Comparative Study of careHPV Assay, Visual Inspection andPap Smears as Primary Screening in Rural China
Zhao Yuqian, Dang Le, Zhang Shaokai, Hu Xiaofeng, Zuo Tingting, Chen Feng, Zhang Xun, Chen Wen, Qiao Youlin△
(1 Department of Cancer Prevention and Treatment, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610041, Sichuan, China.2 National Cancer Center, Cancer Hospital of Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100021, China.3 Department of Cancer Epidemiology, Henan Cancer Hospital, Zhengzhou 450008, Henan, China.4 Shijingshan District of Beijing Center for Disease Control and Prevention, Beijing 100043, China.)
Ⅰ Introduction
Invasive cervical cancer ranks the fourth most common malignant tumor among women around the world, with 85% cervical cancer occurrence and 87% death in less-developed countries and regions estimated in 2012 by International Agency for Cancer Research (IARC)[1]. It has been proven that HPV vaccine and organized screening could prevent cervical invasive cancer effectively[2-3]. Establishing a comprehensive primary screening program with cytology in low-resource settings is difficult due to the lack of qualified cytologists. Visual inspection with acetic acid or Lugol`s iodine (VIA/VILI) is recommended for primary screening in low-resource settings by World Health Organization (WHO) since they are simple and low-cost[4]. However, the sensitivity of VIA/VILI to detect cervical intraepithelial neoplasia 2 or worse lesion (CIN2+) in different studies varies greatly due to its subjectivity[5-7]. Currently, HPV assay is recommended as an option for cervical cancer screening. However, most available HPV tests are expensive, require experienced laboratory personnel and high-quality lab conditions, which is difficult to be implemented in low-resource settings. In recent year, careHPV DNA testing was successfully developed. The accuracy and performance of careHPV DNA testing to detect CIN2+ cervical cancer are better than cytology and much higher than VIA/VILI[8]. In addition, the careHPV test is low-cost and fast[8].
In the year 2009, the central government launched a Free Cervical Cancer Screening Program for 10 million rural women. Pap smears and VIA/VILI are recommended for primary screening. This national screening program has expanded to cover 10 million rural women annually[9]. Our study aimed to evaluate the performance of careHPV DNA assay in low-resource settings, and compare with VIA/VILI and pap smears performed by rural local health providers. Besides, a field-based survey assessing the knowledge and attitudes of the local health providers toward the HPV and integrating HPV assay approach into current cervical cancer prevention programs in low resource settings was conducted.
Ⅱ Materials and Methods
Population
Women living in rural areas of Xinmi County were invited to participant in the cervical cancer screening program during November 2013 and January 2014. Non-pregnant women with no history of CIN, pelvic radiation, or hysterectomy, and could voluntarily provide informed consent were eligible for enrollment. A local health worker took the responsibility to explain the study procedure to all the women. The enrolled women were randomized into 3 arms, as shown in figure 1, and then screened by careHPV DNA assay, Pap smears, or VIA/VILI respectively. Women with any test positive would be referred for colposcopy. The study was approved by the Institutional Review Board Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS).
Screening and Data Collection
The primary screening was done at the township level hospital. On the day of recruiting, eligible women signed the informed consent were given an identity number and assigned to one of the study arms randomly by a pre-set computer program. The pap smears specimens were graded by a local pathologist according to Bethesda 2001classification system, Atypical Squamous Cells of Undetermined Significance (ASC-US) or worse lesion were defined as abnormal cytology. In VIA/VILI, 5% acetic acid were applied to the cervix through embedded cotton swab and then observed after 1 min. If no aceto-whitening was found, then the cervix was swabbed with 5% iodine solution. VIA or VILI exam abnormal women were referred to colposcopy. The careHPV specimen was collected with careHPV cervical sampler into Digene collection media (DCM) and sent to the lab in Xinmi Maternal and Children Health Care Hospital. A local lab assistant was trained by a senior technician from CHCAMS for two days, and then the assay was run by the local lab assistant alone. In careHPV test, results were represented as a ratio of viral load expressed in relative light units (RLU), compared with the mean RLU from a positive control set at 1 pg/mL. Questionnaires of knowledge and attitudes about the cervical cancer screening program were collected from the health providers at the village level.
