Modified insulin-like growth factor 1 containing collagen-binding domain for nerve regeneration
Jian-an Li, Chang-fu Zhao, Shao-jun Li, Jun Zhang Zhen-hua Li, Qiao Zhang, Xiao-yu Yang, Chun-fang Zan
1 Department of Orthopedics, Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Orthopedics, China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics, Affiliated Hospital of Changchun University of Traditional Chinese Medicine, Changchun, Jilin Province, China
Introduction
Insulin-like growth factor 1 (IGF-1), a polypeptide growth factor containing 70 amino acids, has a molecular weight of about 7.5 kDa. This factor is an anabolic peptide hormone with structural similarities to proinsulin (Holman and Baxter, 1996; Twigg and Baxter, 1998; Rosen and Pollak, 1999;Clemmons, 2006). While IGF-1 can be synthesized by most organs during fetal development, it is mainly produced by hepatocytes and the pancreas after birth (Fernandez and Torres-Alemán, 2012). The IGF-1 receptor (IGF-1R), which mediates transmembrane information transfer of IGF-1, is a transmembrane glycoprotein with a tetrameric structure formed by two α and β subunits (Annunziata et al., 2011;Benarroch, 2012).
IGF-1-specific binding to IGF-1R usually triggers two canonical pathways: PI3K/Akt and MEK/ERK (Benarroch,2012; Hayashi et al., 2013). This factor exerts potent effects on neurogenesis and synaptogenesis, as well as neuroprotective, pro-proliferative, and anti-apoptotic actions. It also has the crucial ability to promote axon regeneration and demy-elination (Scolnick et al., 2008; Dyer et al., 2016; Rauskolb et al., 2017). Thus, IGF-1 may be a potential therapeutic factor for peripheral nerve repair. However, in its native form, it is an impractical therapy because of its limited half-life of 8–16 hoursin vivo(Laron, 1999). Thus, short half-life, rapid diffusion, and complications resulting from use of high dosages limit the widespread application of IGF-1. A previous study revealed that many tissues in the body, such as aorta and skin, contain collagens and could therefore be targets of the collagen-binding domain (CBD) (Chen et al., 2007). We hypothesized that CBD could be integrated into IGF-1 to improve its current deficiencies.
In this study, we linked IGF-1 with a CBD deduced from collagenase and expressed the fusion protein using a pET-15b expression system. To verify the biological function of the synthesized protein, we tested its collagen-binding activity and effects on cell proliferation, neural differentiation,and levels of IGF-1R-mRNAin vitro.

Figure 1 Polyacrylamide gel electrophoresis and western blot assay of recombinant proteins.

Table 1 DNA sequences of CBD-IGF-1 and IGF-1
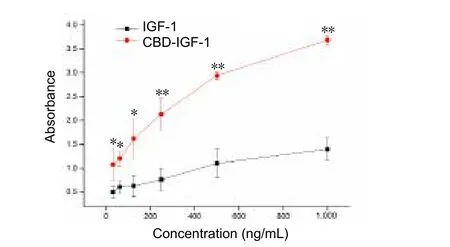
Figure 2 Dose response curve showing binding of IGF-1 and CBDIGF-1 to rat tail collagen.
Materials and Methods
Construction and expression of plasmids
DNA sequences of CBD-IGF-1 and IGF-1 were designed to generate relative proteins (Kim et al., 2015). The two DNA sequences (Table 1) were designed to include restriction endonuclease sites at both ends (underlined). In addition, the DNA sequence of CBD-IGF-1 was designed to include a CBD sequence (TKKTLRT, in red) and glycine sequence (in bold).CBD-IGF-1 DNA sequences and IGF-1 DNA sequences were ligated into a similarly digested pET-15b (+) vector and transformed into E. coli BL21 (DE3) host cells, as described previously (Jacobus and Gross, 2015). Subsequently, 100 μL of host cells carrying recombinant plasmid were plated on solid Luria-Bertani (LB) medium pre-mixed with penicillin(100 μg/mL). Transfected cells grew overnight at 37°C and then monoclonal colonies were selected and plated in 10 mL of liquid LB medium pre-mixed with penicillin. Afterwards,cells grew overnight in a shaker at 150 r/min and 37°C.
