may have been caused by its high mobility and dependence on soil alkalinity.
In 2013,the soil pH indices in the fertilized plots varied from 6.6 to 5.6,and these values were dependent on variations in the fertilization intensity from 720 to 180 Mg ha-1.These pH values were close to the values observed for the sludge.The pH value of the nonfertilized soil remained practically the same at 3.3.
HM accumulation in the stemwood of trees
Figure 3 shows the concentrations of separate metals(Cu,Ni,Pb and Zn)that were measured in the stemwood of all of the investigated tree species after 20 years.Cd and Cr were only found in several wood samples and at concentrations close to the limits of detection or quali fi cation;therefore,they were not subjected to further analysis.
The sampled trees grown in fertilized soil did not exhibit visible symptoms of toxicity.However,in all of the fertilized plots,the accumulated HM concentrations showed exceptional tendencies for individual metal concentrations and tree stems.The results showed that different plants have different levels of susceptibility to the accumulation of various HMs,which is related to differences in the bioavailability of heavy metals/metalloids in soil.HMs can be distributed into three categories:readily bioavailable(Cd,Ni,Zn,As,Se,Cu),moderately bioavailable(Co,Mn,Fe)and least bioavailable(Pb,Cr,U)(Hazrat et al.2013).
Figure 3 shows that Zn presented the highest accumulation in all of the investigated tree stems relative to Cu,Ni and Pb.According to Pesonen et al.(2014),Zn may have occurred in more a bioavailable form than the other metals,which might explain the strong accumulation of Zn in all of the tree samples.In our study,the Zn concentrations in all of the tree stems varied in the nonfertilized plot from 8.3 to 41.4 mg kg-1and in the fertilized plots from 0.7 to 63.2 mg kg-1.
For the individual tree species,the Zn concentrations in the stems of the grey alder,silver birch,and the common aspen×American aspen hybrid were nearly the same and varied from 34.3 to 44.7 mg kg-1.The accumulated Zn concentrations in the European spruce stems were lower at approximately 20.3±3.7 mg kg-1,and box elder accumulated the lowest concentration of Zn at approximately 5.0±0.3 mg kg-1.
Independent of the fertilization intensity,the Pb concentration was highest in the grey alder stems(6.1±0.2 mg kg-1)and lowest in the box elder(4.9±0.4 mg kg-1),and it declined progressively in the following sequence:grey alder>common aspen× American aspen>silver birch>European spruce>box elder.
The highest accumulated Ni concentrations independent of the fertilization intensity were observed in grey alder and common aspen×American aspenhybridstems,valuesthat varied close to 2.0 mg kg-1,and the lowest accumulated Ni concentrationsinallofthefertilizationplotswasfoundinthe silver birch stem at 1.6±0.07 mg kg-1.
The accumulated Cu concentrations independent of the fertilization intensity were highest in the grey alder stems(3.1±0.1 mg kg-1)compared with the other investigated trees(between 0.6 and 1.1 mg kg-1),and the difference was signi fi cant.
In terms of the metal type,the stemwoods in the nonfertilized and fertilized areas generally accumulated HMs in the following sequence:Zn>Pb>Ni>Cu.This tendency became even more noticeable when the variation of accumulation factors was analysed.These factors were calculated according to Eq.(1)and are presented in Fig.4.
In the nonfertilized areas,the accumulation factors of Pb andZnforallofthetreeswerehighatmorethan40%.ThePb accumulation factor was the highest,and its values varied from 44.8 to 82.9%.The Zn accumulation factor was nearly the same for all of the trees and varied between 40.1 and 44.2%.The only exception was box elder,for which the Zn accumulation factor was 13.8±1.4%.The accumulation factors for Cu and Ni were less than those of Pb and Zn,and theirvaluesdidnotexceed25%.Inaddition,theaccumulation factor for Cu in the box elder was 54.8±19.4%.Equivalent accumulation factor values were calculated for Cu and Ni for the silver birch(Cu,7.7±2.3%;Ni,8.4±0.6%)and European spruce(Cu,16.2±3%;Ni,17.1±2.6%).
