Origin of natural sulfur-metal chimney in the Tangyin hydrothermal field, Okinawa Trough: constraints from rare earth element and sulfur isotopic compositions
Hong Cao , Zhi-lei Sun ,*, Chang-ling Liu , En-tao Liu, Xue-jun Jiang , Wei Huang
a The Key Laboratory of Gas Hydrate, Ministry of Land and Resources, Qingdao Institute of Marine Geology, Qingdao 266071, China
b Laboratory for Marine Mineral Resources, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
c College of Marine Science and Technology, China University of Geosciences (Wuhan), Wuhan 430074, China
ABSTRACT
For the first time, we present the rare earth element (REE) and sulfur isotopic composition of hydrothermal precipitates recovered from the Tangyin hydrothermal field (THF), Okinawa Trough at a water depth of 1206 m. The natural sulfur samples exhibit the lowest ΣREE concentrations (ΣREE=0.65×10–6–4.580×10–6) followed by metal sulfides (ΣREE=1.71×10–6–11.63×10–6). By contrast, the natural sulfur-sediment samples have maximum ΣREE concentrations (ΣREE=11.54×10–6–33.06×10–6),significantly lower than those of the volcanic and sediment samples. Nevertheless, the δEu, δCe, (La/Yb)N,La/Sm, (Gd/Yb)N and normalized patterns of the natural sulfur and metal sulfide show the most similarity to the sediment. Most hydrothermal precipitate samples are characterized by enrichments of LREE(LREE/HREE=10.09–24.53) and slightly negative Eu anomalies or no anomaly (δEu=0.48–0.99), which are different from the hydrothermal fluid from sediment-free mid-oceanic ridges and back-arc basins, but identical to the sulfides from the Jade hydrothermal field. The lower temperature and more oxidizing conditions produced by the mixing between seawater and hydrothermal fluids further attenuate the leaching ability of hydrothermal fluid, inducing lower REE concentrations for natural sulfur compared with metal sulfide; meanwhile, the negative Eu anomaly is also weakened or almost absent. The sulfur isotopic compositions of the natural sulfur (δ34S=3.20‰–5.01‰, mean 4.23‰) and metal sulfide samples(δ34S=0.82‰–0.89‰, mean 0.85‰) reveal that the sulfur of the chimney is sourced from magmatic degassing.
Keywords:
Hydrothermal processes
Natural sulfur
Isotopic compositions
REE
Tangyin field
Okinawa Trough
China
1. Introduction
As an important part of Circum-Pacific seismic belt, the Okinawa Trough (OT) is a tectonically active intracontinental back-arc basin generated through the subduction of the Philippine plate under the Eurasian continent (Halbach P et al., 1989). Since the initial discovery of hydrothermal activity in the OT in 1984 (Kimura M et al., 1988), more than 20 known hydrothermal fields have been discovered, such as Jade, Minami-Ensei Knoll and so on (Zeng ZG et al., 2016;Miyazaki J et al., 2017). These fields have received broad research attention, and a series of important issues including mineralogy (e.g. Halbach P et al., 1989; Kimura M et al.,1988; Glasby G P and Notsu K 2003; Halback P et al., 2015;Ishibashi JI et al., 2015), geochemistry (e.g. Gamo T et al.,1987; Zhai SK et al., 2007), isotopes (e.g. Zeng ZG et al.,2000; Lüders V et al., 2001; Terakado Y and Walker RJ,2005; Hongo Y et al., 2007; Nishio R and Chiba H, 2012),organic composition (Zhang QL et al., 2001) and rare earth elements (REE) (e.g. Zeng ZG et al., 2001; Hongo Y et al.,2001) of hydrothermal sulfide, and geochemistry of hydrothermal fluid (e.g. Ishibashi JI et al., 1995; Ishibashi JI et al., 2014; Kawagucci S, 2015) have been discussed in the literature. Moreover, the mineralogy and geochemical signatures (coexisting occurrence of zinc and lead-enriched)suggest that the OT may be a modern analogue for the formation of ancient Kuroko-type volcanogenic massive sulfide (VMS) deposits (Halbach P et al., 1989).
Among all the seafloor hydrothermal sites worldwide,natural sulfur occurs at only a few fields, such as the active Jade hydrothermal field in the OT (Halbach P et al., 1989),the Eastern Pacific Rise 21°N (Styrt MM et al., 1981), and the Kueishantao of Taiwan (Zeng ZG et al., 2007). Although the mineralogy and geochemistry of the OT sulfides have been sufficiently studied, these researches regarding the natural sulfur-bearing hydrothermal fields have been fewer, mainly because of the paucity of relevant hydrothermal deposits. In 2014, a novel type of hydrothermal deposit was discovered in the OT region. The THF is characterized by the coexistence of natural sulfur with metal sulfide, and the presence of hightemperature Fe-Zn sulfide at the bottom of the natural sulfur chimney. This site provides a natural laboratory for further investigation of active seafloor hydrothermal activity.However, little is known about the origin of ore-forming materials within the THF.
This study reports new data of REE and S isotopic compositions in the natural sulfur-metal sulfide chimney from the THF. Our aim is to better constrain the origin of the REE and sulfur, shedding more light on the hydrothermal process of this type of sulfur chimney.