Colposcopy was performed by local doctors. Endocervical curettage (ECC) was performed in a case of the invisible transformation zone. Colposcopy-guided directed biopsies were performed if an abnormal epithelial area on the cervix was observed. Pathological slides were prepared by local doctors. To ensure the accuracy of clinical diagnosis, pathological results were reviewed by a senior pathologist from CHCAMS as the final diagnosis.
Statistical Analysis
Microsoft Access 2010 software was used for data input and management. Double entry validation and logical consistency check were performed. SPSS 23.0 were used for data analysis. Mean and the standard deviation (SD) was calculated for age at screening. Normality test and homogeneity of variance test were performed to detection the characteristics of age distribution. Kruskal-Wallis Test was performed to compare the age of the three arms. Bonferroni test was used to compare the difference between every two groups. A proportion was calculated for a categorical variable. Women were stratified by age (<34, 35~39, 40~44, 45~49, 50~54, 55~59, 60~64). The Cochran-Armitage trend test was used to detect the trend of the positive rate of primary screening results along with the increasing of age group.P<0.05 was considered statistically significant.
Ⅲ Results
Screening Results
A total of 1,993 women younger than 64 years old participated in the screening studies. Among them, 894 women were randomized into careHPV DNA screening, 552 women were screened by VIA/VILI and 547 women were screened by pap smears. The average age with SD was 44.1±7.84 years for all women, and the age range was 21 to 64. The average age with SD for women screened by careHPV assay, VIA/VILI, pap smears were 44.5±8.01, 43.6±8.72, and 44.0±6.51, respectively. No statistically significant difference was found between the age of the three arms (P=0.135).
The age-stratified number of cases, positive rates was presented in table I. For women who had the careHPV assay, 95 (10.6%) were found HPV-positive. Among women had abnormal VIA/VILI, 100 (18.1%) women were found abnormal, and 97.0% (97/100) were a low-grade suspicious lesion, the other 3.0% (3/100) women were a high-grade suspicious lesion. For women who had pap smears, 4.9% (27/547) of them found ASC-US or worse (ASC-US+) cytology, 81.5% (22/27) of them were ASC-US, and 18.5% (5/27) were low-grade squamous intraepithelial lesions (LSIL). The difference between the primary screening positive rates shown statistical significance (χ2=48.647,P<0.001), careHPV DNA testing was higher than pap smears (χ2=14.180,P<0.001) but lower than VIA/VILI (χ2=16.408,P<0.001). As shown in figure 2, the positive rate for careHPV DNA testing was significantly increasing with age (χ2=13.473,Ptrend<0.001), while for women who have had VIA/VILI for primary screening, the positive rate was decreasing with age (χ2=10.818,Ptrend=0.001). No linear trend by age was detected for women had pap smears (χ2=1.837,Ptrend=0.175).
Among women with positive primary screening, 77.5% (172/222) of them came back for colposcopy and/or histology sampling. No statistically significant difference of the follow-up rate was found among the three arms (careHPV 84.2% vs. VIA/VILI 73.0% vs. pap smear 77.5%,χ2=4.398,P=0.111). In total, 5 CIN1 cases, 8 CIN2 cases, and 2 CIN3 cases were found from the 172 women. The overall detection rate of CIN2+ was 0.5%. The detection rates of CIN2+ was 0.7% (6/894) for HPV assay, 0.5% (3/552) for VIA/VILI, and 0.2% (1/547) for pap smears. The CIN2+ detection rates were not significantly different (χ2=1.648,P=0.439).
