Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma
Jenna L. Gollihue, Samir P. Patel, Alexander G. Rabchevsky,
1 Spinal Cord and Brain Injury Research Center, University of Kentucky, Lexington, KY, USA
2 Department of Physiology, University of Kentucky, Lexington, KY, USA
Importance of Mitochondria in Health and Disease
Mitochondrial dysfunction is a hallmark of multiple central nervous system (CNS) diseases such as Alzheimer’s disease, Parkinson’s disease, Alpers syndrome,Leigh syndrome, and myoclonic epilepsy with ragged red fibers, as well as following stroke and traumatic brain or spinal cord injury (Gollihue and Rabchevsky, 2017).Although various therapeutic agents have been used to target mitochondrial dysfunction, there are currently no clinically proven treatments that typically target one or few of the pathophysiological effects of mitochondria dysfunction and uncoupling, including reactive oxygen/nitrogen species release and glutamate excitotoxicity.A novel treatment paradigm that targets mitochondrial dysfunction in injury and disease states is mitochondrial transfer and replacement strategies. There are many reported benefits to replacing mitochondria in injury or disease states, including increasing energy production,calcium buffering capacity, and replacing mitochondrial DNA. While mitochondrial transplantation from one source to recipient cellsin vitrois performed by multiple labs, mitochondrial transplantationin vivois burgeoning in models of acute lung injury and cardiac ischemia, and more recently in CNS models of stroke and Parkinson’s disease. The results show promise in sparing tissues, but such studies have used vastly different methodologies and discerning the optimal transplantation paradigms for certain animal models or outcome measures will continue to be challenging (for comprehensive review, see Gollihue and Rabchevsky, 2017).
Methods of Mitochondrial Transfer or Transplantation
Cell-mediated transfer of mitochondria is reported to be protective in models of acute lung injury and stroke.In these paradigms, a donor cell, whether implanted or endogenous, was found to secrete vesicles containing mitochondria, which were subsequently taken into recipient cells. Mouse bone marrow derived stromal cells (mBMSC)have been instilled into lipopolysaccharide (LPS)-injured mouse lungs and shown to donate their mitochondria into the injured tissue, resulting in increased adenosine triphosphate (ATP) production and cell survival (Islam et al., 2012). The attachment of the mBMSCviaconnexin pore gap junction formation appeared necessary for this donation, with nanotubes formed between the cells and mitochondria-containing vesicles released (Figure 1 nanotube).
Others report that astrocytes release mitochondria-containing vesicles in a mouse model of ischemic stroke (Hayakawa et al., 2016). The mitochondria were then taken into and incorporated within neurons of the stroke infarct area within 24 hours after injury. Further, astrocytes could also be induced to release these mitochondria vesiclesin vitro, and after directly injecting them into mouse brains 3 days after stroke, the mitochondria were successfully incorporated into neurons (Figure 1 vesicle-mediated). Notably, the CD38 protein was found necessary for the vesicle release from astrocytes. Remarkably, in both the Hayakawa and Islam vesicle delivery systems, the recipient cells were not the same cell type as the donor cell.Communication between the two cell types, however,appeared to be vital as Cx43 connexin pore formation or CD38 activation in donor cells were initiated by signals from the injured recipient cells.
Successful mitochondrial transplantationin vivo, by directly injecting isolated autologous mitochondria into ischemic cardiac tissue before reperfusion (Masuzawa et al., 2013), results in improved mechanical function acutely, with benefits seen as soon as 10 minutes after injections. Importantly, confocal microscopy showed incorporation of exogenous mitochondria into cardiomyocytes as quickly as 2 hours after transplantation, and due to such rapid uptake, the authors suggested an actin-dependent internalization (Figure 1 microinjection). Direct injections of autologous mitochondria in a porcine model of cardiac ischemia showed similar beneficial results (Kaza et al., 2017), and there were no signs of increased inflammatory or immune responses attributed to mitochondrial transplants. Exogenous mitochondria labeled with magnetic iron oxide particles were still evident using magnetic resonance imaging 4 weeks after transplant.
