Microglial dynamics during brain development
Microglia are the resident immune cells of the central nervous system(CNS). In the normal state, microglia have a ramified shape and continuously survey the conditions of the brain. In response to various stimuli, some microglia change to an amoeboid shape. This type of microglia is motile and produces several secretory proteins, including inflammatory cytokines and neurotrophic factors, which regulate brain homeostasis. Microglial morphology also changes a great deal in concert with environmental factors during brain development, and related to microglial migration and proliferation, which contribute to the establishment of precise synaptic connectivity and neural circuits.In this review, we focus on the fundamental concepts of microglial fate during brain development, and discuss whether the molecular mechanisms that control microglial morphology are linked to microglial functions.
Microglial behavior changes during brain development:Microglia are CNS-resident macrophages that exhibit heterogeneous and pleomorphic morphology. Normally, the activation status of microglia is determined by their local environment. It is well established that microglial morphology is inextricably linked to their functions(Kettenmann et al., 2011). To date, the traditional view of microglial morphology, ranging between “amoeboid” and “ramified,” has been embraced. In the healthy adult brain, microglia continually extend and retract their ramified processes; this state is called “ramified.” In contrast, at the time of neuronal injury in the adult brain, microglia retract their protrusions; this state is called “amoeboid,” and involves microglia migrating and accumulating at the site of damage. Furthermore, some microglia have “intermediate” forms, which have crossover functions between “amoeboid” and “ramified”. Until recently,it had been thought that ramified microglia are resting forms, and amoeboid microglia are activated one. However, novel technologies,such as single-cell RNA sequencing and two-photon microscope,have revealed that the biological relevance between microglial morphology and functions is not simple for several reasons. First, microglia present heterogeneous population beyond expectation, including morphology, in a region- and age-dependent manner. Second, a different type of microglia is observed in brain even though they have a similar morphology. Finally, the microglia polarization, which is diverted from the concept of M1/M2 polarization in macrophage, is still under discussion although it is a fascinating approach. Therefore,understanding the molecular mechanisms that link microglial morphology to their functions will need to be discussed.
It is noteworthy that this amoeboid shape is observed in both the adult and the developing brain. During neonatal development, the morphology of nascent microglia is similar to that of amoeboid cells.These microglia initially elongate their ramified processes to coordinate with potential intrinsic and extrinsic factors with time, and then become ramified microglia (Perez-Pouchoulen et al., 2015). Thus, it is considered that changes in microglial morphology occur in parallel with normal brain development. During brain development, microglial activity is regulated by several transcription factors that module a variety of differentiation processes. One of the putative candidates involved in the regulation of microglial properties is Runx1. Runx1 is first observed at embryonic day (E)6.5, and is increased around E7.5 in the yolk sac region. These Runx1-expressing cells in the yolk sac infiltrate into the brain and differentiate into mature microglia(Ginhoux et al., 2010). Runx1-expressing cells (i.e., nascent microglia) exhibit amoeboid morphology. In contrast, the morphology of nascent microglia gradually transforms into a ramified shape around 2 postnatal weeks in inverse proportion to Runx1 expression. Therefore, Runx1 may be a potent candidate that controls microglial fate as it is associated with cell shape in normal brain development. It is also known that PU.1 plays an important role in microglial differentiation. PU.1 is a member of the E26 transformation-specifi(ETS) family of transcription factors that regulates a variety of cellular functions,including migration and differentiation. Although PU.1 is normally expressed in both ramified and amoeboid microglia, PU.1 deficiency impairs yolk sac-derived microglia maturation. Thus, PU.1 could be considered an interesting candidate that controls the microglia differentiation involved in their morphology. The remaining question is which signaling pathway is involved in regulating these factors. Previous studies reported that colony stimulating factor 1 receptor (CSF1R)-mediated signaling is essential for microglia survival (Elmore et al., 2014), and modulates some transcription factors, including Runx1 and PU1. Upon stimulation of CSF1R, several intracellular signaling pathways, such as phosphatidylinositol-3-kinase (PI3K)-Akt signaling, are activated. Indeed, Akt changes the phosphorylation status of CCAAT/enhancer binding protein (C/EBP), which is a putative cofactor of Runx1. It is possible that Akt modulates C/EBP function through its phosphorylation. Moreover, Akt increases PU.1 transcriptional activity through its phosphorylation. Thus, Akt could act as a mediator between CSF1R and these factors in microglia. Although Akt regulates microglia shape in a context-dependent manner, other mediators that are involved in microglia regulation must exist. Investigating these mechanisms in the developing brain is thus a challenging process.