Protein purification
After overnight incubation, 1 mL of transfected cells was added to 1 L of YTA medium containing ampicillin (100 μg/mL),which was left to recover at 37°C and 150 r/min to an OD600of 0.8–1.0. Subsequently, it was induced overnight by 1 mL of isopropyl-β-d-thiogalactopyranoside (1 M) at 28°C and 150 r/min. The mixture was pelleted by centrifugation for 30 minutes at 4°C and 4,000 ×g(Heraeus Sepatech, Osterode,Germany). After removal of the supernatant, the remaining pellet was frozen at −80°C for purifying the target protein.
The remaining pellet was resuspended in lysis buffer containing 50 mM phosphate-buffered saline (PBS) and 1 mM phenylmethyl sulfonylfluoride, pH 6.5. Resuspended cells were sonicated for 20 minutes (300 W, 10 s/10 s). The cell lysate was centrifuged for 30 minutes at 4°C and 10,000 ×g. The supernatant was transferred to a fresh tube for future analysis, while the pellet was washed three times with 30 mL of wash buffer (2 M urea, 50 mM PBS, pH 6.5). Following the last wash, the solution was centrifuged under the same conditions. The insoluble cell pellet was resuspended by rocking in 25 mL of dissolving buffer (8 M urea, 50 mM PBS, 5 M βME,pH 6.5) overnight at 4°C. Soluble mixtures were centrifuged at 4°C and 10,000 ×gfor 30 minutes. For the second round of purification, the supernatant was used for CBD-IGF-1 or IGF-1 purification with Ni-NTA affinity chromatography.
Proteins were eluted by linearly decreasing the urea concentration of the dialysate from 6 M to a final concentration of 0 M (6–0 M). The dialysate was changed every day until there was no urea.
Western blot assay
All samples were analyzedviaimmunoblot with anti-IGF-1 antibody to verify the presence of CBD-IGF-1 or IGF-1.Extracted proteins were transferred to nitrocellulose membranes, blocked, and then incubated with rabbit anti-human IGF-1 monoclonal antibody (1:1,000; Abcam, Cambridge,UK) at 4°C overnight. The following day, membranes were rocked for 1 hour at room temperature with goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase(1:500; Abcam). After three washes in Tris-buffered saline with Tween, images of bands were acquired using darkroom development techniques for chemiluminescense.
Collagen-binding assay detected by enzyme linked immunosorbent assay (ELISA)
Type I collagen (in 0.5 M acetic acid) was coated onto 96-well plates. After washing with PBS three times, 100 μL of recombinant IGF-1 or CBD-IGF-1 were added to 96-well plates and incubated at 4°C overnight. After three washes, diluted anti-IGF-1 (1:5,000) was added to the plates and incubated at 37 °C for 1 hour. After three washes, 100 μL of secondary antibody (1:2,000; Earthox, San Francisco, CA, USA) labeled with horseradish peroxidase was added and incubated for 1 hour at 25°C, followed by three washes. The horseradish peroxidase substrate was added and incubated at 37°C for 30 minutes. An Infinite F200 microplate reader (Tecan, Salzburg, Austria) was used to quantify absorbance at 562 nm.
Proliferation effects on PC12 and Schwann cells
As previously described (Zhang et al., 2006), the activity of IGF-1 and CBD-IGF-1 was assayed in both PC12 and Schwann cells (both from Cell Bioscience, Shanghai, China).Briefly, 5 × 104cells in 200 μL of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum were inoculated onto poly-L-lysine-coated 96-well plates for 4 hours. After cells adhered to plates, wells were rinsed with PBS twice and then incubated for 3 days in 200 μL of DMEM containing 10% fetal bovine serum and 15, 31, 62.5, 125, 250,500, or 1,000 ng/mL of IGF-1 or CBD-IGF-1. Cell proliferation was evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Briefly, 20 μL of MTT solution was added to each well and incubated for 4 hours. After centrifugation, the supernatant was removed and 150 μL of dimethyl sulfoxide was added to each well. After incubation for 10 minutes, absorbance values were measured at 570 nm with an Infinite F200 microplate reader.
