Dithenylpyrrole Structure Bridged with Phenyl or Biphenyl Rings: Molecular Configuration and Electrochromic Properties
DAI Yu-Yu LI Wei-Jun YAN Shuan-Ma WANG Shi-Zhao YU YueOUYANG Mi CHEN Li-Tao ZHANG Cheng
Dithenylpyrrole Structure Bridged with Phenyl or Biphenyl Rings: Molecular Configuration and Electrochromic Properties
DAI Yu-Yu LI Wei-Jun*YAN Shuan-Ma WANG Shi-Zhao YU YueOUYANG Mi CHEN Li-Tao ZHANG Cheng*
()
A series of multi-branched dithienylpyrrole (SNS) monomers with rigid phenyl (PhSNS) and biphenyl rings (BPhSNS) as bridges were designed and synthesized, and were fabricated to form cross-linked polymers (pPhSNS, pBPhSNS) by electrochemical polymerization. Cyclic voltammetry (CV) results showed that PhSNS and BPhSNS exhibited similar oxidative properties except for one new higher-potential oxidative peak appearing in the curves of PhSNS. Theoretical calculations indicated that it should be attributed to the different steric configuration between the two dithienylpyrrole (SNS) units in PhSNS. One SNS unit possessed a larger twist angle (40.2°) between thiophene and pyrrole rings than the other one (21.2°), which indicated that PhSNS possessed a relatively larger energy gap (~0.4 eV) between HOMO-1 and HOMO than BPhSNS, for which HOMO and HOMO-1 levels were of almost the same energy. However, both PhSNS and BPhSNS showed similar onset oxidation potentials. The CV curves of pPhSNS and pBPhSNS showed that they presented similar oxidative properties, which enabled their corresponding electrochemical polymers to exhibit similar electrochromic properties. The UV-vis spectra of the corresponding polymers showed that both pPhSNS and pBPhSNS possessed similar optical absorption and similar multicolor switching between yellow (−0.8 V), greyish-green (0.9 V) and gray (1.1 V) colors. Besides, pPhSNS and pBPhSNS showed fast switching times of 0.57 s and 0.93 s at 1100 nm, respectively and reasonable contrasts of 46% and 31% at 1100 nm, respectively. These investigations may help understand the relationship between structural configuration and the electrochemistry/electrochromic properties for polymer electrochromic (PEC) materials research.
Dithienylpyrrole; Molecular configuration; Theoretical calculation; Electorpolymerization; Electrochromic
1 Introduction
Conjugated polymers (CPs) have attracted increasing attention due to their multiple potential applications including sensors1,2, light-emitting diodes3,4, field effect transistors5,6and electrochromic devices (ECDs)7,8. Especially, conjugated polymers as electrochromic materials exhibit superior advantages such as easy of processability, fast switching time, outstanding coloration efficiency and color adjustment9–12. However, to achieve the demand of commercial application, the effort for synthesizing novel, simple and effective electrochromic materials is still important and indispensable13.
Polythiophenes, one of CPs, were investigated widely by the researchers owing to oxidative stability and ease of synthesis or modification14. However, the high oxidation potential of thiophene causes the corresponding polymers to exhibit poor chemical and physical properties15. Extending the conjugation length of thiophene through designing multi-units conjugated molecule may be a good method to decrease the high onset potential of thiophene. A series of these derivatives such as dithienylbenzene16,17and dithienylpyrrole (SNS)18,19were reported and investigated by many researchers. Among them, dithienylpyrrole is one of the most investigated core units owing to the low oxidation potential and easy modification of chemical structure at the N-substitution of the pyrrole unit. Thus, a series of SNS derivatives were designed and studied with-substituted functional group such as alkyl derivatives20, phenyl derivatives21–24and anthraquinone25. Currently, the relationship between molecular configuration of the monomers and electrochemical properties or electrochromic properties in the case of SNS Structure-based monomers and polymers need to be further studied.
Recently, some groups reported that the introduction of rigid units into the main chain or side chain may form the specific twisted configuration and the corresponding polymers might exhibit excellent electrochromic properties26,27. Usluer.26reported a spiro[cyclododecane-1,9′-fluorene] bicarbazole molecular structure by introduction rigid spiro [cyclododecane- 1,9′-fluorene] units into the main structure, which exhibited a specific steric configuration. As a result, the corresponding polymers exhibited high contrast and fast switching time (less than 1 s). Besides, Tieke.27reported that the introduction of rigid terpyridine (tpy) units into the polyiminofluorene acted as ligands for complexing with metal ions (Zn, Co, Ni) and the coordinative interactions enabled the corresponding polymers to form the twisty network structure. The corresponding polymer twisty network was rather rigid and highly porous, which enable rapid ion transfer and fast switching times.
In this work, we introduced different phenyl and biphenyl rigid units into dithienylpyrrole structure to form the phenyl bridged-SNS (PhSNS) and biphenyl bridged-SNS (BPhSNS), respectively. The obtained compounds PhSNS and BPhSNS possessed four thiophene polymerization sites, which is beneficial to generate cross-linked polymer structure and maybe possess microporous structure in the polymer films. These may be expected to obtain excellent electrochromic materials with good film stability and fast color switching. Besides, the different rigid unit of phenyl and biphenyl may enable PhSNS and BPhSNS to exhibit different molecular configuration, which may exhibit different properties for the monomers or the corresponding polymers. PhSNS and BPhSNS were synthesized successfully (Scheme 1), and then they were fabricated to form the cross-linked polymer (pPhSNS, pBPhSNS) by electrochemical polymerization (Scheme 2). The electrochemical, geometric, electronic and optical properties of PhSNS and BPhSNS were studiedsystematically. Correspondingly, the electrochemical and electrochromic character of the resulting polymers (pPhSNS, pBPhSNS) were investigated in this work. The experimental data and results will be showed as followed in detail.