In the fertilized areas,regardless of the fertilization intensity,the accumulation factors decreased signi fi cantly compared with that of the nonfertilized areas.The accumulation factors of Pb and Zn in the fertilized areas reached nearly the same level in all of the trees.In this case,the accumulation factor for Pb varied from 6.0%(box elder)to 11.0%(grey alder),and for Zn,it varied from 4.4%(European spruce)to 14.0%(silver birch).For the box elder,the Zn accumulation factor was low and varied from 1.3 to 1.4%.The Ni accumulation factor was between the values for Pb,Zn and Cu.A consistent decrease was observed in the accumulation factors for the common aspen×American aspen hybrid,silver birch and box elder.For the grey alder and European spruce,the accumulation factors at a fertilization intensity of 720 Mg ha-1were higher than those at a fertilization intensity of 360 Mg ha-1,although the difference was not signi fi cant.A considerable decrease in the Cu accumulation factor was observed with increases in the fertilization intensity,although a signi fi cant decrease in the accumulation factor for the grey alder,common aspen×American aspen hybrid and silver birch stems was observed in all of the fertilization plots.
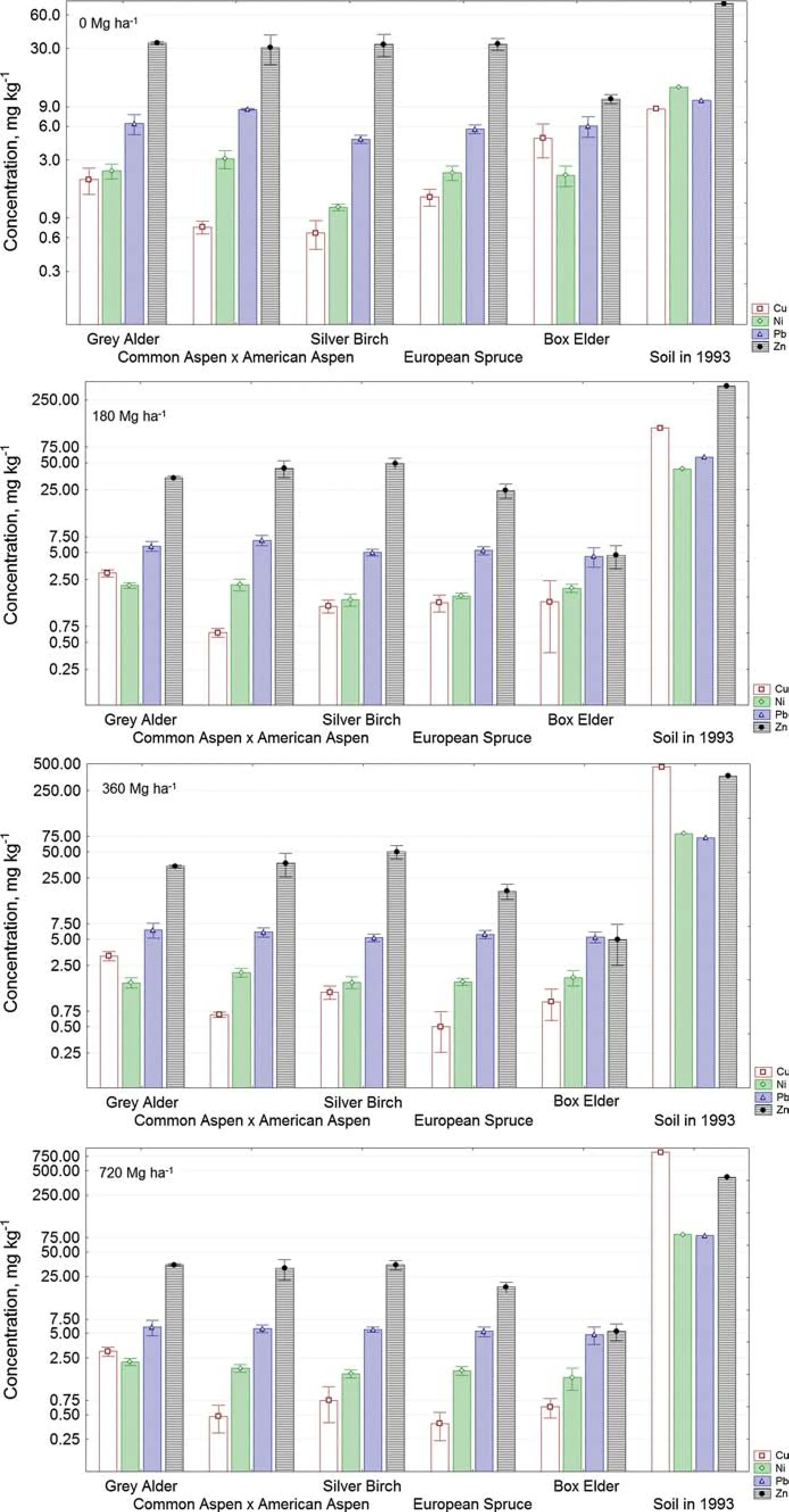
Fig.3 Cu,Ni,Pb and Zn concentrations(mean value,mg kg-1±SD)in the stemwood of the investigated tree species in 2013 and the heavy metal concentrations in the fertilized soil in 1993

Fig.4 Relation of the accumulation factors for Cu,Ni,Pb and Zn(mean±SD)on the intensity of fertilization for all types of trees
Relationship between HM concentrations in the stemwood and soil
Figures 5,6,7 and 8 present the results of the regression analyses on the relationships between the Cu,Ni,Pb and Zn concentrations in the stemwood of all of the tree species and their concentrations in the soil after fertilization.