Immunofluorescence staining
Neurofilament (NF200) and microtubule-associated protein 2 (MAP2) are important cytoskeletal proteins in neurons(Chen et al., 2017). Immunofluorescence experiments were performed to characterize NF200 and MAP2 expression.Briefly, after cells were cultured for 7 days, 20 μL of mouse anti-mouse NF200 or mouse anti-mouse MAP2 (1:200 or 1:100, respectively; Abcam) solution was added to each well and incubated overnight at 4°C. After three washes with PBS,fluorescein conjugated rabbit anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA, USA) was added to each well and incubated in the dark for 1 hour at 37°C. DAPI was added to counterstain nuclei. Images were observed by confocal laser-scanning microscope (Leica, Germany).
Real time-polymerase chain reaction (RT-PCR) for IGF-1R and nerve growth factor (NGF) mRNA expression
RT-PCR was used to analyze mRNA levels of NGF and IGF-1R in Schwann cells at different experimental periods.Total RNA was extracted from cells using TRIzol (Takara Biotechnology, Dalian, China). RT-PCR reactions were performed using a SYBR Premix Ex Taq II (Tli RNaseH Plus)(Takara Biotechnology). Primer sequences (synthesized by Sangon, Shanghai, China) for NGF, IGF-1R, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: NGF forward, 5′-GTC AGT GTG GGT TGG AGA TA-3′, reverse, 3′-GGA GTA TGA GTT CCA GTG CTT G-5′; IGF-1R forward, 5′-AAA CGC TGA CCT CTG TTA CCT CTC-3′, reverse, 3′-CAG GTA GTC CGA AGT AGG CG-5′; and GAPDH forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′, reverse, 3′-TCC ACC ACC CTG TTG CTG TA-5′. Reactions were carried out as follows: 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 56°C for 30 seconds. Amplification was performed using a Stratagene Mx3005p (Agilent, CA, USA). Expression of GAPDH mRNA was determined as an internal control. Final results are expressed as a ratio of the mRNA of interest to that of GAPDH mRNA.
Statistical analysis
Data, expressed as the mean ± SD, were analyzed using IBM SPSS statistics 21.0 (IBM, Armork, NY, USA). The statistical significance of differences was set asP< 0.05 andP< 0.01 by two-tailedt-test.
Results
Construction of CBD-IGF-1 recombinant plasmids and transfection into host cells
Upon harvesting and lysing transfected cells, the soluble mixtures were centrifuged and purified with Ni-NTA affinity chromatography. Coomassie brilliant blue-stained bands of purified proteins were observed at 5–10 kDa on the gel (Figure 1A, B). The presence of CBD-IGF-1/IGF-1 was verified by western blot assay with primary anti-human IGF-1 and secondary antibody conjugated to horseradish peroxidase. As observed in the bands ofFigure 1C, expressed target proteins IGF-1 and CBD-IGF-1 were between 5–10 kDa.
Collagen-binding assay of CBD-IGF-1 and IGF-1
The binding ability of the two recombinant proteins to type I collagen is shown inFigure 2, with collagen-binding activity determined by ELISA. As shown in the binding curves, absorbance at 562 nm for CBD-IGF-1 was significantly higher than for IGF-1. This result indicates an increased amount of CBD-IGF-1 bound to type I collagen.
Effect of CBD-IGF-1 on PC12 and Schwann cell proliferation
Effects of the two proteins on cell proliferation were examined by MTT assay. Within the concentration range from 15 to 250 ng/mL, CBD-IGF-1 groups showed similar proliferation rates to the IGF-1 groups (Figure 3A, B). However, as the concentration increased, the rate of cell proliferation increased. However, at concentrations of 500 ng/mL and 1,000 ng/mL, the proliferation rate of cells cultured with CBDIGF-1 was higher than that of IGF-1.
Effect of CBD-IGF-1 on PC12 cell morphology
PC12 cells were cultured in 96-well plates for 3 days. The morphology of PC12 cells was observed by confocal laser-scanning microscope (TCS SP5, Leica). As displayed inFigure 3C, both proteins promoted cell proliferation and axon growth, which peaked at 500 ng/mL. The cells observed began to differentiate into neurons and immunofluorescence experiments were performed to further verify cell differentiation. As exhibited inAdditional Figures 1and2,MAP2 and NF200 were observed in cells.