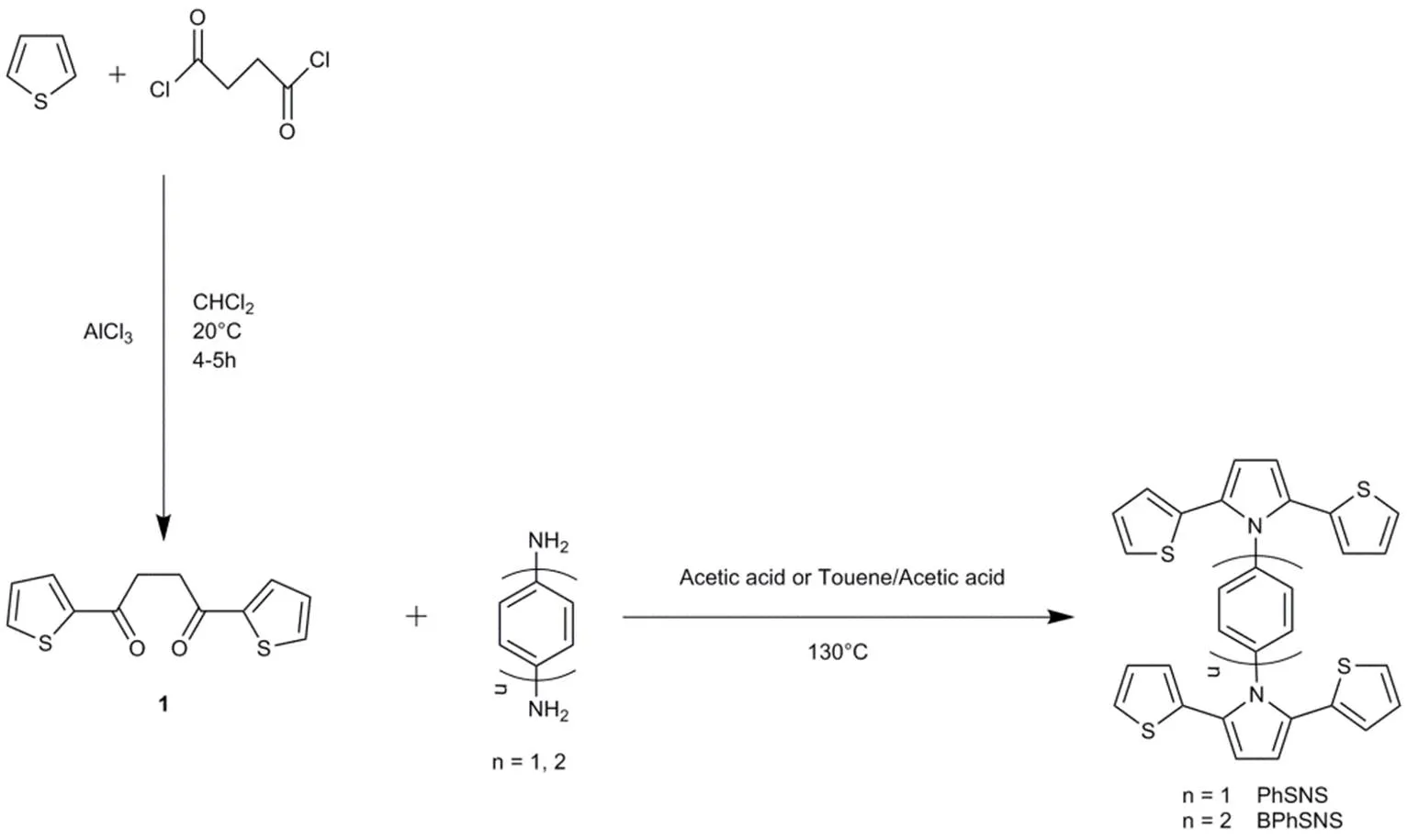
Scheme 1 The synthetic route and chemical structures of PhSNS and BPhSNS.
Scheme 2 Synthetic route and possible cross-linked structure of pPhSNS and pBPhSNS.
2 Experimental
2.1 Chemicals
All the reagents or chemicals were commercial and analytical products without further purification. Indium tin oxide (ITO) glass substrates (CSG HOLDING Co., Ltd.,S≤ 10 Ω∙−1, area: 0.9 cm × 4 cm) were cleaned by ultrasonic in a series of solvents including distilled water, ethanol, methylbenzene and acetone solutions for 15 min, respectively.
2.2 Characterization
1H (500MHz) NMR spectra of the synthesized compounds were recorded on Bruker AVANCE III instrument (Bruker, Switzerland). Mass spectra (MALDI-TOF-MS) analysis was recorded using an AXIMA-CFRTM plus instrument. The DFT calculations of PhSNS and BPhSNS were performed via Gaussian 03 at the B3LYP/6-311+G(,) level. The electrochemical and optical properties of PhSNS and BPhSNS were conducted by CHI660E electrochemical analyzer (Chenhua, China) and Shimadzu UV-1800 spectrophotometer (Shimadzu, Japan), respectively. Electrochemical polymerization of the monomers and electrochemical properties of the corresponding films were performed on CHI660E electrochemical analyzer.The surface morphologies of the films were carried out by S-4800 scanning electron microscope (SEM) (Hitachi, Japan), respectively. Spectroelectrochemistry test, optical contrast and switching time were investigated by Shimadzu UV-1800 spectrophotometer integrated with the CHI660E electrochemical analyzer.
2.3 Synthesis
PhSNS, and BPhSNS were synthesized by two steps as described in Scheme 1. Firstly, 1,4-di(2-thienyl)-butane-1,4-dione (1) was synthesized by Friedel-Crafts reaction14. Secondly, the target compounds were synthesized by the dyhydrative cyclization between 1,4-di(2-thienyl)-butane-1,4-dione (1) and corresponding aniline derivatives as the Knorr-Paal reaction22,28.
2.3.1 1,4-di(2-thienyl)-butane-1,4-dione (1)
A solution of thiophene (9.6 mL, 0.12 mol) and succinychloride (5.4 mL, 0.05 mol) were added dropwise to a suspension containing AlCl3(16.2 g, 0.12 mol) in 100 mL of dichloromethane. After stirring for 4 h at 40 °C, the mixture solution was poured into a mixture of 100 g ice and 10 mL hydrochloric acid and stirred for 30 min. The resulting solution was extracted with CHCl2and washed with NaHCO3solutions and water before desiccation with anhydrous MgSO4. The solvent was evaporated off, and the solid residues were purified by column chromatography to afford white product with a yield of 76%.1H NMR (500 MHz, CDCl3, 25 °C, TMS,): 7.84 (dd,= 3.8, 1.1 Hz, 2H), 7.67 (dd,= 5.0, 1.1 Hz, 2H), 7.17 (dd,= 4.9, 3.8 Hz, 2H), 3.42 (s, 4H). MALDI-TOF MS (mass/): 251.1 [M++ H].
