Adenosine A2A-dopamine D2 receptor heteromers operate striatal function:impact on Parkinson’s disease pharmacotherapeutics
The basal ganglia (BG) assemble a series of deep gray matter structures forming recurrent loops that include the cortex and thalamus, and that participate in the regulation of a plethora of brain functions, including elicitation and learning of reward-and aversive stimuli-associated behaviors,motor activity control and sensorimotor gating (Bromberg-Martin et al., 2010). The striatum is the main input BG structure, thus it receives cortical glutamatergic projections from widespread areas of cortex and projects into other BG nuclei, including globus pallidus pars externa and the BG outputsglobus pallidus pars interna and substantia nigra pars reticulata. On the other hand, the substantia nigra pars compacta-ventral tegmental area (SNpc-VTA) modulates cortical-BG-thalamic circuits by means of dopaminergic innervation of the striatum. Interestingly, the main population of striatal neurons, the medium spiny neurons (MSNs),provide the origin of two different striatal efferent pathways,the direct and indirect pathways (Schiffmann et al., 2007).Both project to the BG outputs, and the direct pathway also projects to brainstem, to the SNpc-VTA. The MSN originating these two pathways are characterized by the differential expression of several key genes. Thus, while MSNs from the direct pathway (direct MSNs) express dopamine D1receptors (D1R) and contain the neuropeptides dynorphin and substance P, indirect MSNs express dopamine D2receptors(D2R) and contain the neuropeptide enkephalin (Fuxe et al.,2007; Schiffmann et al., 2007). Striatal dopamine from SN-pc-VTA projections potentiates direct and inhibits indirect pathway MSN, which leads to a net inhibition of thalamocortical areas.
Interestingly, D1Rs and D2Rs, respectively localized in the direct and indirect MSNs, are functionality tuned by direct receptor-receptor interactions (i.e., heteromerization) established with other endogenously expressed G protein-coupled receptors, for instance adenosine, metabotropic glutamate and cannabinoid receptors, among others. In this way, these heteromers may positively or negatively regulate D1R and/or D2R activation, a fact that may ultimately constitute a very complex scenario in which the control of BG functioning is regulated by a myriad of complex interactions between different neurotrasmistters (Fuxe et al., 2007). From these receptor-receptor complexes, one of the most studied is the one formed by D2R and the adenosine A2Areceptor (A2AR)in the indirect MSN. In such way, it has been shown that reciprocal antagonistic interactions occur within the A2AR-D2R heteromer (Fuxe et al., 2007). Thus, anallostericinteraction was initially described, by which A2AR ligands decrease both the affinity and intrinsic efficacy of D2R ligands (Ferré et al., 2016). And, more recently, an opposite interaction was described, by which D2R agonists decrease the binding of A2AR ligands (Fernández-Dueñas et al., 2013) (Figure 1). In addition, a strong antagonistic interaction (namelycanon-ical) at the adenylyl cyclase (AC) level has been described,which depends on the ability of D2R-mediated Gi activation to inhibit Gs activation elicited by A2AR activation (Ferré et al., 2016) (Figure 1). Interestingly, the occurrence of both kinds of reciprocal antagonistic interactions (canonicalandallosteric) led to conjecture the existence of different populations of receptors: forming and non-forming oligomers.Accordingly, in such a model A2AR-D2R heterodimers would preferentially allow theallostericinteraction, while the formation of D2R-D2R or A2AR-A2AR homodimers would be mainly responsible of thecanonicalinteraction. Alternative-ly, the recent hypothesis of the formation of heterotetrameric structures could explain the simultaneous existence of both types of receptor-receptor interactions (Bonaventura et al., 2015; Ferré et al., 2016) (Figure 1). In fact, most existing experimental data on the biochemical and behavioral effects of A2AR and D2R ligands can be explained in the frame of one main population of striatal A2R forming A2ARD2R heteromers (Taura et al., 2017). However, it is important to note that although the existence of the direct A2ARD2R interaction has been widely accepted for many years,the demonstration of its existence in native tissue was only made recently. First, co-immunoprecipitation experiments and immunoelectron microscopy studies showed the association and co-distribution of D2R and A2AR in rat striatum(Cabello et al., 2009). Next, it was possible to ascertain the close proximity of D2R and A2AR by means of the proximity ligation assay (PLA) in mice and sheep striatum (Trifilieff et al., 2011; Bonaventura et al., 2015), in which it was also observed the ability of a synthetic peptide to specifically disrupt the PLA in sheep striatum (Bonaventura et al., 2015).Finally, the clearest demonstration of A2AR-D2R heteromers in striatal tissue came from a complementary approach using immunoelectron microscopy, PLA and time-resolved Fluorescence Resonance Energy Transfer (TR-FRET) with specific fluorescence ligands in rats (Fernández-Dueñas et al., 2015).
