Development and Validation of a Reduced Chemical Kinetic Mechanism for HCCI Engine of Biodiesel Surrogate
XIAO Jie ZHANG Bo ZHENG Zhao-Lei,*
Development and Validation of a Reduced Chemical Kinetic Mechanism for HCCI Engine of Biodiesel Surrogate
XIAO Jie1ZHANG Bo2ZHENG Zhao-Lei1,*
(1;2)
A simplified mechanism of methyl decanoate and-heptane blend was developed for a homogeneous charge compression ignition engine built from a previously reported detailed mechanism of a methyl decanoate and-heptane. The simplified mechanism with 113 species and 306 reactions was developed using path flux and temperature sensitivity analyses. The simplified mechanism was validated against the experimental data of ignition delay time, in-cylinder pressure, and CO emissions. Results show that the simplified mechanism not only coincides with the ignition delay time of the methyl decanoate and n-heptane, but also the CO emissions, and can reproduce the variation of in-cylinder pressure. The simplified mechanism can be further validated by comparison with the detailed mechanism, which shows that the simplified mechanism can coincide with the in-cylinder pressure and temperature, and can reproduce the variation tendency of the core components. Thus, the reduced mechanism is a reasonable one.
Homogeneous charge compression ignition (HCCI); Biodiesel; Methyl decanoate;Heptane; Reduced mechanism
1 Introduction
As fossil fuel supplies dwindle, the use of biodiesel, a renewable alternative fuel, has been brought into focus. Biodiesel can reduce reliance onimported fossil fuels and contribute to environmentalpreservation by lowering greenhousegas emissions. Using biodiesel in diesel enginesdecreases the emissions of pollutants such as carbonmonoxide, unburned hydrocarbons, and particulate matter1–4.
Most biodiesel fuels are produced from mono-alkyl esters of long-chain fatty acids derived from vegetable oils and animal fats, and these very large molecules aretransformed into esters by reaction of trans-esterification with an alcohol5. Biodiesel is a multiple-component mixture that mainly consists of methyl palmitate (C17H34O2),methyl stearate(C19H38O2),methyloleate (C19H36O2),methyl linoleate (C19H34O2),and methyl linolenate (C19H32O2). Using a chemical kinetic model during simulation has been the most effective way to investigate biodiesel. However, forming a chemical kinetic model that contains all of these species is difficult. Thus, biodiesel surrogate, which is defined using a few simple components to match the characteristics of biodiesel, is a good choice to model real biodiesel.
The main species of biodiesel is methyloleate (C19H36O2) or methyllinoleate(C19H34O2). The present study shows that butyric acid methyl ester have the same chemical structure characteristics as methyl linoleate and methyl linolenate. Thus, methyl butyrate can effectively simulate the characteristics of oxidation of a methyl-ester type of material, and a detailed mechanism for the combustion of methyl butyrate was developed by Fisher6. The results show that the experimental data were lower than the model data by factors of 10 to 50. Vaughn7studied the combustion process of biodiesel droplets under the condition of microgravity. The ignition delay time of pure methyl (i.e. methyl butyrate, methyl decanoate, and methyloleate) and methyl ester of soybean oil were measured. The results show that methyl decanoate is a better biodiesel alternative blend than methyl butyrate; and Gaïl8drew the same conclusion. A detailed mechanism to oxidize methyl decanoate, which contained 3012 species and 8,820 elementary reactions, was developed by Herbinet.9; the model was then compared with the experiments of rapeseed oil in a jet-stirred reactor (JSR). The results show that methyl decanoate can reproduce the early carbon dioxide generation and combustion process of rapeseed oil. Referring to the work of Herbinet, Pasca10studied the combustion process of methyl decanoate using JSR on the base of Herbinet9. The results show that the mechanism of methyl decanoate reproduced the combustion process of biodiesel in good agreement with other types of methyl. A reduced mechanism that contained 713 reactions and 125 species was developed by Seshadri11using the directed relation graph method.