As shown in Fig.5,the accumulated Cu concentrations in the stemwood of three species decreased as its concentration in the soil increased. For the common aspen×American aspen hybrid,a moderate negative correlation was observed(r=-0.54,p<0.05),and for European spruce and box elder,very strong and strong negative correlations were observed, respectively(r=-0.84,p<0.05;r=-0.73,p<0.05).For the grey alder,a moderate positive correlation was observed(r=0.59,p<0.05),and for silver birch,a quadratic relationship was observed.
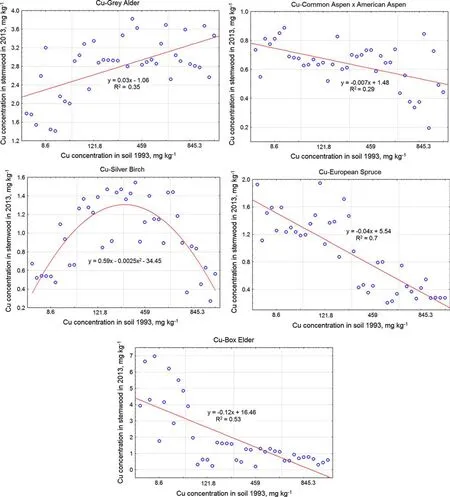
Fig.5 Regression analyses of the Cu concentrations(mg kg-1)in the stemwood of investigated trees,which were dependent on the Cu concentration in the soil after fertilization(mg kg-1)
The accumulated concentrations of Cu in the nonfertilized soil(Cu 8.6 mg kg-1)and soil fertilized at an intensity of 720 Mg ha-1(Cu 845.3 mg kg-1)were nearly the same for silver birch at 0.66±0.02 and 0.74±0.37 mg kg-1,respectively.In areas fertilized with intensities of 180 Mg ha-1(Cu 121.8 mg kg-1)and 360 Mg ha-1(Cu 459 mg kg-1),the accumulated concentrations of Cu in the stemwood of silver birch were 1.26±0.22 and 1.23±0.23 mg kg-1,respectively.In this case,the linear relationship of the results does not imply a lack of relationship between the variables.This correlation between the Cu concentration in the soil and the silver birch stemwood is described as a quadratic relationship.

Fig.6 Regression analyses of the Ni concentrations(mg kg-1)in the stemwood of investigated trees,which were dependent on the Ni concentration in the soil after fertilization(mg kg-1)
The Ni concentration in four tree species(Fig.6)decreased as its concentration in the soil increases.These correlations were negative and weak for grey alder(r=-0.3,p=0.05) and moderate for common aspen×American aspen hybrid(r=-0.58,p<0.05),European spruce(r=-0.49,p<0.5)and box elder(r=-0.54,p<0.5).However,in the silver birch stemwood,the Ni correlation coef fi cient was positive and moderate(r=0.58,p<0.05).