2.3.2 Phenyl bridged-di[2,5-di(2-thienyl)-1H-pyrrole (PhSNS)
Under a nitrogen atmosphere,1,4-di(2-thienyl)-butane-1,4-dione (5 mmol, 1.25 g),p-Phenylenediamine(2 mmol, 0.216 g) and acetic acid(15 mL) were putted into a round bottom flask and refluxed for 72 h. Over the reaction, the acetic acid was removed by NaHCO3solution .Then, the resulting solution was filtrated and the residue was re-dissolved in CHCl3for extraction. Evaporation of the solvent, the crude product was purified by column chromatography with dichloromethane. A white product was obtained.1H NMR (500 MHz, CDCl3, 25 °C, TMS,): 7.36 (s, 4H), 7.14 (dd,= 5.1, 1.1 Hz, 4H), 6.87 (dd,= 5.1, 3.7 Hz, 4H), 6.62 (dd,= 3.6, 1.1 Hz, 4H), 6.55 (s, 4H). MALDI-TOF MS (mass/): 536.75 [M++ H].
2.3.3 Biphenyl bridged-di[2,5-di(2-thienyl)-1H-pyrrole (BPhSNS)
BPhSNS was synthesized by the similar procedure for PhSNS. Finally, a white product BPhSNS was also obtained.1H NMR (500 MHz, CDCl3, 25 °C, TMS,): 7.72 (d,= 8.4 Hz, 4H), 7.41 (d,= 8.4 Hz, 4H), 7.10 (d,= 5.1 Hz, 4H), 6.89–6.83 (m, 4H), 6.62 (d,= 2.5 Hz, 4H), 6.57 (s, 4H). MALDI-TOF MS (mass/): 612.85 [M++ H].
2.4 Prepartion of polymer films
The corresponding polymers pPhSNS and pBPhSNS films were prepared by cyclic voltammetry polymerization for 5 scans between −0.8 to 1.4 V in a conventional three-electrode cell with an ITO-coated glass (Kaivo Optoelectronic Technology Co., Ltd.,s≤ 10 Ω∙□−1, the active area: 9 mm × 20 mm) as working electrode, a platinum sheet and a double-junction Ag/AgCl electrode (silver wire coated with AgCl in saturated KCl solution, 0.1 mol∙L−1tetrabutylammonium perchlorate (TBAP) in acetonitrile solution as the second junction) were applied as the counter electrode and the reference electrode, respectively.The synthesis routes of pPhSNS and pBPhSNS were given in Scheme 2. Meanwhile, the above scheme exhibits the possible cross-linked structure of pPhSNS, and pBPhSNS (Scheme 2), which is attributed to the corresponding monomer with four thiophene sites for polymerization in the peripheral part. The monomer concentration of all polymers is 0.5 mmol∙L−1dichloromethane containing 0.1 mol∙L−1tetrabutylammonium perchlorate (TBAP). All the polymer films measurement was performed at the acetonitrile solution containing 0.1 mol∙L−1TBAP. The electrochemistry experiments were carried out at 25 °C under N2atmosphere.
3 Results and discussion
3.1 Electrochemistry of PhSNS and BPhSNS
Fig.1 exhibited the cyclicvoltammetry curves of PhSNS (0.5 mmol∙L−1) and BPhSNS (0.5 mmol∙L−1) in DCM solution containing 0.1 mol∙L−1TBAP. PhSNS and BPhSNS showed the same onset oxidation potential, which may reflect that HOMO of two compounds was similar according to equations: HOMO = −oxonset (Ag/AgCl) (eV) − 4.66 eV29. Besides, it was obvious that PhSNS displayed two oxidation and reduction peaks compared with BPhSNS possessing only one oxidation and reduction peak. This may be attributed to the different molecular configuration for PhSNS and BPhSNS. The reason was furthermore presented through the theoretical calculation.
3.2 Theoretical calculations
To elucidate the influence of the phenyl and biphenyl unit as a bridge on the geometric and electronic properties of PhSNS and BPhSNS, we conducted theoretical calculations. The DFT calculations were performed via Gaussian 03 at the B3LYP/6-311+G (,) level.Fig.2 and Fig.3 exhibited the optimized structure and calculated spatial electron distribution of HOMO-1, HOMO and LUMO of PhSNS and BPhSNS. From the optimized structure of PhSNS and BPhSNS, it was obvious that PhSNS and BPhSNS presented different molecular configuration. The theoretical results showed that the two SNS groups of PhSNS were different. The sulphur atom in one SNS group of PhSNS faced the phenyl unit and the dihedral angle (40.2°) of this SNS group between thiophene and pyrrole was larger than the dihedral angle (21.2°) of the other SNS group of PhSNS. As a results, the two SNS group of PhSNS displayed different conjugated levels. Thus, PhSNS showed larger energy gap (~0.4 eV) between HOMO−1 and HOMO. On the contrary, there was nearly none difference in steric configurations between the two SNS group in BPhSNS molecule, and of course the HOMO−1 and HOMO of BPhSNS exhibited nearly the similar electron cloud character with nearly the same energy level. This may reflect that PhSNS upon losing one electron from HOMO can furthermore lose one electron from HOMO-1 orbits, which may cause PhSNS to present two oxidation and reduction peaks. Correspondingly, BPhSNS just exhibited one oxidation and reduction peaks due to the similar HOMO−1 and HOMO. Besides, HOMO and LUMO of PhSNS and BPhSNS were very similar, which enabled them to exhibit the similar onset oxidative potential and optical energy gap. This theoretical calculation well explained the electrochemistry behavior of PhSNS and BPhSNS.