The combustion characteristic of a single species is similar to that of biodiesel in some areas, but two key points have to be emphasized. Biodiesel has the characteristic of high relative molecular mass and oxygen content of approximately 10%, but a single species does not. Moreover, a single species cannot match the ratio of C, H, and O of biodiesel. The method of using two components to replace biodiesel is adopted by most researchers because of the limitations of a single species.
Hakka12proposed a blend containing methyl palmitate and-decane (with a molar ratio of 26 : 74) to represent biodiesel. The experimental study was conducted in a JSR. The comparison of unsaturated species showed that the blend could not match biodiesel. Brakora13proposed a blend that contained methyl butyrate andHeptane, with a molar ratio of 1 : 2, to represent biodiesel. The oxygen content of the blend was 11% and the low heat value was 39 MJ·kg−1, both of which were almost identical to those of biodiesel.
Herbinet14proposed a blend containing methyl decanoate andHeptane to represent biodiesel, and a detailed mechanism containing 3787 species and 10217 reactions was formed by incorporating carbon double-bond reaction on the base of detailed mechanism of methyl decanoate. The calculation results were compared with rapeseed oil oxidation experiments in a JSR. The results showed that the model can reproduce the initial formation of CO2, also in the concentration of radical OH. A blend contains five components was proposed by Westbrook15. The mechanism consists of more than 4800 species and almost 20000 reactions. However, this detailed mechanism requires further validation to determine if the model can accurately define the oxidation of different biodiesel fuels. Referring to the work of Herbinet14, Pei16proposed a blend that contained methyl decanoate andheptane, with a molar ratio of 1 : 1, to represent biodiesel, and a detailed mechanism that contained 691 species and 3226 elementary reactions was formed. The ignition delay time of simulation coincided with the experiment in a shock tube.
A skeletal mechanism that consisted of 113 species and 399 reactions was developed by Harun17. The skeletal mechanism can reproduce pressure trends, and the difference with nitrogen oxides is restricted to 20%. Luo18developed a skeletal mechanism that consisted of 115 species and 460 reactions by reducing the tri-component detailed mechanism. The skeletal mechanism can predict the oxidation process of biodiesel. An19developed a similar tri-component skeletal mechanism with 112 species and 498 reactions. The skeletal mechanism can accurately predict the ignition delay time of biodiesel and predict the combustion characteristics of biodiesel.
A blend contained methyl decanoate andHeptane with a molar ratio of 1 : 1 was used to represent biodiesel by Pei16, and a detailed mechanism contained 691 species, 3226 elementary reactions was proposed. The blend not only can match of biodiesel in oxygen content, hydrocarbon ratio and low heat value requirements, but also can reproduce the combustion process of biodiesel. Because of the detailed mechanism contains too many species to coupled three-dimensional modeling, it is necessary to develop a reduced mechanism. Using sensitivity analysis and path flux analysis, the present study obtained a reduced mechanism that contained 113 species and 306 elementary reactions.
2 Development of reduced kinetic model
Path flux analysis and sensitivity analysis methods were adopted in current study. Temperature sensitivity analysis was carried out to retain those elementary reactions that have strong influence on the temperature. Path flux analysis was conducted to retain the principal species and remove unimportant species.
Path flux analysis method was proposed by Sun20. First, production fluxes and consumption fluxes were introduced. The production fluxesA(production fluxes of species A) and consumption fluxesA(consumption fluxes of species A) of speciesA can be calculated as follows:

And the flux of species A related with species B can be calculated as:
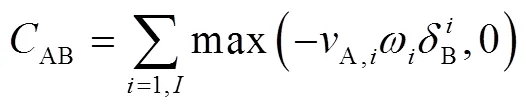
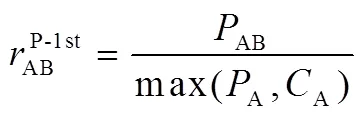
For the second and third generation are defined similiar as first generation. Path flux analysis method is using interaction coefficients to identify the important reaction pathways. Those pathways that have large interaction coefficients are important, and have small interaction coefficients are unimportant.