Fig.7 Regression analyses of the Pb concentrations(mg kg-1)in the stemwood of investigated trees,which were dependent on the Pb concentration in the soil after fertilization(mg kg-1)
This relationship between the accumulated Pb in the stemwood and its concentration in the soil was very weak or weak in most cases(grey alder,European spruce and box elder)(Fig.7),which is partially explained by Pb having the lowest bioavailability to plants(Hazrat et al.2013).Strong and moderate correlations were observed only for the common aspen×American aspen hybrid(r=-0.78,p<0.05)and silver birch trees(r=0.53,p<0.05).Although the correlation was negative for the common aspen×American aspen hybrid,which is consistent with the observations for Cu and Ni,it is positive for the silver birch.

Fig.8 Regression analyses of the Zn concentrations(mg kg-1)in the stemwood of investigated trees,which were dependent on the Zn concentration in the soil after fertilization(mg kg-1)
The relationships(Fig.8)for Zn between the stemwood and soil were nearly the same as for those for Cu(Fig.5),although the statistical signi fi cance for the common aspen×American aspen hybrid(r=0.13,p=0.4)and grey alder(r=0.2,p=0.2)was weak.For the European spruce and box elder,the correlations were negative and moderate(r=-0.57,p<0.05)and strong(r=-0.67,p<0.05),respectively.Moreover,a nonlinear correlation was observed for silver birch,which is consistent with the results for Cu(Fig.5).
The correlation analyses showed that the levels of dependence of the Ni and Pb concentrations in the stemwood on the concentrations in the soil were different.In the case of Ni,this relationship was signi fi cant(p<0.05)for all of the investigated species,whereas for Pb,the relationship was only signi fi cant for the common aspen×American aspen hybrid and silver birch trees.The weak correlation between soil Pb and tree Pb was explained by Hazrat et al.(2013),who indicated that Pb is one of least bioavailable metals to plants.Moreover,the correlation coef fi cient for Pb for the other tree species(Fig.7)was considerably smaller than that for Ni(Fig.6),and the sign of the correlation coef fi cient was different for grey alder and European spruce.In Figs.5–8,the correlation between the HM concentrations in the stemwood and soil was generally strong and statistically signi fi cant,especially for Cu and Ni.Less reliable correlations were observed for Pb and Zn in the stemwood and soil.The correlation analyses suggested that 75% of the correlations between the HMs in the stemwood and their concentrations in the soil were negative and the differences were signi ficant,whereas only 25% of the HMs showed positive correlations between their concentration in the stemwood and the soil.
In general,the correlation analyses con fi rmed that the HM accumulation factors in the stemwood of the investigated tree species decreased with increases in the fertilization intensity.However,changes in the attributes of accumulation that were dependent on the tree species,soil content and metal type were obvious and are discussed below.
Discussion
The analyses of the HM accumulation in the studied stemwoods in relation to the HM concentrations in the soil generated several interesting results.First,all of the investigated trees can be divided according to their susceptibility to the HM,with three species(common aspen×American aspen hybrid,European spruce and box elder)demonstrating decreasing HM accumulation rates with increases in the application of sewage sludge,which was observed in almost all of the investigated cases,and the two local tree species(grey alder and silver birch)demonstrating an increased likelihood of accumulating HM,although the correlation was signi fi cantly weaker.Second,the individual HM accumulation tendency differed among the tree species.For example,the Pb and Zn correlations were weak for all of the studied trees,although these metals accumulated to the highest concentrations.
The results of the investigation showed that when more sewage sludge was applied,the soil pH index also increased.Sewage sludge is rich in humus and nutrients.Moreover,the sludge presents a generally neutral reaction and can neutralize the acidity of peaty soils.According to Watson(2002),the alkalinity of the soil can in fl uence metal sorption.Moreover,alkalinity can tend to limit the uptake of HM by trees because HMs are immobilized in an alkaline soilmatrix,thereby reducing theiruptake.According to the results of this study,the decreased HM values in the stems under increases in the amount of sewage sludge used as fertilizer were generally signi fi cant.