2. Geological Settings
The OT is an incipient intra-continental basin located behind the Ryukyu trench and Ryukyu Islands (Fig. 1), which is formed under the extension of the Asian continental lithosphere behind the Ryukyu arc–trench system, since its initial rifting stage at around 2 Ma (Lee CS et al., 1980;Letouzey J and Kimura M, 1986; Nagumo S et al., 1986; Xu F et al., 2015). The OT, paralleling with the arc of Ryukyu Islands, is about 1200 km long, 230 km wide in the north and 60–100 km wide in the south. It is characterized by active rifting structures and magmatism along the depression(Letouzey J and Kimura M, 1986; Sibuet JC et al., 1987; Feng R et al., 1993; Zheng XL et al., 2015). The water depth in the OT increases gradually from north to south, with the deepest part in the south segment around 2300 m (Huang F 1989;Yang WD et al., 2004; Zhang LF et al., 2014). The volcanic rocks in the OT exhibit both MORB and arc-type characteristics, hinting at involvement of asubducting slab(Yu ZH et al., 2001; Ishizuka H et al., 2008). Sibuet JC et al.(1998) previously documented that the OT is in its early stage of evolution from arc to back-arc activity, which is consistent with the conclusion reported by Kotake Y (2000) that the seafloor spreading in the OT has an extensional rate of only 3.7 ± 0.06 cm/a.
The southernmost area close to the Taiwan Island has the topographically lowermost trough floor throughout the OT with a thinned continental crust less than 20 km thick (Lee CS et al., 1980; Hirata N et al., 1991; Li PL et al., 2000;Klingelhoefer F et al., 2009). Seismic reflection data revealed a minimum crustal thickness of only 8 km at the southernmost area (Klingelhoefer F et al., 2009). This field hosts a major volcanic Alignment-Cross-Backarc Volcanic Trail (Sibuet JC et al. (1998)), or cross-back-arc volcanic trail (CBVT), that might haveo riginated from the subducting Gagua Ridge on the Philippine Sea Plate (Lin JY et al. 2013). The possible signs of magma chambers at the lower crust beneath the CBVT have already been observed (Lin JY et al., 2007).
Both the east and west margins of the OT are delimited by high-angle normal faults sloping to the trough axis(Klingelhoefer F et al., 2009). Furthermore, a central graben exists in the trough axis, paralleling with the trough but in en echelon alignment (Kimura M, 1985). This region is characterized by high3He values in the seawater with(3He/4He)/(3He/4He) air at 1.65 (Ishibashi JI et al., 1995) and high heat flow (up to 2000 mW·m-2), suggesting the presence of shallow magma chambers (Yamano M et al., 1989). The volcanic rocks in the OT include basalt, basaltic andesite,dacitic andesite, and rhyolite (Zhai SK and Gan XQ, 1995).
3. Sampling and Methods
The THF (122°34′E, 24°04′N; 1206 m water depth) was discovered in 2014 during the HOBAB3 cruise (Zeng ZG et al., 2016). This active hydrothermal field is situated on the top of a twin seamount named Yuhua Hill, with a height of approximately 220 m and a width of 1.5 km. This seamount is adjacent to a submarine canyon to the east and is covered with patches of sediment (Zeng ZG et al., 2016). As observed on deck, a natural sulfur sample occurred as a pale yellow (Fig. 2a and 2c), soft and porous material, and is covered with dark gray sediments on its surface. It also has pungent odor and there is a relatively large semi-round channel (Fig. 2b), which is obviously filled with natural sulfur and sulfide. SEM and EDS analysis showed that the native sulfur has very high purity (Fig. 2e and 2g). At the bottom of one of the samples(TY1), a relatively smaller metal sulfide chimney (3 cm×4 cm) was attached to the bottom of the main natural sulfur chimney (Fig. 2d), and pyrite grains were visible in hand specimens (Fig. 2f). The sulfide chimney were dominated by pyrite and sphalerite (Fig. 2h). A large cavity (4 cm×7 cm)can be seen in the natural sulfur chimney (Fig. 2d), likely caused by the eruption of hydrorthermal gas. In addition, the interior wall of the cavity consists of pure natural sulfur.