Questionnaire Survey
In total, 24 health providers from the villages clinics who were responsible for organizing eligible women finished the questionnaires. 45.8%(11/24) of them finished high school and the other 54.2% (13/24) finished the college education. The average years of being working in the village clinics were 15.8 years. As shown in table II, all the health providers have heard of HPV, but the knowledge about HPV and cervical cancer were unsatisfactory since more than half (58.3%) of them thought that all HPV genotypes could cause cervical cancer and 75.0% thought genital bacterial infections could cause cervical cancer. For the knowledge of cervical cancer screening, VIA/VILI and pap smears were better known as the primary screening tests due to the national screening program. The knowledge about HPV assay as the primary screening was unsatisfactory that nearly 30% of them thought that HPV-positive equals to cervical cancer and they were unaware of the recommend HPV screening frequency for HPV-negative women. However, their attitudes about implementing HPV assay into the national program were positive that 79.2% of them were completed agree. The most concerned issue for them was the cost of HPV assay.
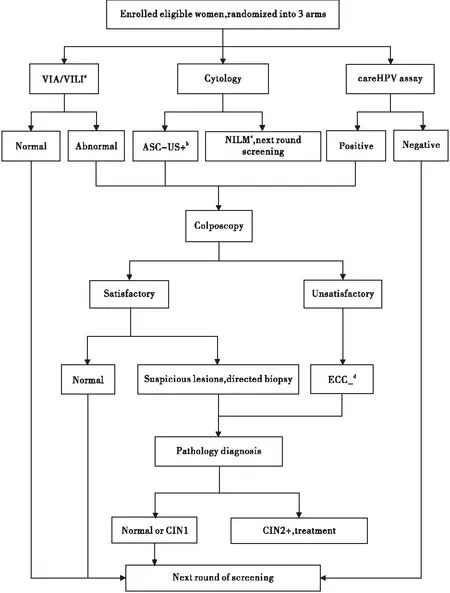
Figure 1. Flowchart of Cervical Cancer Screening Study.
a:VIA/VILI=Visual Inspection with Acetic acid and with Lugol’s Iodine;b:ASC-US+= Atypical Squamous Cells of Undetermined Significance or worse;c:NILM=Negative for Intraepithelial Lesion or Malignancy;d:ECC=Endocervical curettage 95%CI: 95% Confidence Intervals.
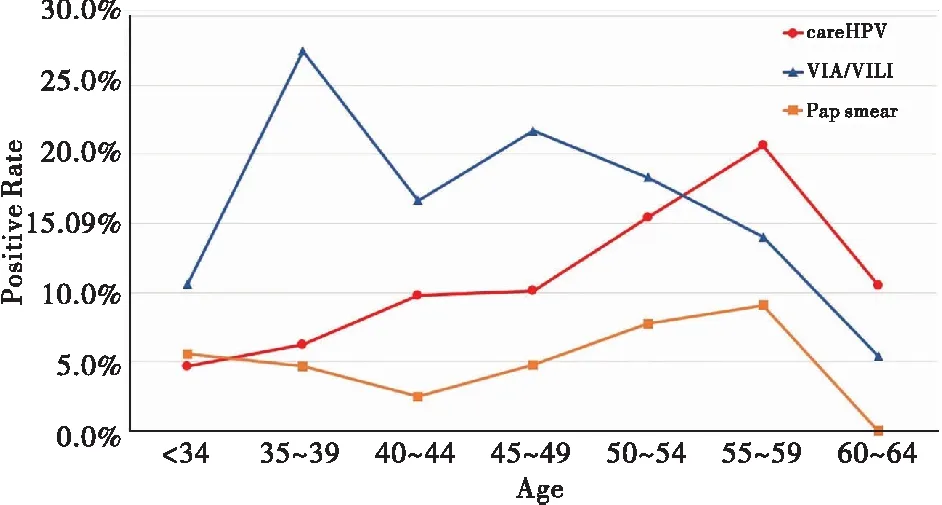
Figure 2. Age-specific positive rate of careHPVDNA assay, VIA/VILI and Pap smears.