Only small concentrations of HMs from the soil accumulated in the tree stems(Fig.3).The distribution of HM concentrations within the stems of hybrid poplars was Zn>Cu>Cd>Ni>Pb>Cr according to Laureysens et al.(2004)and Zn>Cu>Cd>Pb>Cr>Ni according to Pesonen et al.(2014);however,different results were obtained in this investigation for the common aspen× American aspen hybrid,with Zn>Pb>Ni>Cu(in all of the fertilization plots).The same distribution of HMs were observed in all of the fertilization plots for the silver birch stems.According to results obtained by Pesonen et al.(2014),the concentrations were ordered from Zn>Cr>Ni>Pb>Cu>Cd,whereas in the results presented here,Zn and Ni accumulated in the same amounts.The results of this study showed that in almost all of the investigated trees,the sequence of accumulated metals was the same regardless of the fertilization level.Most associated studies have ordered the accumulated concentrations of HMs in different trees by the age distribution and land applications,although in all of the studies,the Zn concentration was almost always highest.For example,Lorenc-Plucinska et al.(2013)found that the accumulated Zn concentrations in young grey alder were highest.The main factors that control the mobility of Zn in soil are the pH,soil organic matter,redox potential,cation exchange capacity,ionic species type and concentration,carbonate content,particle-size distribution and the presence of oxide and hydroxide species(Pinto et al.2014).The levels of the HMs in the trees were also dependent on the in fl uence of these individual variables,and a strong in fl uence of one of these factors can change the distribution of the accumulated HMs in plants.
This study showed low Cu concentrations in the stemwoods,which was likely related to the large decrease in Cu from the soil over the 20 years from 1993 to 2013 compared with that of the other metals(Fig.4).According to other investigations,a low uptake of Cu can limit the biological processes of trees;however,Cu is toxic at high concentrations(Pinto et al.2014).Despite the large decrease in Cu in the soil,the concentration was still high.High concentrations of Cu in the soil can be in fl uenced by the alkalinity of the soil because increases in pH decrease the solubility of Cu in the soil(Wang et al.2013).This study showed that as the amount of sewage sludge increased,the soil pH also increased.This dependency could have the largest in fl uence on Cu accumulation in stemwood.Additionally,the speciation of trace metals in waste appears to be of greater environmental concern than their total concentrations.Potentially relevant risks include the mineralization of organic matter and the release of trace metals into more soluble complexes(Perez-Esteban et al.2013).This study con fi rms that the total concentration of heavy metals in sewage sludge and the soil pH are insuffi cient criteria for the control of plant metal accumulation.
The assessment of the levels of HM in wood after application of sewage sludge shows that does not result in a signi fi cant increase in stemwood HM concentration in the energy tree plantations because the largest proportions of HMs are leached into the ground.Therefore,the use of such wood as fuel in combustion processes will not create environmental problems related to high-HM concentration air pollution or problems related to the use of the ash as fertilizer.However,the use of sewage sludge as a fertilizer for energy tree plantations is controversial because of the potential to increase groundwater pollution,which could be toxic for plants and microorganisms(Perez-Esteban et al.2013).Thus,additional information and investigations on the leaching of HM into deeper soil layers and groundwater are required.
Conclusions
The ability of wood to accumulate HM generally decreased when the intensity of sewage sludge fertilization increased,and the correlation analyses showed that 75% of the results presented a signi fi cant negative correlation between the HM concentration in the stemwood and the soil.At the same fertilization intensity,different trees tend to accumulate certain concentrations of different HMs,which remain at the same level.HM accumulation in mature tree stems is not signi fi cant relative to the overall metabolic balance of harmful materials because in most cases,the concentration of HM in stemwood differs by several orders of magnitude from the concentration in the soil.The levels of HM in the trees showed that there were signi fi cant decreases for Cu and Ni,whereas less reliable correlations were determined for Pb and Zn.Cd and Cr were found only in a few wood samples at concentrations close to the limits of detection or quali fi cation.