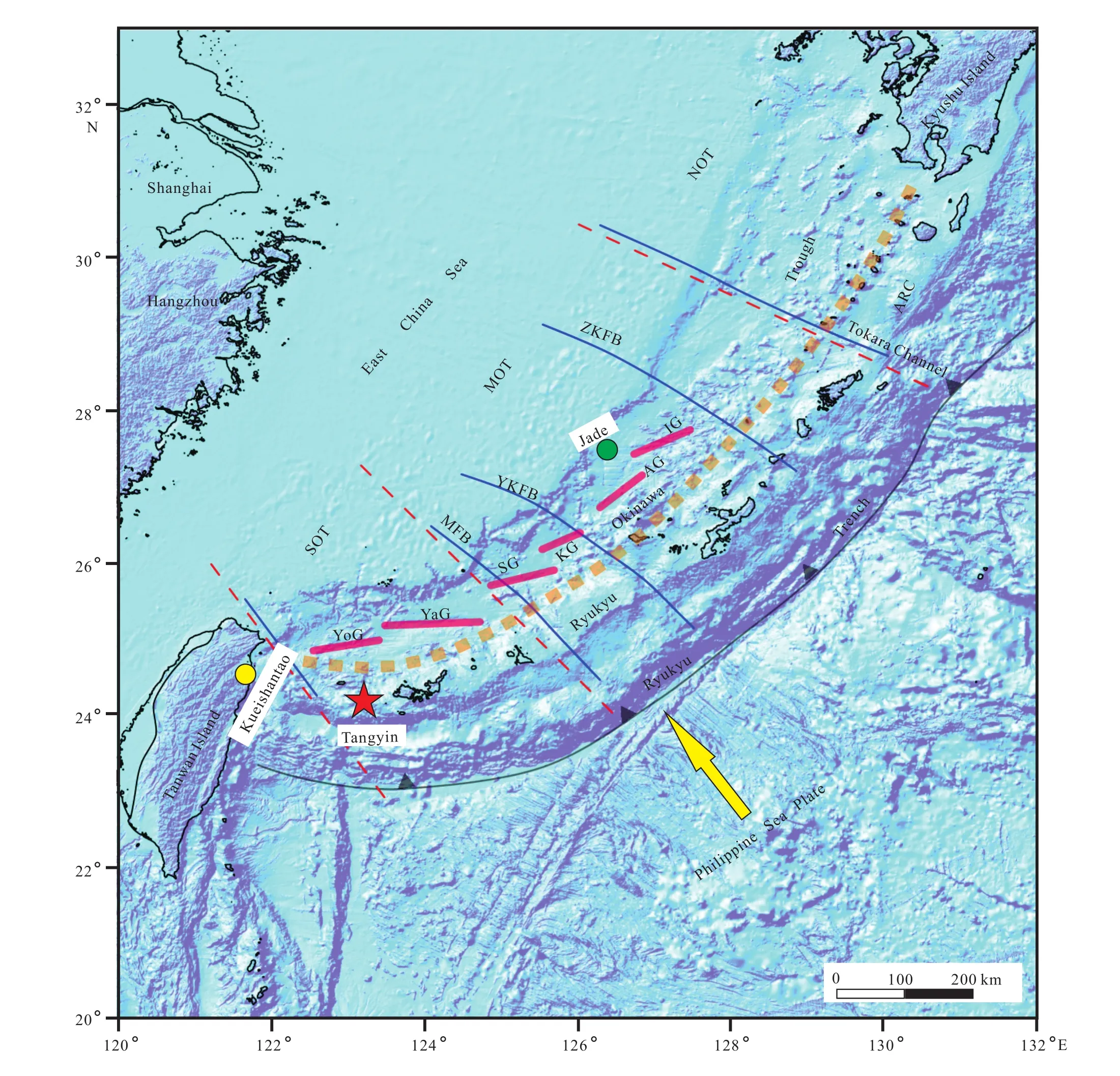
Fig. 1. Location of the Tangyin (delimited by the red star), Jade (orange dot) and Kueishantao (yellow dot) hydrothermal fields along the Okinama Trough. The regional bathymetry is modified from Kimura M (1985), Sibuet M et al.(1987) and Shang LN et al.(2017). The bold orange dashed line indicates the Ryukyu Volcanic Front. Blue lines indicate the major faults: MFB–Miyako Fault Belt; YKFB–Yushan Kume Fault Belt; ZKFB–Zhoushan Kunigami Fault Belt; TFB–Tokara Fault Belt. Red bars show the Okinama Trough central grabens: YoG–Yonaguni Graben; YaG–Yaeyama Graben; SG–Sakishima Graben; KG–Kerama Graben; AG–Aguni Graben; IG–Iheya Graben. NOT–Northern Okinawa Trough; MOT–Middle Okinawa Trough; SOT–Southern Okinawa Trough. Bathymetric data come from the Marine Geoscience Data System(www.marine-geo.org).
For REE analysis, about 40 mg of powdered and dried material from each sample was taken in a Teflon digestion crucible. The powder was digested with 0.5 mL of HNO3,0.5 mL HF and 1.5 mL HCl at 180°C for 24 h. After that the samples were transferred into Teflon vials. When completing digestion and coversion to nitrates, the samples were redissolved in 10 mL 10% double-distilled HNO3to ensure complete homogenization. In the next step, the samples were transferred to polypropylene volumetric flasks and diluted to 40.0 g with deionized water. During the process, a sample to test the recovery along with a parallel sample were selected.At the same time, a parallel native sulfur sample and a native sulfur sample to which was added a standard solution having element concentrations of the same magnitude as the sample were prepared for testing the recovery. This method was used to control the concentration of elements in the samples so that their ratio is 1:1. Finally, the trace elements and REEs were measured with the inductively coupled plasma mass spectrometry (ICP-MS) at the Institute of Oceanology,Chinese Academy of Sciences. Instrument response was calibrated against W-2, an international rock standard, and was cross-checked with other international rock standards (i.e.BIR-1 and BHVO-1), which were run as unknowns at the beginning and end of the analytical sequence.
Before the determination of sulfur isotope composition,sulfide samples were purified to pure BaSO4by the half melting method using carbonate-zinc oxides. V2O5was added to the BaSO4and standards to enhance combustion. Measurements of S isotopic compositions of the samples were analyzed by a MAT 253 gas isotope mass spectrometer, adopting the international standards of canyon diablo troilite (CDT). These analyses were performed at the analytical facilities of the Geology Department at the Chinese Academy of Geological Sciences. All the replicate analyses of sul fi de standards were within ±0.5‰, and the analysis precision was about 0.2‰.
4. Results and discussion
4.1. REE components
The total REE contents (ΣREE) of the hydrothermal precipitate samples display a large variability ranging from 0.65×10–6to 33.06×10–6(Table 1). In this paper, the natural sulfur-sediment samples represent the bulk samples of the sulfur chimney, which contains inclusions of some sediment;however, the natural sulfur samples belong to the pure sulfur that were sampled by hand drilling. The REE concentration of the studied samples has a close relationship with the sample type. Overall, the natural sulfur samples exhibit the lowest ΣREE (varying from 0.65×10–6to 4.58×10–6), followed by metal sulfide samples (varying from 1.71×10–6to 11.62×10–6).The natural sulfur-sediment samples are characterized by the maximum ΣREE (varying from 11.54×10–6to 33.06×10–6)except for the sample TY2 (ΣREE=3.58×10–6).