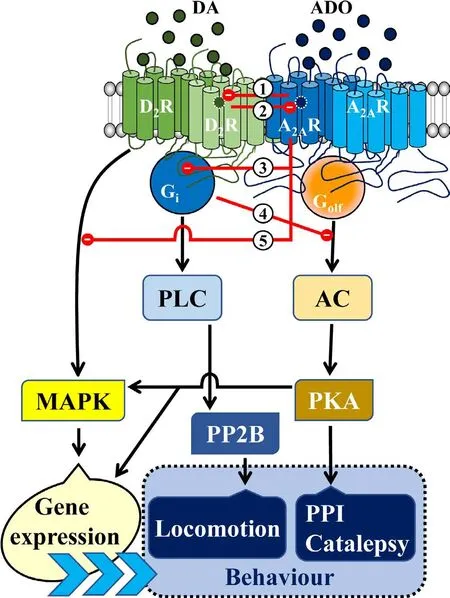
Figure 1 Scheme showing the proposed striatal adenosine A2Areceptor (A2AR)-dopamine D2 receptors (D2R) heteromer-dependent modulation of basal ganglia behavioral outputs.
At this point, we can clearly state that striatal D2Rs form homo- and hetero-complexes with other receptors, for instance the A2AR, and that the above-described receptor-receptor interactions may lead to a fine-tuning regulation of BG function. However, apart from the obvious interest of revealing the functional role of A2AR-D2R heteromers in these brain areas, a high effort is being dedicated to elucidating their role in the pathophysiology and pharmacotherapeutics of Parkinson’s disease (PD). PD is a neurodegenerative disease that affects approximately 1% over the age of 60, which turns to 5% in subjects up to 85 years.The etiology of this pathology is not well determined, and both genetic and environmental factors may be involved. Conversely, it is well-accepted that the main symptoms of PD (bradykinesia,rigidity, resting tremor and posture instability) appear when a large proportion of dopaminergic fibers from the SNpc projecting into the striatum are lost. Therefore, the main PD treatment consists of trying to restore dopamine levels by administrating dopamine-based drugs, from which the precursor L-DOPA has been the most extensively used since the seventies. However, chronic treatment with L-DOPA invariably leads to the occurrence of severe side-effects, such as dyskinesia, thus a lot of efforts are being directed to find out novel non-dopaminergic-based drugs with a lower incidence of these side effects. One of these new approaches consists of the development of selective A2AR antagonists (for review,see Vallano et al., 2011), whose mode of action may consist off acilitating D2R functionor by an independent effect on A2AR not interacting with D2R. Thus, as recently addressed in (Taura et al., 2017), there is evidence for an upregulation of A2AR upon dopamine denervation, which should lead to the presence of a significant population of A2AR not forming heteromers with D2R in PD. Accordingly, either by the direct blockade of A2AR function, which may be over-activatedin PD, or by precluding the negativeallostericinteraction within the A2AR-D2R heteromer, A2AR antagonists have been demonstrated to exert beneficial effects in PD animal models and also in PD patients (Vallano et al., 2011).