CBD-IGF-1 promoted IGF-1R and NGF mRNA expression in Schwann cells
Cell proliferation is the result of signal transduction, which is stimulated by IGF-1R (Fernandez and Torres-Alemán, 2012).Levels of IGF-1R were measured by RT-PCR. Expression of IGF-1-induced IGF-1R mRNA increased rapidly and gradually decreased with time. However, IGF-1R mRNA expression induced by CBD-IGF-1 was maintained at a relatively stable level for a longer period of time. Notably, CBD-IGF-1 significantly promoted NGF mRNA expression (Figure 4).
Discussion
Neurotrophic factors play an important role in the repair of nerve injury (Zhang et al., 2000; Lee et al., 2003; Yates et al., 2004; Fernandez and Torres-Alemán, 2012; Chaker et al., 2015; He et al., 2016; Sacchetti and Lambiase, 2017; Wen et al., 2017). Among neurotrophic factors, IGF-1 is a pleiotropic factor that can promote proliferation and differentiation of neurons (Nieto-Estévez et al., 2016). However, like other growth factors, neurotrophic factors are difficult to maintain at the target site for long periods of time because of their diffusion and short half-life. Moreover, a large dose of neurotrophic factors may cause harmful consequences.To overcome these obstacles, many novel drug carriers have been developed to immobilize a drug on target cells, protein,or protein regions. Results of a previous study investigating the four segments of 116-kDaClostridium histolyticumcollagenase showed that the S3 segment was the binding domain (Matsushita et al., 1998). In a later study, the same researchers found that the CBD specifically recognized and combined with the triple-helical region of collagen’s normal conformation; notably, this binding ability could be enhanced by calcium (Matsushita et al., 2001). The CBD exhibits a broad substrate spectrum because it can combine with any tissue containing type I collagen (Toyoshima et al.,2001). Although, when fusing binding domains to growth factors, it is also important to confirm that the recombinant proteins retain their activities in addition to binding collagen (Yang et al., 2009; Egawa et al., 2011).
We hypothesized that CBD could be integrated into IGF-1 protein to improve its current deficiencies for clinical application. In the present investigation, we constructed fusion proteins containing the CBD deduced from collagenase. As shown inFigure 1, the molecular weight of the fusion protein is between 5 to 10 kDa. This is important, as reports suggest that the human immune system recognizes recombinant proteins as endotoxins or antigens when they are larger than 10 kDa (Petsch and Anspach, 2000; Kim et al., 2015). It is also worth noting that function and binding ability can be improved by adding amino acids between growth factors and binding domains (Yang et al., 2009; Egawa et al., 2011).Although CBD binds to the intertwined region of the triple helix, it cannot unwind the helix (Philominathan et al., 2012).
Purified proteins were confirmed to be CBD-IGF-1 or IGF-1 by western blot assay. IGF-1 promotes proliferation of nerve cells by binding to IGF-1R, which can activate two canonical pathways: PI3K/Akt or MAPK (Mairet-Coello et al., 2009;Yuan et al., 2015). To determine if recombinant proteins retained their IGF-1R binding activity and acquired the ability to bind type I collagen, we analyzed IGF-1R mRNA levels and performed binding assays for CBD-IGF-1. The results confirmed the affinity of CBD-IGF-1 towards type I collagen and increased ability to bind collagen. IGF-1R mRNA levels rose rapidly in Schwann cells incubated for 4 hours, and peaked after 1 day. Importantly, levels of IGF-1R mRNA stimulated by CBD-IGF-1 decreased more slowly in the following days.After incubation with the fusion protein, there was also a dramatic increase in amounts of NGF mRNA similar to that observed for IGF-1R mRNA. These results suggest that the half-life of CBD-IGF-1 is longer than that of IGF-1.