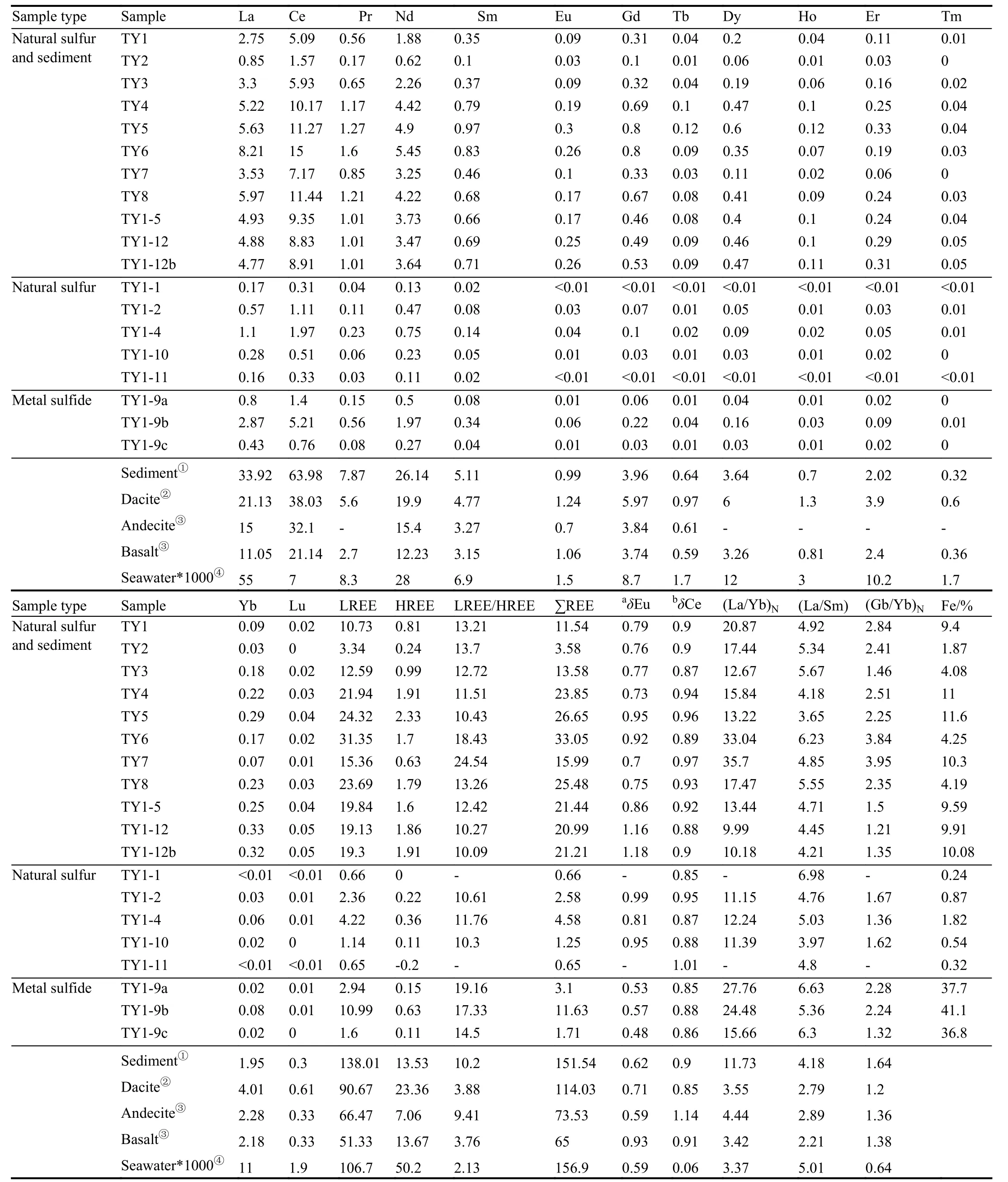
Table 1. REE concentration data (10-6) and parameters of the hydrothermal deposit from the Tangyin hydrothermal field.
The REE Chondrite-normalized distribution patterns of the samples show an enrichment of light rare earth element(LREE) (Fig. 3). The degrees of fractionation between the LREEs and HREEs are obvious and highly variable, and the LREE/HREE ratios range between 10.09 and 24.53, with(La/Yb)N=9.99–35.70, showing that the studied samples are characterized by a relative enrichment of LREE and depletion of HREE. The substitution of REEs into metal sulfides appears to be strongly influenced by the larger ionic radii of the REEs (e.g., Alt J C, 1988; Mills R A and Elderfield H,1995). The great fractionation difference between the LREE and HREE may be attributed to the presence of abundant Fe;this speculation is supported by the significant positive correlation between LREE and Fe (Fig. 4a). Meanwhile, it is worth noting that the(La/Sm)Nvalue of the studied samples ranges from 3.65 to 6.98, but (Gd/Yb)Nfrom 1.21 to 3.95,suggesting that the extent of fractionation of LREE is higher than that of HREE.