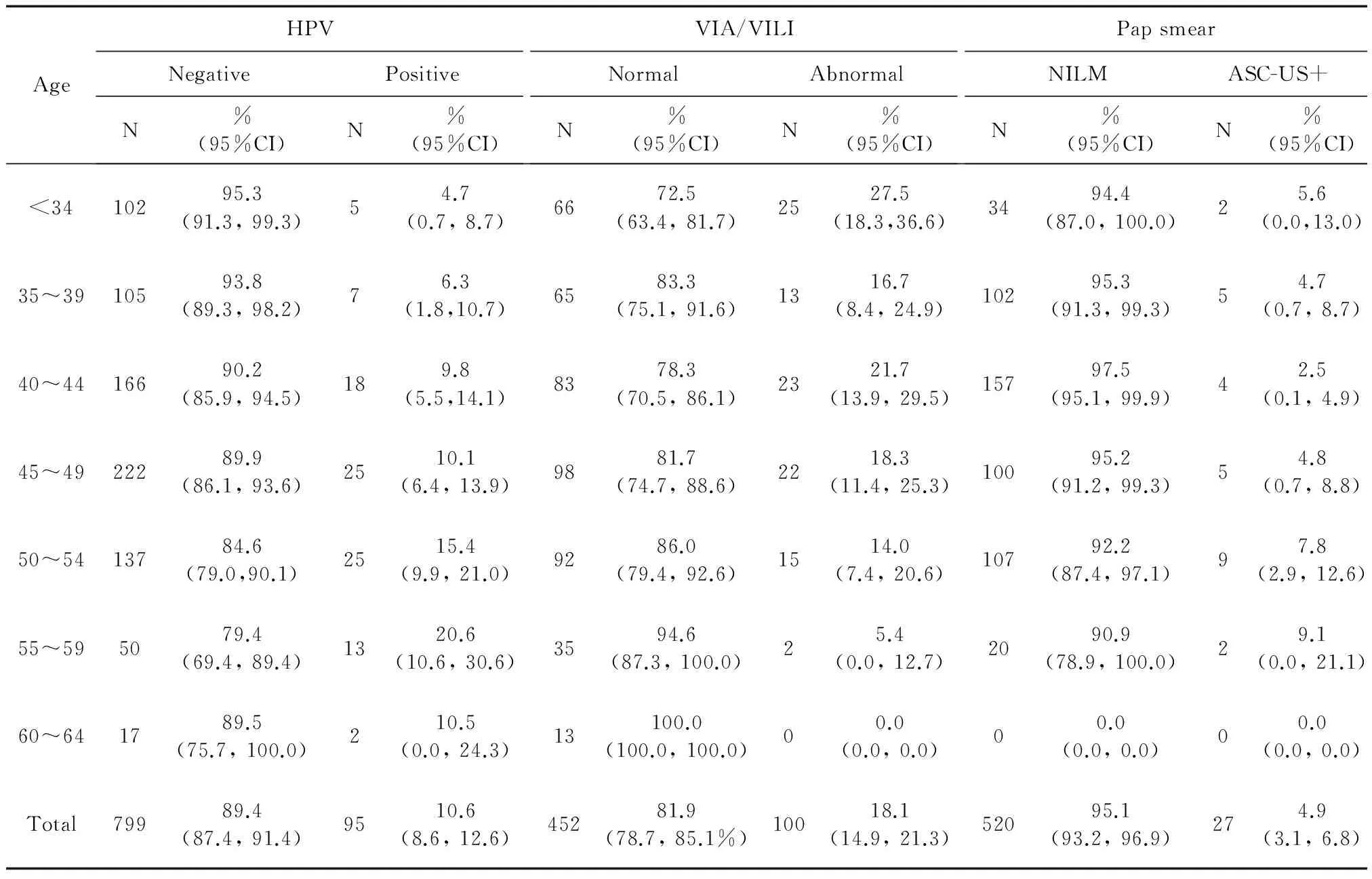
AgeHPVNegativePositiveN%(95%CI)N%(95%CI)VIA/VILINormalAbnormalN%(95%CI)N%(95%CI)PapsmearNILMASC-US+N%(95%CI)N%(95%CI)<3410295.3(91.3,99.3)54.7(0.7,8.7)6672.5(63.4,81.7)2527.5(18.3,36.6)3494.4(87.0,100.0)25.6(0.0,13.0)35~3910593.8(89.3,98.2)76.3(1.8,10.7)6583.3(75.1,91.6)1316.7(8.4,24.9)10295.3(91.3,99.3)54.7(0.7,8.7)40~4416690.2(85.9,94.5)189.8(5.5,14.1)8378.3(70.5,86.1)2321.7(13.9,29.5)15797.5(95.1,99.9)42.5(0.1,4.9)45~4922289.9(86.1,93.6)2510.1(6.4,13.9)9881.7(74.7,88.6)2218.3(11.4,25.3)10095.2(91.2,99.3)54.8(0.7,8.8)50~5413784.6(79.0,90.1)2515.4(9.9,21.0)9286.0(79.4,92.6)1514.0(7.4,20.6)10792.2(87.4,97.1)97.8(2.9,12.6)55~595079.4(69.4,89.4)1320.6(10.6,30.6)3594.6(87.3,100.0)25.4(0.0,12.7)2090.9(78.9,100.0)29.1(0.0,21.1)60~641789.5(75.7,100.0)210.5(0.0,24.3)13100.0(100.0,100.0)00.0(0.0,0.0)00.0(0.0,0.0)00.0(0.0,0.0)Total79989.4(87.4,91.4)9510.6(8.6,12.6)45281.9(78.7,85.1%)10018.1(14.9,21.3)52095.1(93.2,96.9)274.9(3.1,6.8)

Table II. Knowledge and attitudes about HPV and HPV screening for cervical cancer of local health providers.

(continued)
Ⅳ Discussion
In the presented study, we found that in rural China, the CIN2+ detection rate of careHPV was higher than the conventional pap smear and VIA/VILI, though the difference was not statistically significant. If all women with positive primary screening result should be referred to colposcopy, the referral rate of HPV DNA testing would be at a medium level compared with VIA/VILI and pap smears. Besides, although the local health providers from village clinics were positive about HPV testing for cervical cancer screening, it is urgent to educate them with adequate knowledge about HPV DNA testing and the screening strategy.
Although liquid-based cytology has been proven to be more accurate than conventional pap smears[10], due to the limited resources in rural China, liquid-based cytology is currently not an option for the national large population cervical cancer screening program. It has been reported that the prevalence of abnormal cytology is 17.4% for women living in rural China[11]ranging between 8.9%~25.7%. In previous studies, the sensitivity could be as excellent as 81.0%~94.2%, and the yield of CIN2+ by cytology could be 3.4% in the laboratory with strict quality control and experienced cytopathologists[6,11-12]. The cytology abnormity rate in our study performed by county level doctors is 4.9% and the CIN2+ detection rate is only 0.2%, which is much lower than the previous studies in a higher level laboratory. The pooled data of the