Mitochondria isolated from transgenic cell lines with fluorescently labeled mitochondria have been directly microinjected into rat spinal cords and observed within a variety of host cell types after 24 and 48 hours (Gollihue et al., 2017), though mechanisms of uptake were not investigated. Alternatively, direct injections of isolated mitochondria conjugated to Pep-1, a cell penetrating peptide, into rat brains in a model of 6-hydroxydopamine (6-OHDA) induced Parkinson’s disease have been visualized up to 12 weeks post injection (Chang et al., 2013; Changet al., 2016). It was posited that Pep-1 was essential for incorporation, unlike other reports of successful incorporation when directly injecting isolated mitochondria.
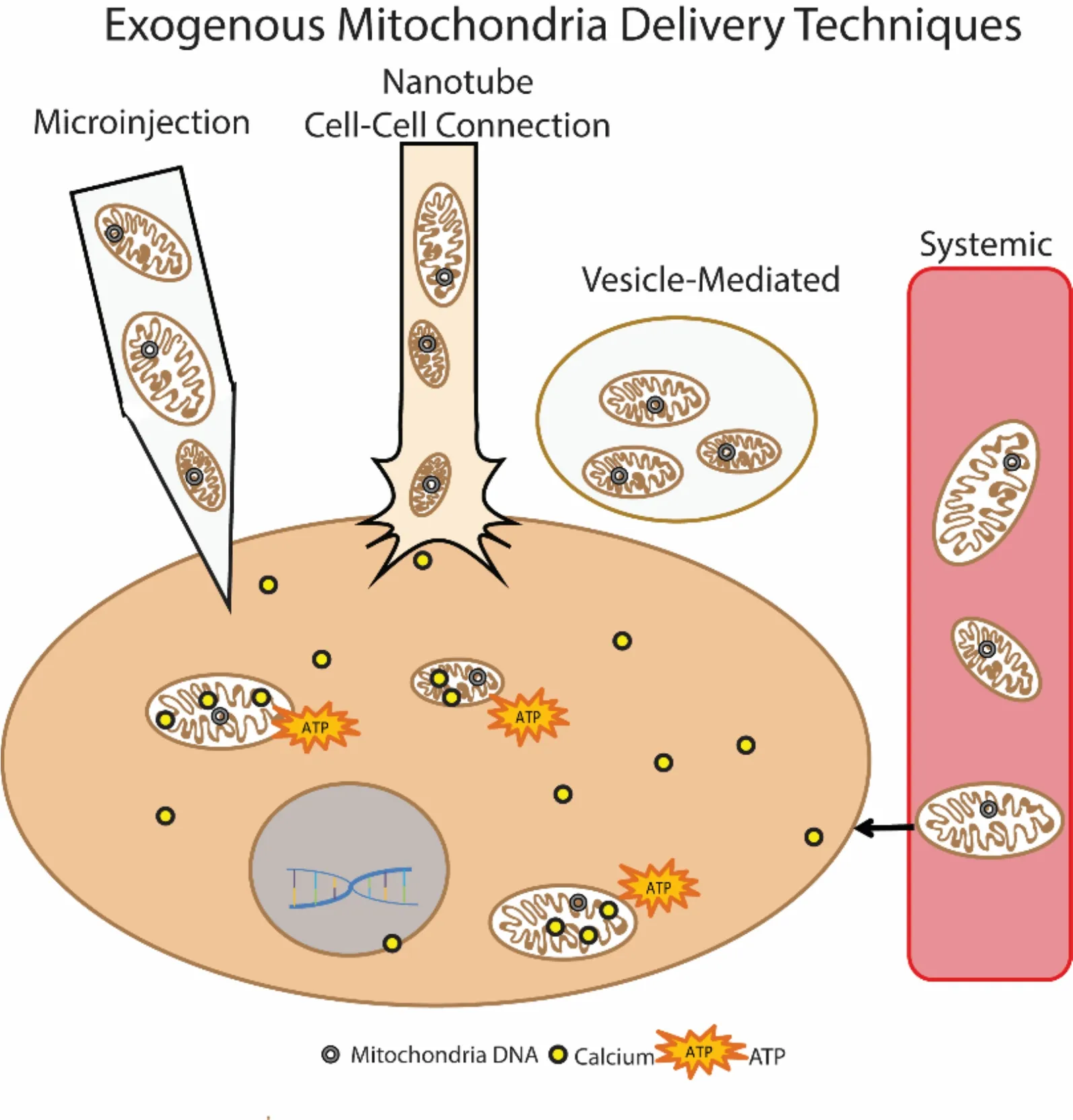
Figure 1 Exogenous mitochondria delivery techniques.