Compared with the natural sulfur chimney (LREE=0.65×10–6–4.58×10–6, mean 1.81×10–6; LREE/HREE=10.30–11.76,mean 10.89; (La/Yb)N=11.15–12.24, mean 11.59; (Gd/Yb)N=1.36–1.67, mean=1.55), the LREE in metal sulfides (Fe-rich sulfides, under review) (LREE=1.712×10–6–11.63× 10–6, mean 5.18; LREE/HREE=14.50–19.16, mean=17; (La/Yb)N=15.66–27.76, mean=22.63; (Gd/Yb)N=1.32–2.28, mean=1.95) is higher and varies considerably, the fractionation between LREE and HREE, in LREE and in HREE is distinct, implying that the formation process of the metal sulfide is relatively slow compared to the natural sulfur. This might have been caused by the high leaching efficiency under the seafloor due to the long duration of fluid-rock interaction during the precipitation of metal sulfides in the THF. In addition,contrary to the high temperature hydrothermal fluid, the leaching ability of low temperature hydrothermal fluid is weak, which might be another reason for the lower REE content in the natural sulfur chimney than that of the metal sulfide.
Conversely, when compared with the natural sulfur chimney in the Kueishantao hydrothermal field (sulfur content is 99.96%, ΣREE=4.46×10–9–21.00×10–9, LREE/HREE=6.26–13.54; Zeng ZG et al., 2007) and in the Kusatsu-Shirane crater lake (sulfur content is 99.71%, the ΣREE is 12.1×10–6,and the LREE/HREE is 3.17; Kargel JS et al., 1999), the ΣREE in the natural sulfur chimney from the THF is clearly higher than that at the Kueishantao and lower than that at the Kusatsu-Shirane. Compared with the metal sulfide from the Jade hydrothermal field (ΣREE=1.04×10–6to 4.88×10–6,LREE/HREE=1.04–6.84, Zeng ZG et al., 2001), the ΣREE in the metal sulfide (ΣREE=1.71×10–6– 11.63×10–6; LREE=1.71×10–6–11.63×10–6, LREE/HREE=14.50–19.16) from our study area is slightly higher. These results may imply that the persistence time of fluid-rock interaction under the seafloor in the THF is longer than those in the Kueishantao and Jade hydrothermal fields. Meanwhile, there is a close relationship between the ΣREE and Fe contents (Fig. 4b), indicating that the absolutely high ΣREE and its relatively varying range in the hydrothermal deposits are likely related to the presence of Fe-rich sulfide minerals. This is also demonstrated by the fact that Fe-rich sulfide minerals generally have a wide range of precipitation temperatures (possibly from <100°C to >300°C)(e.g., Fouquet Y et al., 1988; Hannington M et al., 1991).
Compared with the volcanic rock (mainly dacite, andesite and basalt; Zhai SK and Gan XQ, 1995; Li WR et al., 1997),sediment (Zhao Y, 1983), and seawater in the OT, the ΣREE of all the hydrothermal samples are significantly lower, and the δEu, δCe, (La/Yb)N, La/Sm, and (Gd/Yb)Nin the natural sulfur chimney and metal sulfide are comparable to the values in the sediment in the OT (Fig. 5). Furthermore, the normalized REE patterns of the hydrothermal samples display the most similarity to the sediment, followed by those of the dacite, andesite and basalt.
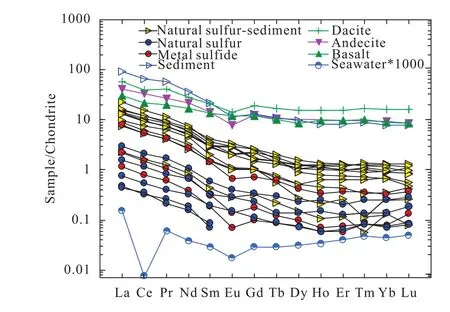
Fig. 3. Chondrite-normalized REE patterens of natural sulfur samples from the Tangyin hydrothermal field. REE values of seawater, dacite, andesite, basalt and sediment are from Gillis KM et al.(1990), Zhai SK and Gan XQ (1995), Li WR et al. (1997), and Zhao Y (1983) respectively.
Except for the sample TY1-12 (δEu=1.16) and TY1-12b(δEu=1.18), all other samples only exhibit a slight negative Eu anomaly or even no anomaly (δEu=0.48 –0.99). Among them, the metal sulfides show the most distinguished negative Eu anomaly (δEu=0.48–0.57), followed by the natural sulfursediment samples (δEu=0.73 –0.95), then the natural sulfur samples, which is characterized by a slightly negative Eu anomaly (δEu=0.89–0.99). Moreover, all the studied samples,except for the sample TY1-11 (δCe=1.01), are characterized by slight Ce negative anomalies (δCe=0.85 –0.97). Overall,regarding the proxies of δEu and δCe, the studied samples are comparable to the natural sulfur ball found in the Kueishantao hydrothermal field (δEu=0.31–0.68, δCe=0.99–1.20, Zeng ZG et al., 2011), and the metal sulfide from the Jade hydrothermal field (δEu=0.58–0.71, δCe=0.78–0.98, Zeng ZG et al., 2001)respectively.