Sensitivity analysis investigates the effect of parameter change on the solution of mathematical models. In chemical kinetics, models are usually based on differential equations and results are concentration-time curves, reaction rates, and various kinetic features of the reaction21. It will be known the effect of an elementary reaction on the solution by using sensitivity method. The sensibility is defined as:
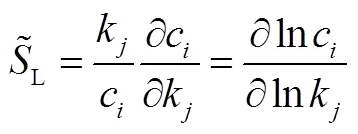
The parameters of the engine used in this study were similar to those in the work of Pei.16, as shown in Table 1, and those parameters were kept constant during the simulation. The relative molecular mass, mass fraction of oxygen and low heat value of biodiesel and MD-NH (methyl decanoate andheptane were mixed with a molar ratio of 1 : 1) are given in Table 2, thus, methyl decanoate andheptane were mixed with a molar ratio of 1 : 1 to be close to biodiesel.
2.1 Reaction path flux analysis
Pei16have carried out a research on the reaction path of biodiesel at low temperature, and Zhang22have carried out a similar research at high temperature. The main reaction process of biodiesel fuel surrogate can be known according to these two reaction path. The same reactants may be obtained in different ways; thus, elementary reactions that have a strong influence or sensitivity to reactants are retained, and those that have a weak influence or sensitivity to reactants are removed. Different intermediate species are introduced after introducing elementary reactions. The path of consumption and acquisition of additional intermediate species need to be analyzed to make the model form a closed cycle.
Methyl decanoate andheptane are oxidized by an oxygen molecule and form radical HO2. The relevant elementary reaction is as follows:
R615 MD + O2= MDMJ + HO2,
R627 MD + O2= MD2J + HO2,
R3022C7H16+ O2= C7H15-1 + HO2
Radical H, OH, and HO2gradually increase along with reaction development. Thus, these radicals accelerate the reaction of fuel dehydrogenation. The main elementary reaction is as follows:
R612 MD + H = MDMJ + H2,
R613 MD + HO2= MDMJ + H2O2,
R614 MD + OH = MDMJ + H2O,
R622 MD + H = MD2J + H2,
R623 MD + HO2= MD2J + H2O2,
R3019C7H16+ H = C7H15-1 + H2,
R3020C7H16+ OH = C7H15-1 + H2O,
R3021C7H16+ HO2= C7H15-1 + H2O2.
2.1.1 MDMJ consumption path flux analysis
The MDMJ is mainly consumed by heterogeneous reaction R928. The relevant elementary reaction is as follows:
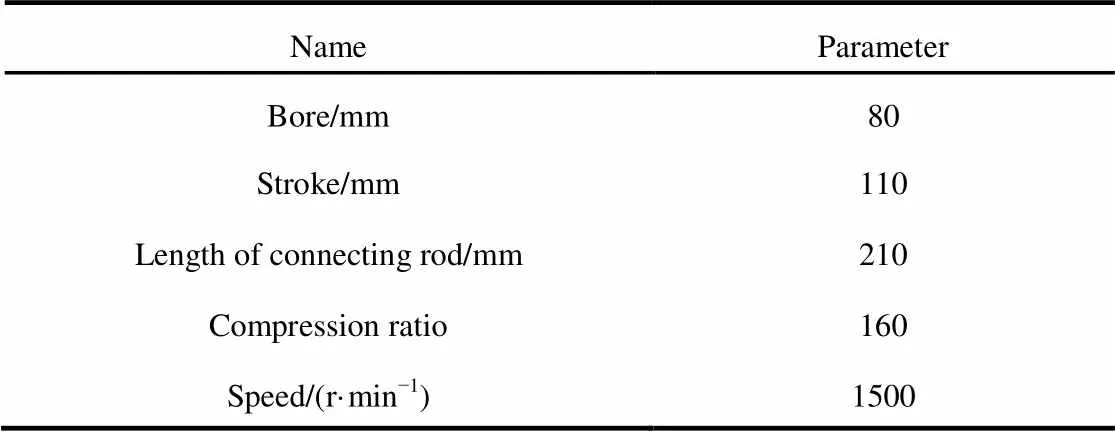
Table 1 HCCI engine parameters.