The biological activities of fusion proteins were examined using an MTT assay. With increasing concentrations, rates of cell proliferation increased. Thus, CBD-IGF-1 exhibited similar biological activities to IGF-1in vitro. Interestingly, at concentrations of 500 ng/mL and 1,000 ng/mL, the proliferation rate of cells cultured with CBD-IGF-1 was higher than that of IGF-1. One possible reason for this phenomenon was that cells secreting type I collagen and CBD-IGF-1 bound to type I collagen, leading to a high local concentration. Morphological observation of PC12 cells showed that CBD-IGF-1 promoted cell proliferation and axon growth. Immunofluorescence experiments conducted to verify neuronal differentiation showed increased MAP2 and NF200 in cells, indicating that the fusion protein can promote neural differentiation of PC12 cells. This result, which is similar to previous research showing potent actions of IGF-1 on axon regeneration (Fernandez and Torres-Alemán, 2012), demonstrated that the recombinant proteins retain their biological activity. Moreover,addition of the CBD promoted maintenance of a relatively high concentration of IGF-1 to promote cell proliferation.
The primary limitation of this research is the need to test the immunogenicity and biological activity of CBD-IGF-1in vivo. As such, furtherin vivostudies are currently underway to determine the clinical efficacy of CBD-IGF-1.
In summary, we synthesized a fusion protein containing IGF-1 and CBD, and provided a preliminary evaluation of its effectiveness. Our results achieved the goal of a targeted and long-lived recombinant CBD-IGF-1 protein, which maybe an effective neurotrophic factor suitable for clinical use.
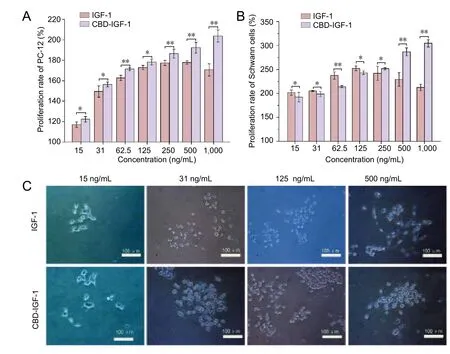
Figure 3 Effect of CBD-IGF-1 on PC12 and Schwann cellproliferation.

Figure 4 Effect of CBD-IGF-1 on IGF-1R and NGF mRNA levels in Schwann cells detected by RT-PCR.
Author contributions:JAL, Chang-fu Zhao (CFZ), SJL, Chun-fang Zan(CFZ) and XYY searched literatures and wrote the paper. XYY and JAL designed the study, obtained funding, and provided technical support.Chang-fu Zhao (CFZ), SJZ, JZ, and Chun-fang Zan (CFZ) searched literatures and participated in follow-up experiments. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the National Natural Science Foundation of China, 81350013; a grant from the Jilin Provincial Science and Technology Plan of China, 20160101027JC, SC201502001;and the Graduate Innovation Fund of Jilin University in China, No.2017031, 2017176. The conception, design execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of this funding organization.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Aldo Calliari, Universidad de la República, Uruguay.
Comments to authors:The manuscript is an interesting work intended to develop a collagen-targeted neurotrophic factor as a starting point to develop a pharmacological tool for nerve repair. The manuscript intended to develop an pharmacological tool (a recombinant molecule resulting from the fusion of IGF and a collagen binding domain from a bacterial collagenase), designed to efficiently target the nerve tissue. Overall, the research is well conduced and the results are clearly described.
Additional file:
Additional figure 1:Immunofluorescence staining of MAP2.
Additional figure 2:Immunofluorescence staining of NF200.
Annunziata M, Granata R, Ghigo E (2011) The IGF system. Acta Diabetol 48:1-9.
Benarroch EE (2012) Insulin-like growth factors in the brain and their potential clinical implications. Neurology 79:2148-2153.
Chaker Z, Aid S, Berry H, Holzenberger M (2015) Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging cell 14:847-856.
Chen B, Lin H, Wang J, Zhao Y, Wang B, Zhao W, Sun W, Dai J (2007)Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials 28:1027-1035.
Chen T, Yu Y, Tang LJ, Kong L, Zhang CH, Chu HY, Yin LW, Ma HY(2017) Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites. Neural Regen Res 12:433-439.
Clemmons DR (2006) Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol 6:620-625.
Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D (2016) The role of insulin-like growth factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 325:89-99.
Egawa EY, Kato K, Hiraoka M, Nakaji-Hirabayashi T, Iwata H (2011)Enhanced proliferation of neural stem cells in a collagen hydrogel incorporating engineered epidermal growth factor. Biomaterials 32:4737-4743.
Fernandez AM, Torres-Alemán I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13:225-239.