Most hydrothermal samples from the THF are characterized by negative Eu anomalies, distinctively different from the hydrothermal fluid from sediment-free mid-oceanic ridges (Klinkhammer GP et al., 1994; Hongo Y et al., 2007)and back-arc basins such as the Manus Basin (Craddock PR et al., 2010), Lau Basins (Bach W et al., 2003; Charlou JL et al.,1990), and other areas in the OT (Hongo Y and Nozaki Y,2001), but comparable with the sulfide from the Jade hydrothermal field (Liu YG et al., 2004; Zeng ZG et al.,2001). Previous studies have demonstrated that temperature plays an important role in controlling the ratio of Eu3+/Eu2+.For instance, in a reducing environment with high temperature, Eu usually exists in the form of Eu2+(Sverjensky DA, 1984); in this case, there is a distinct Eu anomaly. The obvious negative anomaly of sulfide can be attributed to the larger ionic radii of Eu2+rather than those of Fe, Cu, and Zn,in preventing the Eu2+entering into the crystal lattice of the metal sulfide (Alt JC, 1988; Mills RA and Elderfield H, 1995;Hongo Y et al., 2007). In contrast, in the low-temperature fluid system, a large proportion of the Eu is in a trivalent state because divalent Eu is only stable at temperatures above 250°C (Sverjensky DA, 1984), which induces an indistinct negative Eu anomaly. The negative Eu anomalies and positive Gd anomalies displayed in the studied samples are considered to be signals of the low-temperature seawater (Baar H et al.,1985), which suggests that oxidation was increasing with decreasing temperature within the mixing conditions of seawaters and hydrothermal fluids. As a result, Eu is present as Eu (III), and the natural sulfur samples just display slightly negative Eu anomalies.
4.2. Sulfur sources of the hydrothermal chimney
As shown in Table 2 and Fig. 6, the δ34S values of the samples ranges from –0.82‰ to +5.01‰, displaying a relatively wide variation. In addintion, the range of δ34S values has a close relationship with the sample type. In details, the metal sulfides exhibit the lowest δ34S value(δ34S=0.82‰–0.89‰, mean 0.85‰), followed by the natural sulfur-sediment samples (2.63‰–4.48‰, mean 3.52‰). In contrast, the pure natural sulfur samples are characterized by the maximum δ34S value (ranging between 3.20 ‰ and 5.01‰, mean 4.23‰).
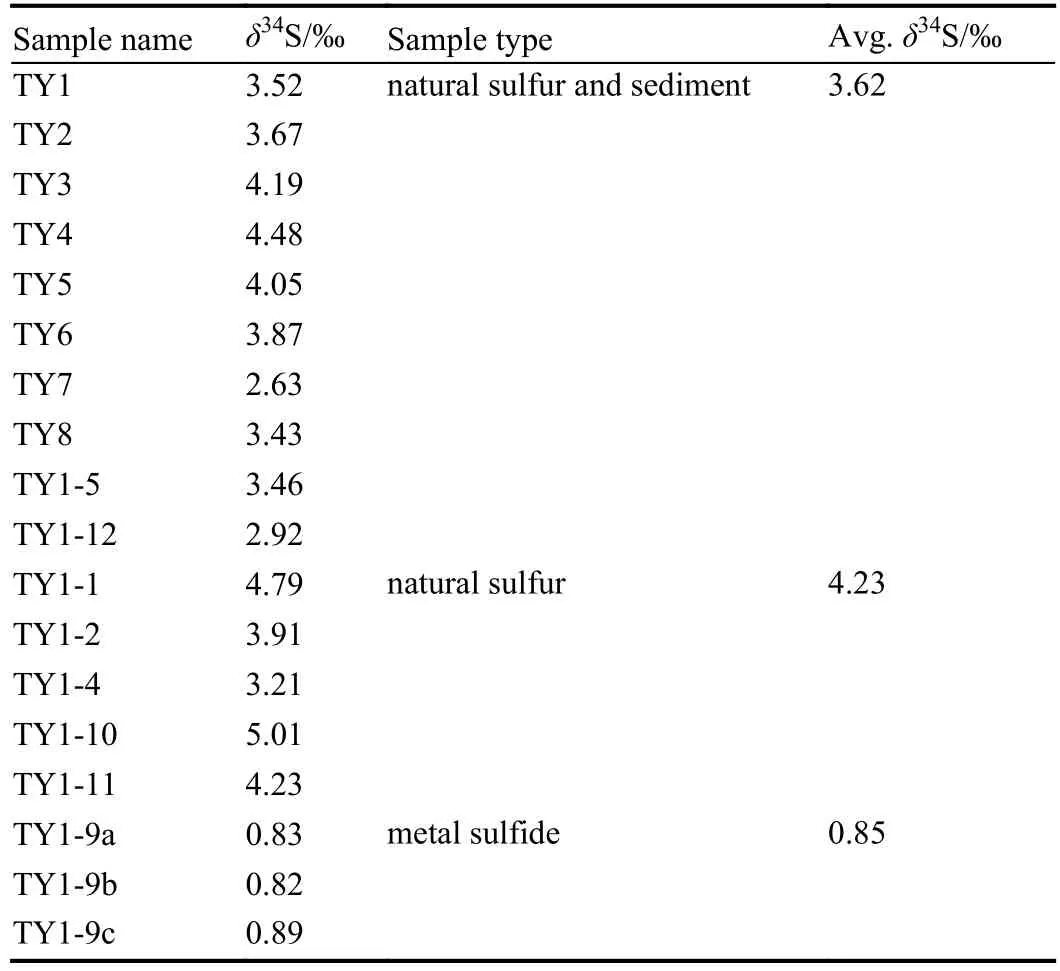
Table 2. The S isotopic composition of the hydrothermal samples from the THF.
It is worth noting that the range of δ34S values in the studied samples is smalller than that of the Onsen hydrothermal field in the Desmos crater, Manus Basin (Fig. 7).This indicates that the sulfur sources of the THF samples differ from those in the Kueishantao, Jade, Lau Basin, and Onsen hydrothermal fields. Specifically, the δ34S values of the natural sulfur chimney in the THF are slightly higher than those in the Kueishantao and Lau Basin hydrothermal fields,but significantly lower than those in the Jade hydrothermal field. Moreover, compared with the other hydrothermal fields,the range of δ34S values in this area is more concentrated. We therefore speculate that the THF has a relatively simple sulfur source.