The fi ndings of this study show that the accumulated concentrations of Zn,Pb,Ni and Cu in the tree stems in the plots fertilized with sewage sludge were signi fi cantly lower than in the nonfertilized plots.The export of HM to the aboveground biomass only accounted for a small proportion of these metals contained in high-moor peatland soil;therefore,the use of this wood for energy production will not exceed the permitted limits of heavy metal pollution in the air and ash.
Berra M,Dell‘orso M,Mangialardi T,Polini AE,Piga L.(2010).Chemical and environmental characterization of fl y ash from woody biomass combustion.Proceedings Venice,Third International Symposium on Energy from Biomass and Waste.Venice,Italy 8-11
Bramryd T(2013)Long-term effects of sewage sludge application on the heavy metal concentrations in acid pine(Pinus sylvestris L.)forests in a climatic gradient in Sweden.For Ecol Manage 289:434–444
Brunner I,Luster J,Madeleine S,Günthardt-Goerg FB(2008)Heavy metal accumulation and phytostabilisation potential of tree fi ne roots in a contaminated soil.Environ Pollut 152:559–568
Chandra R,Cho W,Kang H.(2016).Phytoextraction potential of four poplar hybrids under greenhouse conditions.Forest Science and Technology,1–8(Published online:03 Aug 2016)
Chang FC,Ko CH,Tsai MJ,Wang YN,Chung CY(2014)Phytoremediation of heavy metal contaminated soil by Jatropha curcas.Ecotoxicology 23:1969–1978
Dickinson NM,Turner AP,Watmough SA,Lepp NW(1992)Acclimation of trees to pollution stress:cellular metal tolerance traits.Ann Bot 70:569–572
Dimitriou I,Eriksson J,Adler A,Aronsson P,Verwijst T(2006)Fate of heavy metals after application of sewage sludge and wood-ash mixtures to short-rotation willow coppice.Environ Pollut 142:160–169
Evangelou Michael WH,Conesa HM,Robinson BH,Schulin R(2012)Biomass Production on Trace Element-Contaminated Land:a Review.Environ Eng Sci 29:9
Gradeckas A,Kubertavičiené L,Gradeckas A(1998)Utilization of wastewater sludge as a fertilizer in short rotation forests on cut away peatlands.Baltic Forestry 2:7–13
Hazrat A,Ezzat K,Muhammad AS(2013)Phytoremediation of heavy metals—Concepts and applications.Chemosphere 91:869–881
Hossain MA,Pukclai Piyatida,Jaime A.Teixeira da Silva,Fujita M.(2012).Molecular mechanism of heavy metal toxicity and tolerance in plants:central role of glutathione in detoxi fi cation of reactive oxygen species and methylglyoxal and in heavy metal chelation.Journal of botany,37
Labrecque M,Train I,Teodorescu S,Daigle S(1995)Effect of wastewater sludge on growth and heavy metal bioaccumulation of twoSalixspecies.Plant Soil 171:303–316
Laureysens I,Blust R,De Temmerman L,Lemmens C,Ceulemans R(2004)Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture:I.Seas Var leaf wood bark Conc Environ Pollution 131:485–494
Lettens S,Vandecasteele B,DeVos B,Vansteenkiste D,Verschelde P(2011)Intra-and inter annual variation of Cd,Zn,Mn and Cu in foliage of poplars on contaminated soil.Sci Total Environ 409:2306–2316
Lorenc-Plucinska G,Walentynowicz M,Niewiadomska A(2013)Capabilities of alders(Alnus incana and A.glutinosa)to grow in metal-contaminated soil.Ecol Eng 58:214
Maciejewska A,Veringa H,Sanders J,Peteves SD.(2006).Co- fi ring of biomass with coal:constraints and role of biomass pre-treatment.European Communities,2006.EUR–Scienti fi c and Technical Research Series ISSN 1018-5593
McLaughlin MJ(2001)Bioavailability of metals to terrestrial plants.Geoderma 122:143–149
Moffat AJ,Armstrong AT,Ockleston J(2001)The optimization of sewage sludge and e fl uent disposal on energy crops of short rotation hybrid poplar.Biomass Bioenerg 20:161–169