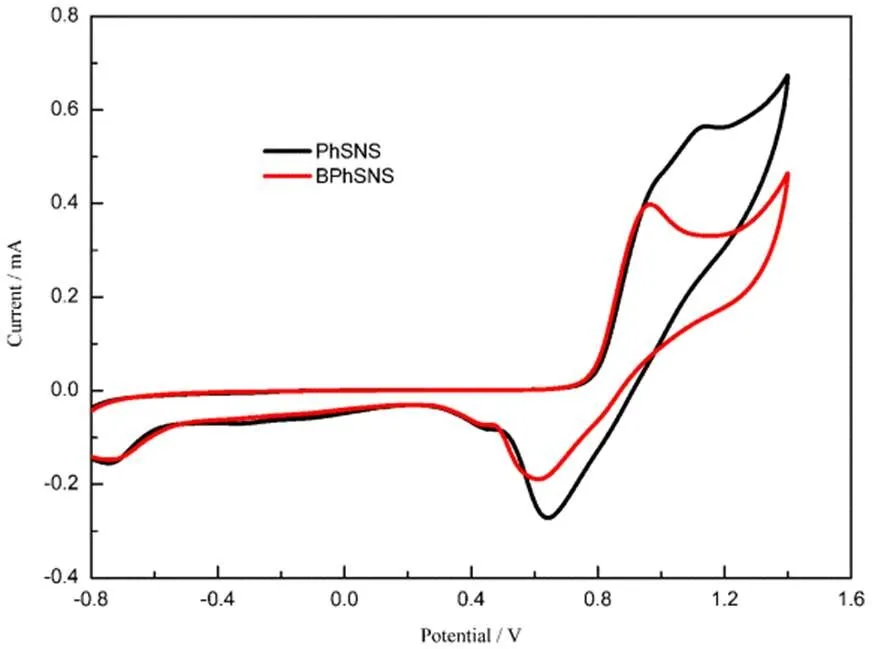
Fig.1 Cyclic voltammetry curves of PhSNS (0.5 mmol∙L−1) and BPhSNS (0.5 mmol∙L−1) in DCM solution containing 0.1 mol∙L−1 TBAP at a scan rate of 100 mV∙s-1.

Fig.2 Optimized conformation of PhSNS and calculated spatial electron distribution of HOMO−1, HOMO and LUMO of PhSNS.
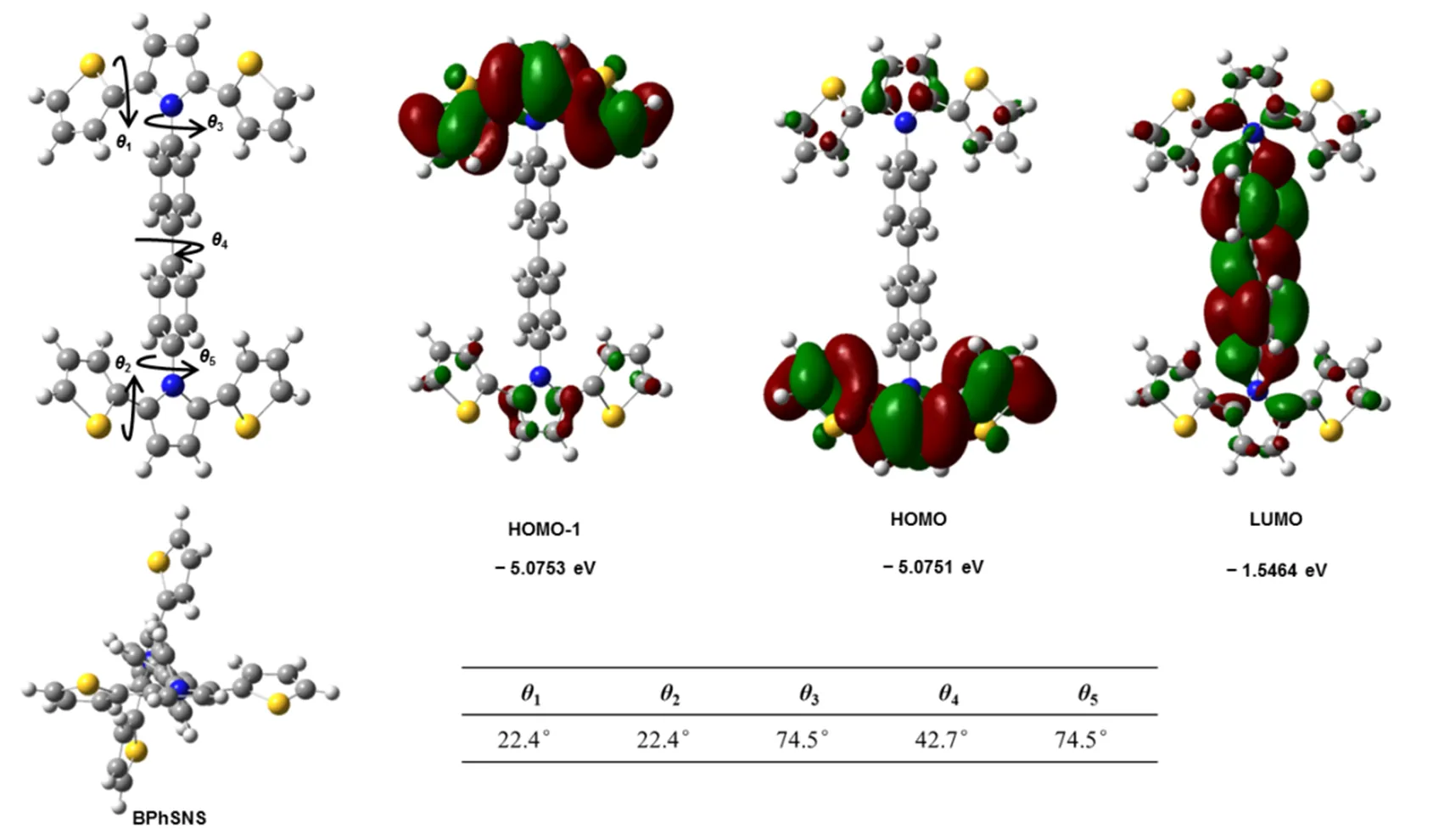
Fig.3 Optimized conformation of BPhSNS and calculated spatial electron distribution of HOMO−1, HOMO and LUMO of BPhSNS.
3.3 UV-vis absorption of PhSNS and BPhSNS
Fig.4 exhibited the UV-vis absorption curves of PhSNS and BPhSNS monomer in dichloromethane (DCM) solution. Obviously, the maximum absorption peak of PhSNS showed blue-shift slightly compared with BPhSNS. The reason may be that one SNS of PhSNS possessed relatively larger twisted angle of 40.2° between thiophene and pyrrole unit and so as to this SNS of PhSNS presented low conjugated levels compared with that of BPhSNS, as seen from theoretical calculations. Besides, as shown in the above figure,PhSNS and BPhSNS exhibited similar the onset value of the absorption peak (onset). We may obtain similargaccording tog= 1240/onset. This result was consistent with theoretical calculations.