nationwide cervical cancer screening program was reported by Song et al that the positive rate for pap smears is 3.9%[13], which is similar to our study. It implied that although by good quality control and extensive training, cytology could be an effective way for cervical cancer screening, but currently it is not an ideal option for population screening in rural China. VIA/VILI is another option for the national cervical cancer screening program in rural China. In the national reports of the first round of cervical cancer screening[13], the overall abnormal rate for primary VIA/VILI is 11.1%, which is much lower than our data. In our study, the positive rate of VIA/VILI is nearly twice as that of HPV assay and three times of pap smears, but the detection rates of CIN2+ was lower than HPV assay. The positive predict value of VIA/VILI is far from satisfactory.
The accuracy of HPV assay as primary screening have been proved[14-16]and introduced in guidelines worldwide[17-19]. However, the high cost, high requirement of lab personnel and facilities makes it impossible to be implemented in large population screening programs in resource-limited countries and regions. Previous studies reported excellent performance of careHPV in clinical trials[8, 20]performed by well-trained lab personnel in organized screening programs, and studies have been conducted to implement careHPV assay in developing countries[21-24]. A cluster randomized controlled trial in India reported similar results to our study, that the highest positive rate was found in VIA arm, the lowest was for cytology[24]. No significant differences were found for high-grade lesions detection rates. Both of our real-world studies at a lower level of health resource suggested that for large population screening program, extensive training and good quality control are needed to improve the quality, and careHPV DNA assay is feasible at low-resource settings performed by the local health provider. An age trend of abnormal VIA/VILI and HPV-positive were observed. Similarly, Kang et al and Zhao et al reported an increasing HPV prevalence with age[25-26]. The birth cohort effect and lower clearance rate for older women may partly explain, but further follow-up studies are needed. It may suggest that an age-specific screening strategy might be reasonable to integrate HPV assay into the national screening program, especially for areas that could not provide HPV assay for all women. Further studies comparing the CIN2+ detection rate between different screening strategies in real-world are needed.