Mitochondria have also been transplanted using systemic delivery approaches (Figure 1 systemic). The same group that directly injected mitochondria into rabbit hearts also tested vascular perfusion of mitochondria in a rabbit model of focal cardiac ischemia (Cowan et al.,2016). Mitochondria were isolated from human cardiac fibroblast cells and were perfused through the coronary artery after 30 minutes of cardiac ischemia. This study compared direct injection to systemic delivery techniques and revealed that either delivery method resulted in functional protection of cardiac tissue. In a CNS-targeted paradigm isolated mitochondria were delivered systemicallyviatail vein injections in a mouse model of Parkinson’s disease and found to increase behavioral recovery close to control levels (Shi et al., 2017). The fluorescent exogenous mitochondria were identified in multiple different organs and tissue types, including the brain, heart, liver, kidney, and muscle tissue. Systemic delivery showed that mitochondria reached both targeted as well as untargeted tissues. Additionally, they posited that the uptake mechanism was endocytosis, as mitochondria were not incorporated into red blood cells,which lack endocytosis abilities.
Mechanism of Incorporation
Various groups have shown successful mitochondria transplantation using various techniques that have similar results; mitochondrial incorporation into host tissues with beneficial outcomes (for comprehensive review see Gollihue and Rabchevsky, 2017). However, one question that few agree upon is not whether the mitochondria are incorporated, but rather how they are incorporated within the cell. While the very nature of mitochondrial compaction during the isolation protocols may contribute to their facilitated intracellular delivery, various mechanisms have been testedin vitro, with the most decisive stemming from studies showing actin-dependent mechanisms for incorporation of mitochondria (Pacak et al., 2015). However, it is not known whether thein vitromechanisms of incorporation apply toin vivomodels.
Islam et al. (2012) showed that blocking endocytosis,using dynamin, inhibited mitochondrial transfer from mBMSC to injured lung epithelium. This led to the theory that endocytosis was responsible for the uptake of mitochondria-containing vesicles (Figure 1 vesicle-mediated). They also reported that blocking the docking and formation of gap junction channels between the cells inhibited mitochondrial transfer, indicating communication between the injured cell and the donor cell was crucial.
A possible incorporation mechanismin vivofor either mitochondrial organelles or vesicles could be a phenomenon known as massive endocytosis (Hilgemann et al.,2013). This entails internalization of very large portions of the plasma membrane, and would allow for internalization of things as large as whole organelles or vesicles.Once internalized, the mitochondria are released into the cytosol.
Comparing Delivery Methods
While various transfer or transplantation methods showed successful incorporation into host tissues, each came with their own set of caveats. First, the integrity and/or health of the transplanted mitochondria was established either by electron microscopy for morphology and purity (Cowan et al., 2016; Hayakawa et al., 2016;Gollihue et al., 2017; Kaza et al., 2017), measures of mitochondrial respiration and energy production (Masuzawa et al., 2013; Cowan et al., 2016; Hayakawa et al., 2016;Gollihue et al., 2017; Kaza et al., 2017), maintenance of fluorescence membrane potential dyes (Hayakawa et al., 2016; Shi et al., 2017), and calcium buffering (Shi et al., 2017). Injection of mitochondria into tissues should be mindful of possible adverse effects due to various damage associated molecular patterns that could incite inflammatory or immune responses, although no signs of such overt reactions have been noted following direct injections (Masuzawa et al., 2013; Kaza et al., 2017).
Isolated mitochondria themselves are at risk and vulnerable to possible adverse effectors in the extracellular environment including reactive oxygen and nitrogen species. Conjugating mitochondria to a carrier peptide allows for the transfer of the mitochondria across the plasma membrane, which could decrease the exposure time to the extracellular environment in addition to increasing incorporation efficiency (Chang et al., 2016).Another approach would be to deliver mitochondria within either vesicles or liposomes (Figure 1), though a caveat to this technique is the difficulty in packaging high numbers of mitochondria within liposomes. Vesicles that are expelled from cells may contain other elements in addition to mitochondria, making it difficult to discern benefits afforded by mitochondrial transfer when other secretory molecules may play a part.