3.4 Electropolymerization
Fig.5 presented the multisweep voltammogram (10 scans) curves during electrochemical polymerization of PhSNS (0.5 mmol∙L−1) and BPhSNS (0.5 mmol∙L−1) in dichloromethane (DCM) solution containing 0.1 mol∙L−1TBAP at a potential scan rate of 100 mV∙s−1. Obviously, PhSNS and BPhSNS showed similar cyclic voltammetry except for one new oxidative peak appearing at the higher potential in the first curve of PhSNS. With the number of cycles increasing, the cyclic voltammetry curves of PhSNS and BPhSNS were similar, which was attributed to the formation of similar cross-linked polymers structure through cyclic voltammetry polymerization. With the number of cycles increasing, oxidation peaks and their reverse reduction peaks appeared at the lower potentials due to the formation of the corresponding polymers coated on the ITO/glass surface. Besides, the current densities also increased with the number of cycles increasing, which indicated that their polymer films were well adhered on the electrode30.
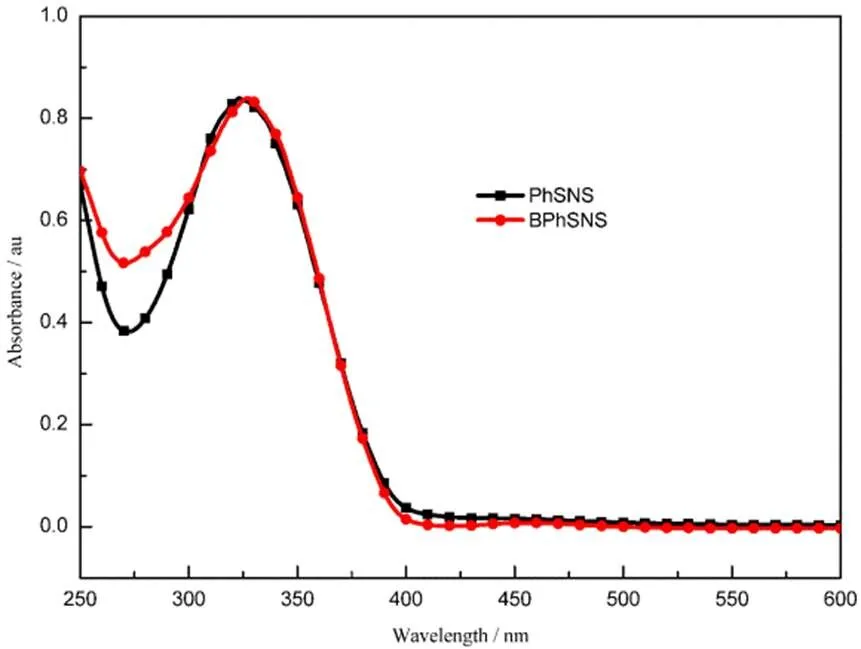
Fig.4 UV-Vis absorption spectra of PhSNS and BPhSNS in DCM solution.
Concentration: 10−5mol∙L−1.

Fig.5 Multisweep voltammogram (10 scans) curves for polymerization of (a) PhSNS (0.5 mmol∙L−1) and (b) BPhSNS (0.5 mmol∙L−1) in DCM solution ontaining 0.1 mol∙L−1 TBAP at a scan rate of 100 mV∙s−1.
3.5 Morphological charcterisation
The morphological characterisation of dithienylpyrrole derivatives with N-substituted benzene was rarely studied. Fig.6 depicted the SEM of pPhSNS and pBPhSNS polymers films. It was obvious that all films exhibited particle morphology. The reason may be that the dithienylpyrrole structure by introducing the rigid phenyl or biphenyl units was inclined to form relatively low degree of polymerization polymers during the electropolymerizaiton process of PhSNS and BPhSNS, which may enable the corresponding polymers to exhibit the particle instead of fibril and golobule-like morphology.
3.6 Electrochemistry of pPhSNS and pBPhSNS
Fig.7 exhibited the CV curves of pPhSNS and pBPhSNS polymers films at different scan rate between 50 to 200 mV∙s−1in acetonitrile solution containing 0.1 mol∙L−1TBAP. It is clear that both pPhSNS and pBPhSNS polymers showed similar two oxidation and reduction peaks, which may be attributed to the similar SNS structure-based cross-linked polymer structure. As a result, the resulting polymers pPhSNS and pBPhSNS displayed multicolor behavior of yellow, greyish-green and gray. Besides, the peak currents of the three polymers were proportional to the potential scan rate, as seen from the inset of Fig.7. This indicated that the electrochemical process of pPhSNS and pBPhSNS were reversible and not diffusion limited31.
3.7 Spectroelectrochemistry
Fig.8 exhibited the UV-vis absorbance spectra of pPhSNS and pBPhSNS films at different applied voltages in acetonitrile solution containing 0.1 mol∙L−1TBAP without the monomer. The two polymers pPhSNS and pBPhSNS showed similar spectroeletrochemical curves. At the neutral state, the absorption peak at about 400nm was the main absorption of the two polymers and they presented yellow color, which was attributed to the–* transition of the polymer backbone. With the increase of potential, the absorption decreased at 400 nm and two new absorption peaks appeared at about 600 nm and 1100 nm, which was ascribed to the electron transition of ‘polaron’ and ‘bipolaron’, respectively. Besides, as shown in the inset of Fig.8, both pPhSNS and pBPhSNS presented the similar multicolor color switching from yellow (−0.8 V) to greyish-green (0.9 V) and the final color was gray (1.1 V).