A successful screening program does not only depend on the screening approach, but also the coverage of the target population. A health provider has a strong influence on women's decisions to uptake screen for cervical cancer[27-28]. It has been reported that knowledge of cervical cancer and its prevention are significantly associated with screening uptake[29]. So that information about cervical cancer and public education of how to prevent it as well as screening services available should be provided to women correctly. The local health providers investigated in our study are directly responsible for recruiting eligible women and are the propagandist of the national screening program. By the questionnaire survey, we found that the local health providers have heard of the HPV and HPV assay, but are not acquainted with it. Although most of them are aware of the primary screening approaches, their knowledge of HPV assay for cervical cancer screening program is not satisfactory. Fortunately, their attitudes about integrating HPV assay into the national program are positive. Our data suggested that in the future national screening program, more attention should be paid to educate the local health providers about HPV assay.
The presented study is a pilot study for implementing HPV assay into the national screening program. The clinical examinations are all performed by the local doctors, except for the histology diagnosis, which best present the true performed of careHPV in rural areas. It is the first real-world evaluation and comparison of the careHPV assay with currently recommended pap smear and VIA/VILI in the national screening program. Limitations of this study are lack of the knowledge and attitude survey for the screened women, that the acceptance and preference of HPV assay are not evaluated. Besides, a straightforward screening algorithm that all women with positive primary screening referred to colposcopy limited us to evaluate possible triage approaches for HPV-positive women. Lower cost strategies for triage of HPV DNA-positive women are reported[30], including high-risk HPV genotyping, E6 protein, etc. A demonstration study of HPV assay as primary screening provided by county-level hospitals in 21 study sites across the country is ongoing. The HPV-positive women are randomly referred to reflex cytology, VIA/VILI or directly colposcopy examination, for the purpose of evaluating practice primary and triaging approaches to reduce colposcopy examination for large population screening.
In conclusion, our study proved it is possible to apply careHPV assay performed by simply trained lab personnel in low-resource settings. Education for implementing HPV assay in local health providers is needed.
Acknowledgement
We sincerely appreciate the job of administrative staff from the Maternal and Children Health Hospital in Xinmi County, including Mrs. Fengxian Zheng, from the Department of Health Care, Mr. Guoqi Gao, the secretary of the hospital, and Mrs. Caihong Li, the director of the hospital for coordinating this study. We also thank the women and health providers who participant in our study.
[1] Ervik MFL, Ferlay J, Mery L, et al. Cancer Today. International Agency for Research on Cancer [EB/OL] https://gco.iarc.fr/today/fact-sheets-cancers?cancer=16.2017-01-10.
[2] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015 [J]. CA Cancer J Clin, 2015,65(1):5-29.
[3] Gustafsson L, Pontén J, Zack M, et al. International incidence rates of invasive cervical cancer after introduction of cytological screening[J]. Cancer Causes Control, 1997,8(5):755-763.
[4] Sauvaget C, Fayette JM, Muwonge R, et al. Accuracy of visual inspection with acetic acid for cervical cancer screening[J]. Int J Gynaecol Obstet, 2011,113(1):14-24.
[5] Basu PS, Sankaranarayanan R, Mandal R, et al. Visual inspection with acetic acid and cytology in the early detection of cervical neoplasia in Kolkata, India[J]. Int J Gynecol Cancer, 2003,13(5):626-632.
[6] Zhao FH, Zhang WH, Pan QJ, et al. A study of cervical cancer screening algorithms[J]. Zhonghua Zhong Liu Za Zhi, 2010,32(6):420-424.