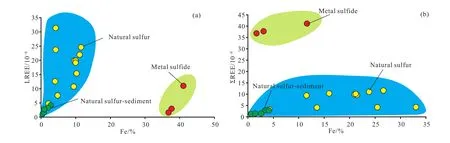
Fig. 4. (a) Correlation between the LREE and Fe of hydrothermal precipitates. (b) Correlation between the ΣREE and Fe of hydrothermal precipitates.
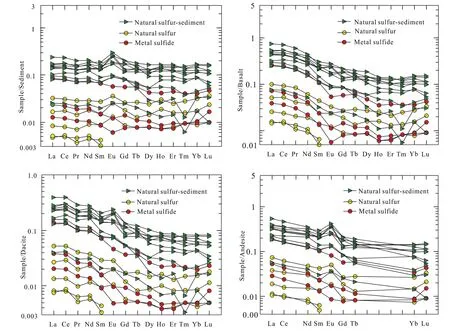
Fig. 5. Normalized REE patterns of natural sulfur samples from the THF. REE values of seawater, dacite, andesite, basalt and sediment are from Gillis KM et al. (1990), Zhai SK and Gan XQ(1995), and Li WR et al. (1997) respectively.
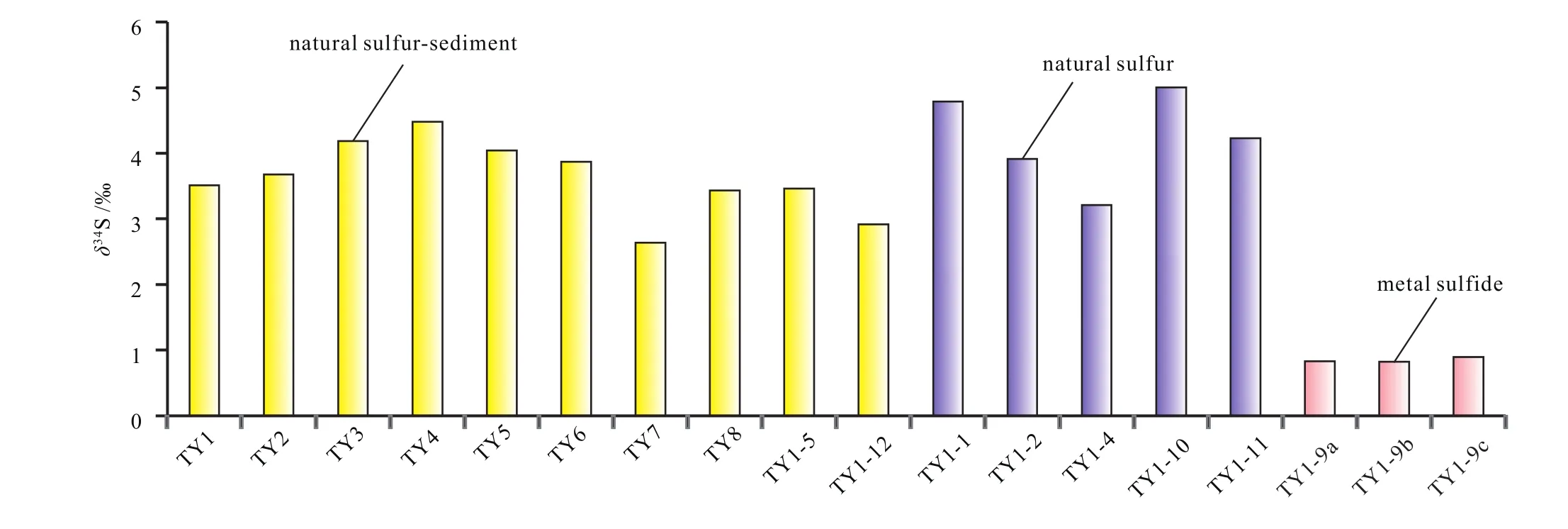
Fig. 6. Distribution of the δ34S of different hydrothermal precipitates from the THF: yellow bar indicates natural sulfur-sediment, purple bar indicates natural sulfur, and pink bar indicates the metal sulfide.