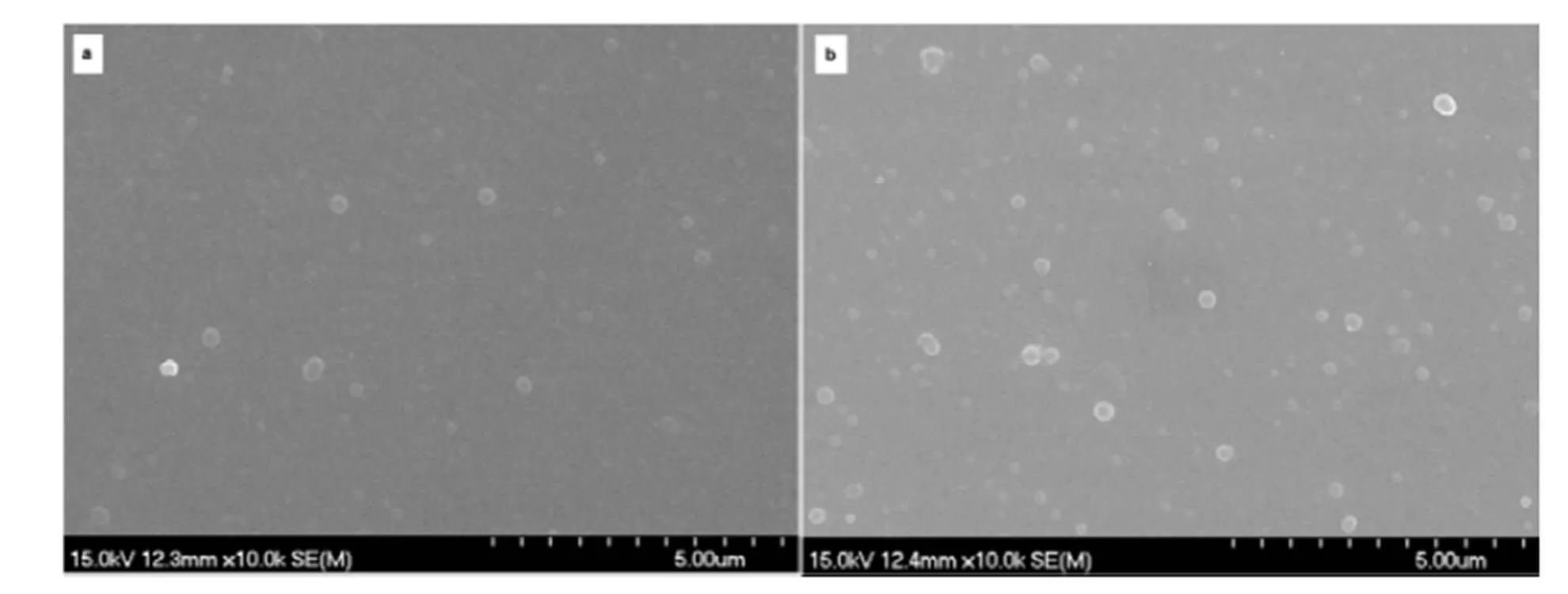
Fig.6 SEM of (a) pPhSNS and (b) pBPhSNS polymers films.
Fig.7 Cyclic voltammetry curves of (a) pPhSNS and (b) pBPhSNS in monomer free acetonitrile solution containing 0.1 mol∙L−1TBAP at different scan rates between 50 and 200 mV∙s−1.
Insets of (a) and (b) were the anodic and cathodic peak current versus scan rate curves of pPhSNS and pBPhSNS.

Fig.8 UV-vis absorbance curves of pPhSNS and pBPhSNS polymers in applied potential between -0.8 to 1.3 V in monomer free acetonitrile solution containing 0.1 mol∙L−1 TBAP.
Insets were the switching color of pPhSNS and pBPhSNS polymers.
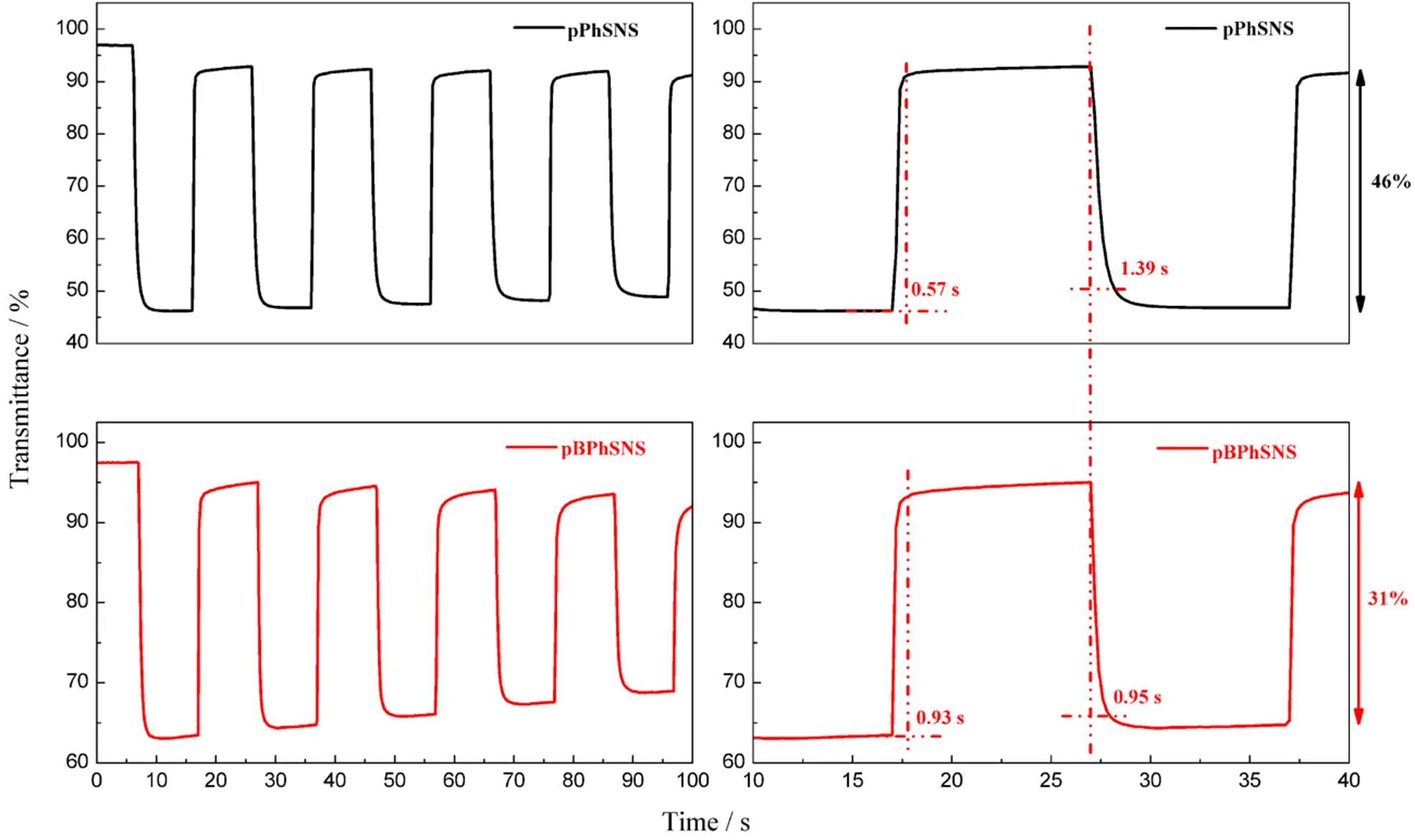
Fig.9 Optical contrast and switching time of pPhSNS and pBPhSNS polymers films monitored at 1100 nm in acetonitrile solution containing 0.1 mol∙L−1 TBAP between -0.8 to 1.1 V with a residence time of 10 s.