Orlandi M,Pel fi ni M,Pavan M,Santilli M,Colombini MP(2002)Heavy metals variations in some conifers in Valle d’Aosta(Western Italian Alps)from 1930 to 2000.Microchem J 73:237–244
Perez-Esteban J,Escolastico C,Moliner A,Masaguer A(2013)Chemical speciation and mobilization of copper and zinc in naturally contaminated mine soils with citric and tartaric acids.Chemosphere 90:276–283
Pesonen J,Kuokkanen T,Kaipiainen E,Koskela J,Jerkku I,Pappinen A,Villa A(2014)Chemical and physical properties of short rotation tree species.Eur J Wood Products 72:769–777
Pinto E,Aguiar AARM,Ferreira IM(2014)In fl uence of Soil Chemistry and Plant Physiology in the Phytoremediation of Cu,Mn,and Zn.Crit Rev Plant Sci 33:351–373
Planquart P,Bonin G,Prone A,Massiani C(1999)Distribution,movement and plant availability of trace metals in soils amended with sewage sludge composts:application to low metal loadings.Sci Total Environ 241:161–179
Pulford ID,Watson C(2003)Phytoremediation of heavy metal contaminated land by trees–a review.Environ Int 29:529–540
Pulford ID,Riddell-Black D,Stewart C(2002)Heavy metal uptake by willow clones from sewage sludge-treated soil:the potential for phytoremediation.Int J Phytorem 4:59–72
Rascio N,Navari-Izzo F(2011)Heavy metal hyperaccumulating plants:how and why do they do it?And what makes them so interesting?Plant Sci 180:169–181
Reimann C,Arnoldussen A,Finne TE,Nordgulen FKU,Englmaier P(2007)Element contents in mountain birch leaves,bark and wood under different anthropogenic and geogenic conditions.Appl Geochem 22:1549–1566
Salt CA,Hipkin JA,Davidson B.(1996).Phytoremediation—a feasible option at Lanarkshire Steelworks?Proceedings of a discussion meeting,Glasgow,Glimmerveen,I.Institute of chartered foresters,Edingburg,51
Smilde KW(1981)Heavy-metal accumulation in crops grown on sewage sludge amended with metal salts.Plant Soil 62:3–14
Turner AP.(1994).The responses of plants to heavy metals.In:Ross SM.Toxic metals in soil—plant systems.Chichester:Wiley,pp.153–87
Turner AP,Dickinson NM(1993)Survival of Acer pseudoplatanus L.(sycamore)seedlings on metalliferous soils.New Phytology 12:509–521
Unterbrunner R,Puschenreiter M,Sommer P,Wieshammer G,Tlustos P,Zupan M,Wenzel WW (2007)Heavy metal accumulation in trees growing on contaminated sites in Central Europe.Environ Pollut 148:107–114
Wang C,Yang ZF,Yuan XY,Browne P,Chen LX,Ji JF(2013)The in fl uences of soil properties on Cu and Zn availability in soil and their transfer to wheat(Triticum aestivum L.)in the Yangtze River delta region China.Geoderma 193:131–139
Watson C.(2002).The phytoremediation potential of Salix:studies of the interaction of heavy metals and willows.PhD thesis,University of Glasgow
Wilson B,Pyatt FB(2007)Heavy metal bioaccumulation by the important food plant,Olea europaea L.,in an ancient metalliferous polluted area of Cyprus.Bull Environ Contam Toxicol 78:390–394
 Journal of Forestry Research2018年2期
Journal of Forestry Research2018年2期
- Journal of Forestry Research的其它文章
- Effect of species composition on ecosystem services in European boreal forest
- Analysis of SSR loci and development of SSR primers in Eucalyptus
- Optimal and synchronized germination of Robinia pseudoacacia,Acacia dealbata and other woody Fabaceae using a handheld rotary tool:concomitant reduction of physical and physiological seed dormancy
- Genetic effects of historical anthropogenic disturbance on a longlived endangered tropical tree Vatica mangachapoi
- Genetic variation in relation to adaptability of three mangrove species from the Indian Sundarbans assessed with RAPD and ISSR markers
- Cloning and characterization of geranylgeranyl diphosphate synthetase from Pinus massoniana and its correlation with resin productivity