Noteworthy, to date, just one A2AR antagonist has been approved for human use and introduced into clinics (Vallano et al., 2011); while others have not survived clinical trials(for several reasons) or are still under investigation. In view of that, and within the A2AR-D2R heteromerization context,it would seem likely to revisit the pharmacological strategy used to select A2AR-based drugs for PD therapeutics. Accordingly, we propose that it would be important to characterize thein vivoefficacy of the putative effects of A2AR ligands, considered to be implemented in PD therapeutics, on the striatal A2AR forming and not forming heteromers with D2R. An example of this kind of approach is the recent study we set to readdress thein vivopharmacological properties of the A2AR antagonist SCH442416 (Taura et al., 2017). Of note, we took advantage of genetic manipulation, using wild-type and D2R or A2AR deficient mice (D2R–/–and A2AR–/–, respectively), in order to dissect the role of A2AR-D2R heteromerization on the behavioral effects of this ligand. First,a significant but partial reduction of activity was observed when evaluating spontaneous locomotor activity in D2R–/–or A2AR–/–mice, thus pointing to the existence of neuroadaptations that counteract the respective loss of D2R- and A2AR-mediated tonic stimulation and inhibition of psychomotor activity. When examining the effects of SCH442416-mediated activation of locomotion, we observed a substantial diminished effect in D2R–/–mice, which would be consistent with a dependence on theallostericinteraction within the A2AR-D2R heteromer (Taura et al., 2017). On the other hand, our data also provided further support to the importance of A2AR mediation in the behavioral effects secondary to the activation or interruption of D2R signaling, such as inhibition of sensorimotor gating or catalepsy, respectively,consistent with a dependence on thecanonicalinteraction within the A2AR-D2R heteromer. Thus, SCH442416 counteracted the inhibitory effect the D2R agonist sumanirole on prepulse inhibition (PPI) and the cataleptic effect of the D2R antagonist haloperidol. On the other hand, the A2AR agonist CGS21680-induced catalepsy was completely counteracted by the genetic blockade of A2AR and only a partial counteraction was observed in D2R–/–mice (Taura et al., 2017). Nevertheless, SCH442416 can counteract CGS21680-mediated catalepsy in D2R–/–mice (unpublished data), indicating that this can be used as a method to evaluate the potency and efficacy of A2AR antagonists on A2AR not forming heteromers with D2R.
We have recently proposed a heuristic model of the operation of the A2AR and D2R in the indirect MSN that integrates the information available in the literature together with our more recent behavioral results (see Taura et al., 2017 and Figure 1). The model proposes that D2R mainly signals by activating phospholipase C (PLC) through α β γ subunit-dependent mechanism, thus activating the Ca2+/calmodulin-dependent protein phosphate calmodulin (PP2B),which leads to enhancing locomotor activity (Figure 1).Similarly, A2AR mainly signals through activation of AC and protein kinase A (PKA), which facilitates PPI and catalepsy(Figure 1). In addition, D2R and A2AR activation also modify gene expression by G protein-independent or dependent mitogen-activated protein kinase (MAPK) activation (Figure 1). In the frame of the A2AR-D2R heteromer, it seems clear that these signaling cascades are determined by the receptor that is predominantly activated and the consequent predominant interaction,allostericorcanonical(Taura et al., 2017)(Figure 1). Obviously, these interactions should be absent in the frame of the A2AR not forming heteromers, which should be independent from D2R signaling.
Overall, the accumulation of data since the early 1990’s regarding the interactions between D2R and A2AR has prompted to a more comprehensive representation of the functioning of the A2AR-D2R heteromer in the BG context. However,although the formation of A2AR-D2R heteromers in native tissue and their significant functional and pharmacological significance is becoming generally accepted, we need to determine their role in the pathophysiology and treatment of PD and other neuropsychiatric disorders, particularly in view of the possible changes in the stoichiometry of A2AR and D2R, when attempting to develop novel pharmacological tools such as new A2AR antagonists.
This work was supported by MINECO/ISC III (SAF2014-55700-P and PIE14/00034), IWT (SBO-140028) and Fundació la Marató de TV3 (Grant 20152031) to FC. Also, FC and VFD belong to the “Neuropharmacology and Pain” accredited research group (Generalitat de Catalunya,2014 SGR 1251), and by the intramural funds of the National Institute on Drug Abuse to SF.