3.8 Kenetic studies
Kinetic studies were carried out to elucidate the optical contrast and switching time of the polymer films. Fig.9 depicted the contrast and switching time of pPhSNS and pBPhSNS polymers at 1100 nm under a repeated potential between −0.8 to 1.1 V with a residence of 10 s in a monomer-free acetonitrile solution containing TBAP. As seen in the above figure, the contrasts of pPhSNS and pBPhSNS polymer films at 1100 nm were calculated as 46% and 31%, respectively. Obviously, the contrasts of pPhSNS and pBPhSNS showed small difference, which may be attributed to pPhSNS with the higher doping levels in the oxidation state compared with pBPhSNS polymer films. As shown in Fig.5, this may be that the peak current intensity of PhSNS compound was higher than that of BPhSNS during the electropolymerizaiton of the corresponding monomer, which may reflects that the injected charge of PhSNS was more than BPhSNS during the electropolymerization process at the same cycle numbers. Thus, the resulting polymers pPhSNS may exhibit compact structure, which may display higher doping levels. Besides, the switching time of the two polymers were also calculated in Fig.9. Both pPhSNS and pBPhSNS showed similar fast discoloring time of 0.57, 0.93 s and coloring times of 1.39, 0.95 s, respectively. Compared with pSNS32,33and N-submitted Phenyl pSNS34derivatives, pPhSNS and pBPhSNS presented superior electrochromic properties. Especially, the switching time of them has been improved about 1 s, which was attributed to the formed similar cross-kinked polymer structure through introducing phenyl and biphenyl groups as a bridge.
4 Conclusions
A series of multi-branched dithienylpyrrole (SNS) monomers with rigid phenyl (PhSNS) and biphenyl rings (BPhSNS) as a bridge were designed and synthesized, and then they were fabricated to form the cross-linked polymer by electrochemical polymerization. The cyclic voltammetry results of PhSNS and BPhSNS exhibited that them showed similar oxidative properties except for one new oxidative peak appearing at the higher potential of PhSNS. Theoretical calculation results indicated that it should be attributed to the different molecular configuration between the two dithienylpyrrole (SNS) in PhSNS′s case. The UV-Vis absorption curves presented that PhSNS displayed blue-shift slightly compared with BPhSNS. However, both PhSNS and BPhSNS showed similar onset oxidation potential. PhSNS and BPhSNS were inclined to form the similar cross-linked polymer structure through electrochemical polymerization. The cyclic voltammetry curves of pPhSNS and pBPhSNS showed that both them presented similar oxidative properties. This enabled their corresponding polymers to exhibit similar electrochemistry and electrochromic properties. The UV-Vis spectra of the corresponding polymers showed that both pPhSNS and pBPhSNS possessed similar optical absorption and similar multicolor switching of yellow (−0.8 V), greyish-green (0.9 V) and gray (1.1 V). Besides, pPhSNS and pBPhSNS showed fast switching time of 0.57 and 0.93 s at 1100 nm, respectively and reasonable contrast of 46% and 31% at 1100 nm, respectively. These investigations may be of some supports to the understanding of the relationship between structural configuration and electrochemistry/electrochromic properties for PEC materials research.
(1) Thomas, S. W.; Joly, G. D.; Swager, T. M.2007,, 1339. doi: 10.1021/cr0501339
(2) Ji, X. F.; Yao, Y.; Li, J. Y.; Yan, X. Z.; Huang, F. H.2013,, 74. doi: 10.1021/ja3108559
(3) Thompson, B. C.; Kim, Y. G.; McCarley, T. D.2006,, 12714. doi: 10.1021/ja061274a
(4) Zhou, H. X.; Yang, L. Q.; You, W.2012,, 607. doi: 10.1021/ma201648t
(5) Chen, H. J.; Guo, Y. L.; Yu, G.; Zhao, Y.; Zhang, J.; Gao, D.; Liu, H. T.; Liu, Y. Q.2012,, 4618. doi: 10.1002/adma.201201318
(6) Chen, Z. Y.; Lee, M. J.; Ashraf, R. S.; Gu, Y.; Albert-Seifried, S.; Nielsen, M. M.; Schroeder, B.; Anthopoulos, T. D.; Heeney, M.; McCulloch, I.2012,,647. doi: 10.1002/adma.201102786
(7) Schwendeman, I.; Hickman, R.; Sonmez, G.; Schottland, P.; Zong, K.; Welsh, D. M.; Reynolds, J. R.2002,, 3118. DOI: 10.1021/cm020050y
(8) Nielsen, C. B.; Angerhofer, A.; Abboud, K. A.; Reynolds, J. R.2008,, 9734. doi: 10.1021/ja7112273
(9) Beaujuge, P. M.; Reynolds, J. R.2010,, 268. doi: 10.1021/cr900129a
(10) Sassi, M.; Salamone, M. M.; Ruffo, R.; Patriarca, G. E.; Mari, C. M.; Pagani, G. A.; Posset, U.; Beverina, L.2016,, 5240. doi: 10.1002/adfm.201601819
(11) Bian, G. F.; Hu, B.; Ouyang, M.; Wang, P. J.; Lu, X. J.; Dai, Y. Y.; Zhang, C.2015,, 1888. [边高峰, 胡 彬, 欧阳密, 王萍静, 吕晓静, 戴玉玉, 张 诚. 物理化学学报, 2015,, 1888.] doi: 10.3866/PKU.WHXB201509062
(12) Ouyang, M.; Fu Z. Y.; Lu, X. J.; Chen, H. L.; Hu, B.; Xia, X. F.; Zhang C.2013,, 996. [欧阳密, 付志艳, 吕晓静, 陈欢乐, 胡 彬, 夏旭峰, 张 诚. 物理化学学报, 2013,, 996.] doi: 10.3866/PKU.WHXB201302282
(13) Gunbas, G.; Toppare, L.2012,, 1083. doi: 10.1039/c1cc14992j
(14) Alkan, S.; Cutler, C. A.; Reynolds, J. R.2003,, 331. doi: 10.1002/adfm.200304307
(15) Hwang, J.; Son, J. L.; Shim, Y. B.2010,, 1286. doi: 10.1016/j.solmat.2010.03.027
(16) Yang, C.; Abley, M.; Holdcroft, S.1999,, 6889. doi: 10.1021/ma990937o
(17) Zhang, C.; Hua, C.; Wang, G. H.; Ouyang, M.; Ma, C. A.2010,, 50.doi: 10.1016/j.jelechem.2010.04.009
(18) Otero, T. F.; Carraco, J.; Figuras, A.; Brillas E.1994,, 231. doi: 10.1016/0022-0728(93)03155-I
(19) Camurlu, P.; Gultekin, C.2012,, 142. doi: 10.1016/j.solmat.2012.07.031
(20) Pinar, C.; Cemil, G.; Zeynep, B.2012,, 50. doi: 10.1016/j.electacta.2011.11.079
(21) Wu, T. Y.; Liao, J. W.; Chen, C. Y.2014,, 245. doi: 10.1016/j.electacta.2014.10.116
(22) Tarkuc, S.; E. Sahmetlioglu, C.; Tanyeli, I. M.; Toppare, A. L.2006,, 5412. doi: 10.1016/j.electacta.2006.02.011
(23) Wang, G.; Fu, X. K.; Huang, J.; Wu, C. L.; Wu, L.; Deng, J.; Du, Q. L.; Zou, X. C.2011,, 6352. doi: 10.1016/j.electacta.2011.05.023
(24) Kiralp, S.; Camurlu, P.; Gunbas ,G.; Tanyeli, C.; Akhmedov, I.; Toppare, L.2009,, 1082. doi:10.1002/app.29544
(25) Wang, G.; Fu, X. K.; Huang, J.; Wu, L.; Du, Q.