Systemic delivery of mitochondria provides the added benefit of a more diverse incorporation profile, being taken into multiple organs and tissues, which could be beneficial depending upon the mitochondrial dysfunction phenotype. For instance, systemic delivery of mitochondria after cardiac ischemia results in some exogenous mitochondrial colocalization within cardiomyocytes,but a larger amount of mitochondria were found in the interstitial spaces. If mitochondrial dysfunction is wide spread, such as in a genetic mitochondrial disorder, then this technique may have more effect. However, in injury or diseases in which mitochondria dysfunction is more focal, for instance around an injury site, then direct trans-plantation to concentrate healthy mitochondria within that injured tissue may be more applicable (Cowan et al.,2016).
There are also caveats to be considered concerning the source of donor mitochondria; whether to isolate from cell culture or attempt autologous transplants. Transgenic labeling of mitochondria in cell culture before transplantation can provide an indelible marker for visualization in situ (Islam et al., 2012; Gollihue et al., 2017).However, only non-autologous mitochondrial transplantation can be performed when using such a labeling paradigm. An additional benefit of using non-autologous transplant is that the exogenous mitochondrial DNA can be quantified in tissues using quantitative real-time PCR.While labeling isolated mitochondria before transplantation with a MitoTracker dye or rhodamine derivative can be used for autologous transplants (Masuzawa et al., 2013), the fidelity of these markers to label only the transplanted mitochondria and not endogenous mitochondria is uncertain.
Conclusions
As more studies utilize mitochondrial transplantation for therapeutics in various pathophysiological conditions,comparisons will lend to optimal techniques for both delivery and functional incorporation. Although there are still many questions as to the exact mechanisms by which mitochondria are both incorporated into cells and exert beneficial effects, transplantation strategies as therapeutics for nervous system dysfunctions such as stroke, Parkinson’s disease and spinal cord injury are stimulating,though still in the experimental modeling stages. However, the McCully group is beginning to study clinical efficacy of mitochondrial transplant in pediatric cardiac dysfunction. Accordingly, depending upon the type of injury or disease model and the different tissues involved(i.e., cardiacvs. nervousvs. lung), the various delivery methods (Figure 1) can be refined for optimal effects.When precise mitochondrial incorporation mechanisms are elucidated, then delivery approaches can be targeted to specific tissues or cell types in future studies. Major advancements in the field of mitochondrial transplantation will require unveiling the intricacies involved in successful transplantation and functional incorporation within cells. Consequent translation of these findings to the clinical setting may provide novel and impactful ways for mitochondrial transplantation to both protect and repair damaged tissues associate with CNS disease and trauma.
Author contributions:JLG, SPP and AGR wrote the paper.
Conflicts of interest:None declared.
Financial support:This work was funded by NIH R21NS096670(AGR), University of Kentucky Spinal Cord and Brain Injury Research Center Chair Endowment (AGR), NIH/NINDS 2P30NS051220.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Chang JC, Liu KH, Chuang CS, Wu SL, Kuo SJ, Liu CS (2013) Transplantation of Pep-1-labeled mitochondria protection against a 6-OHDA-induced neurotoxicity in rats. Changhua J Med 11:8-17.
Chang JC, Wu SL, Liu KH, Chen YH, Chuang CS, Cheng FC, Su HL,Wei YH, Kuo SJ, Liu CS (2016) Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res 170:40-56.e3.
Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, Friehs I, Wu Y, Levitsky S, Del Nido PJ, Packard AB, McCully JD (2016) Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One 11:e0160889.
Gollihue JL, Rabchevsky AG (2017) Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35:70-79.
Gollihue JL, Patel SP, Mashburn C, Eldahan KC, Sullivan PG, Rabchevsky AG (2017) Optimization of mitochondrial isolation techniques for intraspinal transplantation procedures. J Neurosci Methods 287:1-12.
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535:551-555.
Hilgemann DW, Fine M, Linder ME, Jennings BC, Lin MJ (2013)Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts. Elife 2:e01293.
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18:759-765.
Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD (2017) Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg 153:934-943.
Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C,Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD (2013) Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304:H966-982.
Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, Del Nido PJ,Cowan DB, McCully JD (2015) Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open 4:622-626.
Shi X, Zhao M, Fu C, Fu A (2017) Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 34:91-100.
- 中国神经再生研究(英文版)的其它文章
- Dyslipidemia modulates Müller glial sensing and transduction of ambient information
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology
- Neuroprotective effects of statins against amyloid βinduced neurotoxicity
- A new direction for Alzheimer’s research
- DNA plasticity and damage in amyotrophic lateral sclerosis