Víctor Fernández-Dueñas, Sergi Ferré, Francisco Ciruela*
Unitat de Farmacologia, DepartamentPatologia i Terapèutica Experimental, Facultat de Medicina, IDIBELL, Universitat de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain(Fernández-Dueñas V, Ciruela F)
Institut de Neurociències, Universitat de Barcelona, Barcelona,Spain (Fernández-Dueñas V, Ciruela F)
Integrative Neurobiology Section, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, MD, USA (Ferré S)
*Correspondence to:Francisco Ciruela, fciruela@ub.edu.
orcid:0000-0003-0832-3739 (Francisco Ciruela)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Isabel Liste, Instituto de Salud Carlos III, Spain.
Comments to authors:In the present manuscript Fernández-Dueñas et al. reviewed the current state of knowledge of the interaction between the D2 dopamine receptor and A2A adenosine receptor, and how this interaction can control the activity of striatum neurons in control and pathological conditions such as Parkinson’s disease. The article is well written,clear, interesting to the research field and possibly for the development of future pharmacotherapy of Parkinson`s disease.
Bonaventura J, Navarro G, Casadó-Anguera V, Azdad K, Rea W,Moreno E, Brugarolas M, Mallol J, Canela EI, Lluís C, Cortés A,Volkow ND, Schiffmann SN, Ferré S, Casadó V (2015) Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2receptor heterotetramer. Proc Natl Acad Sci U S A 112:E3609-3618.
Bromberg-Martin ES, Hikosaka O, Nakamura K (2010) Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30:6262-6272.
Cabello N, Gandía J, Bertarelli DC, Watanabe M, Lluís C, Franco R,Ferré S, Luján R, Ciruela F (2009) Metabotropic glutamate type 5,dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J Neurochem 109:1497-1507.
Fernández-Dueñas V, Gómez-Soler M, Morató X, Núñez F, Das A,Kumar TS, Jaumà S, Jacobson KA, Ciruela F (2013) Dopamine D(2)receptor-mediated modulation of adenosine A(2A) receptor agonist binding within the A(2A)R/D(2)R oligomer framework. Neurochem Int 63:42-46.
Fernández-Dueñas V, Taura JJ, Cottet M, Gómez-Soler M, López-Cano M, Ledent C, Watanabe M, Trinquet E, Pin JP, Luján R, Durroux T,Ciruela F (2015) Untangling dopamine-adenosine receptor-receptor assembly in experimental parkinsonism in rats. Dis Model Mech 8:57-63.
Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A,Lluís C, Casadó V, Volkow ND (2016) Allosteric mechanisms within the adenosine A2A-dopamine D2receptor heterotetramer. Neuropharmacology 104:154-160.
Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF (2007) Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav 92:210-217.
Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S (2007) Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83:277-292.
Taura J, Valle-León M, Sahlholm K, Watanabe M, Van Craenenbroeck K, Fernández-Dueñas V, Ferré S, Ciruela F (2017) Behavioral control by striatal adenosine A2A-dopamine D2receptor heteromers.Genes Brain Behav doi: 10.1111/gbb.12432.
Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch JA (2011)Detection of antigen interactions ex vivo by proximity ligation assay:endogenous dopamine D2-adenosine A2Areceptor complexes in the striatum. Biotechniques 51:111-118.
Vallano A, Fernandez-Duenas V, Pedros C, Arnau JM, Ciruela F (2011)An update on adenosine A2Areceptors as drug target in Parkinson’s disease. CNS Neurol Disord Drug Targets 10:659-669.
- 中国神经再生研究(英文版)的其它文章
- Neuroprotective effects of statins against amyloid βinduced neurotoxicity
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Dyslipidemia modulates Müller glial sensing and transduction of ambient information
- Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma
- A new direction for Alzheimer’s research
- DNA plasticity and damage in amyotrophic lateral sclerosis