The functional architecture of the neural circuits regulated by microglia:Recent studies have reported that pathological stimuli trigger microglial activation. Severe brain injuries, such as ischemic, excitotoxic, and neurodegenerative insults, result in microglial activation,followed by altering aspects of these cells.In vivoimaging analyses have revealed that ramified microglia are highly motile even in normal conditions (Nimmerjahn et al., 2005). Usually, microglia processes survey their microenvironment; upon sensing a brain injury,they are transformed into a different type of microglia. This machinery is intimately connected to brain homeostasis, and its deficiency aggravates the environment of the brain. Interestingly, microglia behaviors are gradually altered during the developmental stages. During this period, microglia play important roles in the construction and maintenance of neuronal connectivityviasynaptogenesis and synapse pruning. It has been shown that microglial properties change dramatically around the first 3 postnatal weeks (Perez-Pouchoulen et al., 2015). Interestingly, it is believed that microglia maturation occurs around 2 to 3 postnatal weeks as a result of a change in the gene expression pattern. At this time, postnatal mice experience enormous environmental stresses compared to those in the fetus period. For instance, postnatal mice usually open their eyes around 10 postnatal days. After opening their eyes, light stimulation activates the optic nerve, which contributes to the formation of robust synapses. If these neurons do not receive the adequate amount of light stimulation,however, they cannot form functional synapses. At this time, microglia sense immature synapses and engulf and eliminate them (Schafer et al., 2012). Consequently, environmental stimulation contributes to the establishment of sophisticated neural circuits. As postnatal mice are exposed to enormous stimulation, this process may also contribute to the production of microglial diversity through their regionand time-dependent activation.
Several genes in microglia function as key factors that control neuronal connectivity. For instance, CX3C chemokine receptor 1 (CX-3CR1), which is one of the chemokine receptors and expresses only microglia in the CNS, regulates synapse pruning around 2 postnatal weeks. Loss of CX3CR1 increases dendritic spines and attenuates the frequency of spontaneous excitatory postsynaptic current (sEPSC)in CA1 pyramidal neurons (Paolicelli et al., 2011), suggesting that defect in CX3CR1 causes immature formation of neuronal connectivity. Furthermore, CX3CR1 plays an important role in chemotaxis in the brain. After sensing abnormal debris such as Aβ, microglia recruit and engulf them to protect the brain from their toxicity. Since phagocytosis and chemotaxis are required for actin reorganization, it is likely that CX3CR1 is implicated in the regulation of the cytoskeleton. Indeed, CX3CR1 deficiency modulates microglial morphology in response to stroke (van der Maten et al., 2017). Therefore, CX3CR1 may control synapse pruning, chemotaxis, and morphologyviaregulating the cytoskeleton. P2Y12, which is a purinergic receptor, also regulates synaptic pruning in the developing brain. Usually, P2Y12 is downregulated after inflammatory stimulation and increases mi-croglial process dynamics rapidly. Upon blocking neural activity by monocular deprivation, microglial hyper-ramification is observed in the visual cortex (Sipe et al., 2016). Moreover, P2Y12 disruption decreases the number of ramified microglia induced by monocular deprivation, suggesting that microglial morphology in the visual cortex is dependent on light stimulation. In addition, recent studies have reported that the complement pathway is involved in synapse pruning when neural circuits are connected in the developing brain.Complement component 1q (C1q), which is an initial protein of the complement cascade, is synthesized from retinal ganglion cells,and its metabolic product, complement 3 (C3), stimulates microglia through complement receptor 3 (CR3) on the microglial membrane,leading to enhancement of synapse pruning and phagocytosis (Stevens et al., 2007). Interestingly, transforming growth factor-β (TGF-β)secreted from astrocytes upregulates C1q expression in neurons.These findings demonstrate that the complement pathway controls microglia in coordination with neurons and astrocytes. Another interesting target involved in microglia dynamics is triggering receptor expressed on myeloid cells 2 (TREM2). Usually, TREM2 is expressed in microglia and acts as a sensor of various lipids associated with damaged neurons. In addition, TREM2 is known to be a risk factor of Alzheimer’s disease; mutation in TREM2 increases the accumulation of Amyloid beta (Aβ) in the brain. The TREM2 expression pattern during the postnatal period from 1 to 14 days is gradually changed in a region-specific manner (Chertoff et al., 2013). TREM2 adaptor protein, DAP12, is also associated with CSF1R. Although CSF1R activates survival signaling in microglia, the TREM2-DAP12 complex enhances phagocytosis and the inflammatory response. Therefore, it is likely that there is some crosstalk between TREM2 and the CSF1R pathway that is associated with regulating microglial functions.