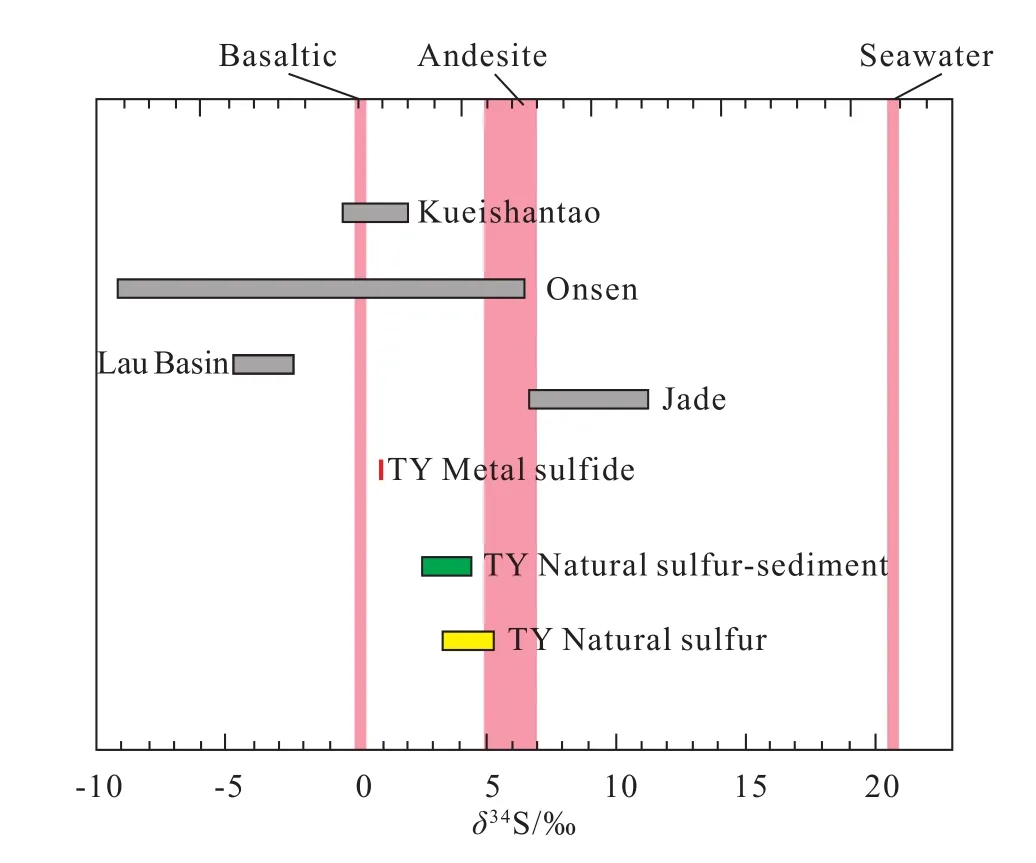
Fig. 7. Sulfur isotope values of natural sulfur-metal sulfide samples from different seafloor hydrothermal fields: red bar indicates metal sulfide; green bar indicates natural sulfur-sediment; yellow bar indicates natural sulfur. Andesite from Ueda A and Sakai H (1984) and Woodhead JD et al. (1987), Mid-Ocean Ridge Basalt from Sakai H et al. (1984), seawater from Rees CE et al. (1978), Kueishantao natural sulfur from Zeng ZG et al. (2007), Jadenatural sulfur from Marumo K and Hattori KH (1999) and Zeng ZG et al. (2002), Lau Basin natural sulfur from Herzig PM et al. (1998), and Onsen natural sulfur from Gena KR et al. (2006).
Previous studies have shown that the δ34S values of island arc lava (andesite and rhyolite) vary from +5‰ to +7‰ (Ueda A and Sakai H, 1984; Woodhead JD et al., 1987). By contrast,the δ34S values of the samples from the THF are much lower than that in the island arc lava, which excludes the possibility that the sulfur comes directly from the hosted andesite in this area. Also, the sulfur isotopic composition of the metal sulfides has the same distribution range as that of the midocean ridge basalt (δ34S=+0.1‰ ± 0.5‰; Gena KR et al.,2006), hinting that the sulfur of the chimney at the THF is sourced from deep magma. This can be explained by deep magma degassing. However, this hypothesis needs more lines of evidence, especially from radioactive isotope dating of the hydrothermal fluid herein. Compared with the metal sul fi des,theδ34S values of the natural sulfur in our samples are relatively heavier, implying that there might be more contributions of sulfur from the reduction of seawater sulfates to the hydrothermal fluid during the precipitation of natural sulfur, whereas less contributions to the metal sul fi des.
5. Conclusions
(1) The REE concentrations (ΣREE=0.65×10–6–4.58×10–6)in the natural sulfur chimney from the THF are obviously higher than that of the Kueishantao hydrothermal field, but lower than that of the Kusatsu-Shirane crater lake. The ΣREE in the metal sulfide (ΣREE=1.71×10–6–11.63×10–6) from the same area is higher than that from the Jade hydrothermal field. The chondrite-normalized REE patterns of most samples show an enrichment in LREE and a slightly negative Eu anomaly (δEu=0.48–0.99), which is similar to those of the sediment and followed by dacite, andesite and basalt in the OT.
(2) Largely owing to the short duration of the fluidrock/sediment interaction and the REE’s lesser susceptibility to leaching in low-temperature hydrothermal fluids than in high-temperature hydrothermal fluids, natural sulfides generally exhibit lower REE concentrations than metal sulfides.
(3) The sulfur isotopic compositions of both the natural sulfur and the metal sulfides are characterized by the similar feature to the underlying magma source, suggesting that the sulfur in this area might be originated from magmatic degassing. This hypothesis needs further support especially from geochemical evidence of the hydrothermal fluid in this area.
Acknowledgements
We gratefully acknowledge the support by the captains,crew, and scientific parties who participated on board the HOBAB3 cruise for indispensable cooperation in investigation and sampling the THF. This study was supported by the Natural Science Foundation of China(41606086), the Taishan scholar Special Experts Project(ts201712079), the National Key Research and Development Program (2017YFC0307704), and the Marine Geological Survey project of China Geological Survey (DD20160218,DD20160344).
- China Geology的其它文章
- Deep Continental Scientific Drilling Engineering Project in Songliao Basin: progress in Earth Science research
- Main technical innovations of Songke Well No.2 Drilling Project
- Preliminary results of environmental monitoring of the natural gas hydrate production test in the South China Sea
- Episodic crustal growth in the Tanzania Craton: evidence from Nd isotope compositions
- Exploration and research progress of shale gas in China
- Overview on hydrothermal and hot dry rock researches in China