[7] Li N, Ma CP, Sun LX, et al. Evaluation on the visual inspection with Lugol’s iodine in cervical cancer screening program[J]. Zhonghua Liu Xing Bing Xue Za Zhi, 2006,27(1):15-18.
[8] Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China[J]. Lancet Oncol, 2008,9(10):929-936.
[9] Yu S, Yang CS, Li J, et al. Cancer Prevention Research in China[J]. Cancer Prev Res (Phila), 2015,8(8):662-674.
[10] Strander B, Andersson E A, Milsom I, et al. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program[J]. Cancer Cytopathol, 2007,111(5):285-291.
[11] Pan QJ, Hu S, Zhang X, et al. Pooled analysis of the performance of liquid-based cytology in population-based cervical cancer screening studies in China[J]. Cancer Cytopathol, 2013,121(9):473-482.
[12] Pan QJ, Li L, Qiao Y. Comparative study on liquid-based cytology for cervical carcinoma screening in a high-risk area of China[J]. Zhonghua Zhong Liu Za Zhi, 2001,23(4):309-312.
[13] Song B, Wu JL, Song L, et al. Analysis on the status of cervical cancer screening for rural women in 2012[J]. Chinese Journal of Women and Children Health. 2015,6(1):4.
[14] Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test[J]. Gynecol Oncol, 2015,136(2):189-197.
[15] Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials[J]. Lancet, 2014,383(9916):524-532.
[16] Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China[J]. Lancet Oncol, 2010,11(12):1160-1171.
[17] Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: A review of current American Cancer Society Guidelines and Current Issues in cancer screening[J]. CA Cancer J Clin, 2015,65:30-54.
[18] World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention [M].WHO Press, 2013:1-6.
[19] Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance[J]. Gynecol Oncol, 2015,136(2):178-182.
[20] Kang LN, Jeronimo J, Qiao YL, et al. Optimal positive cutoff points for careHPV testing of clinician-and self-collected specimens in primary cervical cancer screening: an analysis from rural China[J]. J Clin Microbiol, 2014, 52(6):1954-1961.
[21] Trope LA, Chumworathayi B, Blumenthal PD. Preventing cervical cancer: stakeholder attitudes toward CareHPV-focused screening programs in Roi-et Province, Thailand[J]. Int J Gynecol Cancer, 2009, 19(8):1432-1438.
[22] Labani S, Asthana S, Sodhani P, et al. Care HPV cervical cancer screening demonstration in a rural population of north India[J]. Eur J Obstet Gynecol Reprod Biol, 2014, 176:75-79.
[23] Gage JC, Ajenifuja KO, Wentzensen N, et al. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria[J]. Int J Cancer, 2012, 131(12):2903-2909.
[24] Sankaranarayanan R, Nene BM, Dinshaw KA, et al. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India[J]. Int J Cancer, 2005,116(4):617-623.
[25] Kang LN, Castle PE, Zhao FH, et al. A prospective study of age trends of high-risk human papillomavirus infection in rural China[J]. BMC Infect Dis, 2014,14:96.
[26] Zhao YQ, Zhao FH, Hu SY, et al. Multi-center cross-sectional study on type-specific human papillomavirus infection among Chinese women[J]. Zhonghua Liu Xing Bing Xue Za Zhi, 2015, 36(12):1351-1356.
[27] Bessler P, Aung M, Jolly P. Factors affecting uptake of cervical cancer screening among clinic attendees in Trelawny, Jamaica[J]. Cancer Control, 2007, 14(4):396-404.
[28] Ncube B, Bey A, Knight J, et al. Factors associated with the uptake of cervical cancer screening among women in portland, Jamaica[J]. N Am J Med Sci, 2015,7(3):104-113.
[29] Lyimo FS, Beran TN. Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: three public policy implications[J]. BMC Public Health, 2012,12:22.
[30] Qiao YL, Jeronimo J, Zhao FH, et al. Lower cost strategies for triage of human papillomavirus DNA-positive women[J]. Int J Cancer, 2014,134(12):2891-2901.