Conclusions:Here, we examine the broad aspects of microglial implications in brain development (Figure 1). Several decades ago, we did not have a good approach to observing living microgliain vivo.Technological advancements, however, now allow us to observe them in the living state. For instance, the two-photon microscope is available to observe living microglia on the surface of mice brain. In addition, the simple imaging technique is a good approach for quantification of morphological changes and chemotaxis (Tsuruta et al.,2017). We are also able to manipulate the neurons and glial cell activity using optogenetics. These novel techniques are useful to unveil the unknown functions of microglia and to shed light on new ways in which to understand the mechanisms that link microglia to the architecture of the neural network.
We apologize to the many authors whose papers could not be cited due to space limitations. I would like to thank the members of our laboratory for their helpful discussion, and Drs. Ban Sato and Darina Obukhova from University of Tsukuba for critical reading of this manuscript.
Tomomi Okajima, Fuminori Tsuruta*
Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan (Okajima T, Tsuruta F)
PhD Program in Human Biology, School of Integrative and Global Majors, University of Tsukuba, Tsukuba, Ibaraki, Japan; Life Science Center of Tsukuba Advanced Research Alliance (TARA), University of Tsukuba, Tsukuba, Ibaraki, Japan (Tsuruta F)
*Correspondence to:Fuminori Tsuruta, Ph.D.,
tsuruta.fuminori.fn@u.tsukuba.ac.jp.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-Shar-eAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
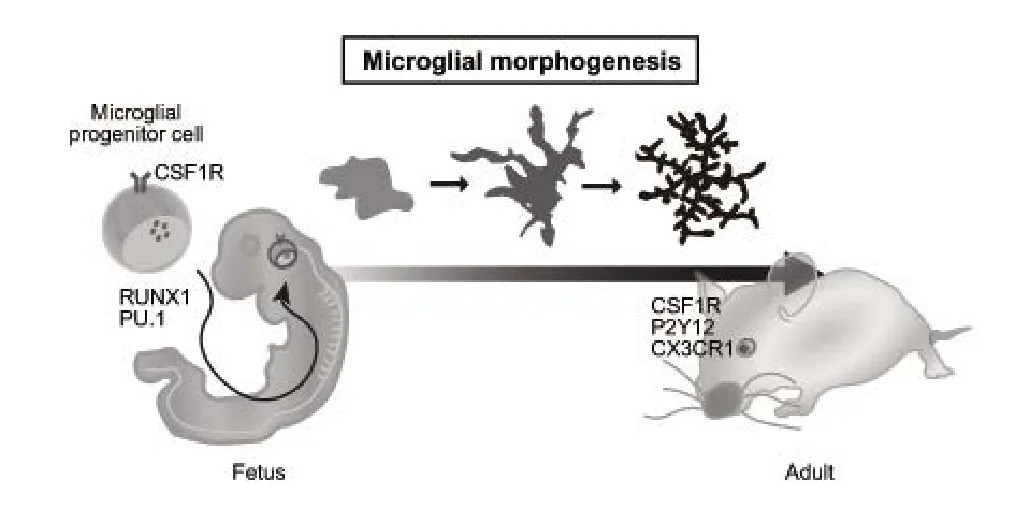
Figure 1 Microglial development.
Open peer review report:
Reviewer:Chih-Li Lin, Chung Shan Medical University, China.
Comments to authors:This perspective review discusses microglia behaviors during brain development. It is a well-written and well-organized short manuscript that deals with molecular mechanisms of microglial regulation in the development of brain.
Chertoff M, Shrivastava K, Gonzalez B, Acarin L, Gimenez-Llort L (2013)Differential modulation of TREM2 protein during postnatal brain development in mice. PLoS One 8:e72083.
Elmore MR, NajafiAR, Koike MA, Dagher NN, Spangenberg EE, Rice RA,Kitazawa M, Matusow B, Nguyen H, West BL, Green KN (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82:380-397.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF,Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841-845.
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461-553.
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314-1318.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT(2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456-1458.
Perez-Pouchoulen M, VanRyzin JW, McCarthy MM (2015) Morphological and phagocytic profile of microglia in the developing rat cerebellum(1,2,3). eNeuro 2:ENEURO.0036-0015.2015.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012)Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691-705.
Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK(2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun 7:10905.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164-1178.
Tsuruta F, Okajima T, Yano S, Chiba T (2017) Quantification of endosome and lysosome motilities in cultured neurons using fluorescent probes. J Vis Exp doi: 10.3791/55488.
van der Maten G, Henck V, Wieloch T, Ruscher K (2017) CX3C chemokine receptor 1 deficiency modulates microglia morphology but does not affect lesion size and short-term deficits after experimental stroke. BMC Neurosci 18:11.
- 中国神经再生研究(英文版)的其它文章
- Neuroprotective effects of statins against amyloid βinduced neurotoxicity
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Dyslipidemia modulates Müller glial sensing and transduction of ambient information
- Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma
- A new direction for Alzheimer’s research
- DNA plasticity and damage in amyotrophic lateral sclerosis