2010,, 6933. doi: 10.1016/j.electacta.2010.07.012
(26) Usluer, O.; Koyuncu, S.; Demic, S.; Janssen, R. A. J.2011,, 333. doi: 10.1002/polb.22190
(27) Maier, A.; Rabindranath, A. R.; Tieke, B.2009,, 3668. doi: 10.1021/cm901158t
(28) Just, P. E.; Chane-Ching, K. I. and Lacaze, P. C.2002,, 3467. doi: 10.1016/S0040-4020(02)00328-9
(29) Hu, B.; Lv, X. J.; Sun, J. W.; Bian, G. F.; Ouyang, M.; Fu, Z. Y.; Wang, P. J.; Zhang, C.2013,, 1521.doi: 10.1016/j.orgel.2013.03.024
(30) Carbas, B. B.; Önal, A. M.2012,, 38.doi: 10.1016/j.electacta.2012.01.026
(31) Nie, G. M.; Yang, H. J.; Chen, J.; Bai, Z. M.2012,, 2167.doi: 10.1016/j.orgel.2012.05.055
(32) Camurlu, P.; Karagoren, N.. 2013,, 847. doi: 10.1016/j.reactfunctpolym.2013.03.014
(33) Pozo-Gonzalo, C.; Pomposo, J. A.; Alduncin, J. A.; Salsamendi, M.; Mikhaleva, A. I.; Krivdin, L. B.; Trofimov, B. A.2007,, 4784. doi:10.1016/j.electacta.2007.01.050
(34) Tarkuc, S.; Sahmetlioglu, E.; Tanyeli, C.; Akhmedov, I. M.; Toppare, L.2006,, 5412. doi: 10.1016/j.electacta.2006.02.011
苯/二联苯为桥的噻吩-吡咯-噻吩结构:分子构型及电致变色性质
戴玉玉 李维军*闫拴马 王士昭 俞 越 欧阳密 陈丽涛 张 诚*
(浙江工业大学化学工程学院,绿色化学合成技术国家重点实验室培育基地,科技部能源材料及应用国际科技合作基地,杭州 310014)
合成了两种以苯或二联苯为桥键单元的噻吩-吡咯-噻吩衍生物(PhSNS、BPhSNS),并通过电化学聚合形成具有交联结构的聚合物薄膜(pPhSNS、pBPhSNS)。两种单体的循环伏安曲线表明,相比BPhSNS的一个氧化峰,PhSNS表现出两个氧化峰,理论计算结果表明这可能主要是由于PhSNS化合物中的两条噻吩-吡咯-噻吩具有不同的分子构型,其中一条噻吩-吡咯-噻吩中噻吩与吡咯单元的扭曲角为21.2°,另一条噻吩-吡咯-噻吩中噻吩与吡咯单元的扭曲角为40.2°,这使得PhSNS的HOMO-1和HOMO表现出比较大的能隙(~0.4 eV),因而导致出现两个峰;BPhSNS结构中两条噻吩-吡咯-噻吩的分子构型相同,使得HOMO-1和HOMO很相似,因此其电化学只出现一个峰。然而,两个单体表现出相同的起始氧化还原电位,同时相应的聚合物表现出相似的氧化还原曲线,使得两种聚合物薄膜表现出相似的电致变色性质。光谱电化学表明,pPhSNS和pBPhSNS表现出相似的光学吸收及相似的颜色变化(黄色-灰绿色-灰色);此外,pPhSNS和pBPhSNS表现出快速的响应速度(1100 nm 处分别为0.57和0.93 s),同时表现出合理的光学对比度(1100 nm处分别为46%和31%);这些研究将为关联分子构型与单体的电化学、聚合物的电致变色性质三者之间的关系提供一定的借鉴与帮助。
噻吩-吡咯-噻吩;分子构型;理论分析;电化学聚合;电致变色
O646
10.3866/PKU.WHXB201705252
March 30, 2017;
May 11, 2017;
May 25, 2017.
Corresponding authors. ZHANG Cheng, Email: czhang@zjut.edu.cn. LI Wei-Jun, liwj@zjut.edu.cn; Tel: +86-571-88320929.
The project was supported by the National Natural Science Foundation of China (51603185, 51673174, 51573165, 51273179), Zhejiang Provincial Natural Science Foundation, China (LY17E030001, LY15E030006, LY12B03008), and Special Fund for Scientific Research from Zhejiang University of Technology, China (3817101101T).
国家自然科学基金(51603185, 51673174, 51573165, 51273179), 浙江省自然科学基金(LY17E030001, LY15E030006, LY12B03008)及浙江工业大学科研专项基金(3817101101T)资助项目