Hayashi Y, Yamamoto N, Nakagawa T, Ito J (2013) Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol Cell Neurosci 56:29-38.
He XZ, Wang W, Hu TM, Ma JJ, Yu CY, Gao YF, Cheng XL, Wang P (2016)Peripheral nerve repair: theory and technology application. Zhongguo Zuzhi Gongcheng Yanjiu 20:1044-1050.
Holman SR, Baxter RC (1996) Insulin-like growth factor binding protein-3: factors affecting binary and ternary complex formation. Growth Regul 6:42-47.
Jacobus AP, Gross J (2015) Optimal cloning of PCR fragments by homologous recombination in Escherichia coli. PLoS One 10:e0119221.
Kim DG, Kim EY, Kim YR, Kong IS (2015) Construction of chimeric human epidermal growth factor containing short collagen-binding domain moieties for use as a wound tissue healing agent. J Microbiol Biotechnol 25:119-126.
Laron Z (1999) Somatomedin-1 (recombinant insulin-like growth factor-1): clinical pharmacology and potential treatment of endocrine and metabolic disorders. Biodrugs 11:55-70.
Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE,Sakiyama-Elbert SE (2003) Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol 184:295-303.
Mairet-Coello G, Tury A, DiCicco-Bloom E (2009) Insulin-like growth factor-1 promotes G(1)/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci 29:775-788.
Matsushita O, Koide T, Kobayashi R, Nagata K, Okabe A (2001) Substrate recognition by the collagen-binding domain of Clostridium histolyticum class I collagenase. J Biol Chem 276:8761-8770.
Matsushita O, Jung CM, Minami J, Katayama S, Nishi N, Okabe A (1998)A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J Biol Chem 273:3643-3648.
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C (2016) IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci 10:52.
Petsch D, Anspach FB (2000) Endotoxin removal from protein solutions.J Biotechnol 76:97-119.
Philominathan ST, Koide T, Matsushita O, Sakon J (2012) Bacterial collagen-binding domain targets undertwisted regions of collagen. Protein Sci 21:1554-1565.
Rauskolb S, Dombert B, Sendtner M (2017) Insulin-like growth factor 1 in diabetic neuropathy and amyotrophic lateral sclerosis. Neurobiol Dis 97:103-113.
Rosen CJ, Pollak M (1999) Circulating IGF-I: New perspectives for a new century. Trends Endocrinol Metab 10:136-141.
Sacchetti M, Lambiase A (2017) Neurotrophic factors and corneal nerve regeneration. Neural Regen Res 12:1220-1224.
Scolnick JA, Cui K, Duggan CD, Xuan S, Yuan XB, Efstratiadis A, Ngai J(2008) Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron 57:847-857.
Toyoshima T, Matsushita O, Minami J, Nishi N, Okabe A, Itano T (2001)Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res 42:281-290.
Twigg SM, Baxter RC (1998) Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem 273:6074-6079.
Wen SY, Li AM, Mi KQ, Wang RZ, Li H, Liu HX, Xing Y (2017) In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity. Neural Regen Res 12:1716-1723.
Yang Y, Zhao Y, Chen B, Han Q, Sun W, Xiao Z, Dai J (2009) Collagen-binding human epidermal growth factor promotes cellularization of collagen scaffolds. Tissue Eng Part A 15:3589-3596.
Yates JM, Smith KG, Robinson PP (2004) The effect of brain-derived neurotrophic factor on sensory and autonomic function after lingual nerve repair. Exp Neurol 190:495-505.
Yuan H, Chen R, Wu L, Chen Q, Hu A, Zhang T, Wang Z, Zhu X (2015)The regulatory mechanism of neurogenesis by IGF-1 in adult mice.Mol Neurobiol 51:512-522.
Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF (2000) Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci 12:4171-4180.
Zhang R, Ruan D, Zhang C (2006) Effects of TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus cells in medium with different serum concentrations. J Orthop Surg Res 1:9.
- 中国神经再生研究(英文版)的其它文章
- Neuroprotective effects of statins against amyloid βinduced neurotoxicity
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Dyslipidemia modulates Müller glial sensing and transduction of ambient information
- Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma
- A new direction for Alzheimer’s research
- DNA plasticity and damage in amyotrophic lateral sclerosis