Table 2 Comparison table of biodiesel and MD-NH.
MD-NH: methyl decanoate andheptane were mixed with a molar ratio of 1 : 1.
R928 MDMJ = MD2J.
2.1.2 MD2J consumption path flux analysis
As shown in Fig.122, MD2J is mainly consumed by the following reactions:
R3084 C7H15O2-3 + MD2J = C7H15O-3 + MD2O,
R741 C7H15-1 + MP2D = MD2J,
R849 MD2J + O2= MD2D + HO2,
R2455 MD2J + O2= MD2O2.
MD2O reacts with CH3O, as a result, MD2O2is formed. MD2O2turn into MD2OOH4J through heterogeneous reaction R2490. Then, MD2OOH4J is oxidized by oxygen, MDKET24 is formed. The MDKET24 is consumed by two consecutive decomposition reactions. The relevant elementary reaction is as follows:
R2490 MD2O2= MD2OOH4J,
R2593 MD2OOH4J + O2= MD2OOH4O2,
R2624 MD2OOH4O2= MDKET24 + OH,
R2655 MDKET24 = MDKET24O + OH.
MDKET24O is decomposed into C6H13CHO and MP2OXO3J through reaction R2686. C6H13CHO is consumed by radical O2, OH, and CH3O2, then C6H13CO was formed. Subsequently C6H13CO turn into C2H5and C2H4through three consecutive decomposition reactions. MP2OXO3J is decomposed into CH2CO and CH3OCO. The relevant elementary reaction is as follows:
R2686 C6H13CHO + MP2OXO3J = MDKET24O,
R2982 C6H13CHO + CH3O2= C6H13CO + CH3O2H,
R2975 C6H13CHO + O2= C6H13CO + HO2,
R825 C6H13-1 + CO = C6H13CO,
R2976 C6H13CHO + OH = C6H13CO + H2O,
R764 C2H4+ PC4H9= C6H13-1,
R475 PC4H9= C2H5+ C2H4,
R2708 CH2CO + CH3OCO = MP2OXO3J.
MD2D, which is the product of reaction R849, decomposes into MD3D2J and MF24D.MF24D reacts with H atom, as a result, MF4D2J and MF4D3J are formed. MF4D2J and MF4D3J turn into MF4DMJ through a heterogeneous reaction. MF4DMJ decomposes into CH2O and C4H7CO. The relevant elementary reaction is as follows:

Fig.1 Consumption rate of MD2J.
R2002 MD2D + OH = MD3D2J + H2O,
R2215 H + MF24D = MF4D2J,
R2217 H + MF24D = MF4D3J,
R2354 MF4DMJ = MF4D3J,
R2355 MF4D2J = MF4D3J,
R2213 CH2O + C4H7CO = MF4DMJ.
2.1.3 C7H15-1 consumption path flux analysis
C7H15-1 is oxidized by an oxygen molecule, and C7H15O2-3 is formed. C7H15O2-3 reacts with oxygen, and C7KET12 is obtained. Then, C7KET12 decomposes into C5H11-1. An oxygen molecule is subsequently added to C5H11-1, thereby forming C5H11O2-1. Then, C5H11O2-1 turn into C5H10OOH1-3 through a heterogeneous reaction, and C5H10OOH1-3 is consumed by decomposing reaction. The relevant elementary reaction is as follows:
R3023 C7H15-1 + O2= C7H15O2-3,
R3025 C7KET12 = C5H11CO + CH2O + OH,
R828 C5H11-1 + CO = C5H11CO,
R2445 C5H11-1 + O2= C5H11O2-1,
R2475 C5H11O2-1 = C5H10OOH1-3,
R2563 C5H10OOH1-3 = C4H8-1 + CH2O + OH.
Fuel is oxidized to small CH2O molecules through the reaction discussed in the preceding paragraphs. CH2O is oxidized to CO2during the high temperature stage. Combined with the reaction path of high temperature, the relevant elementary reaction is as follows:
R54 CH2O + OH = HCO + H2O,
R220 CH2CHO + O2= CH2O + CO + OH,
R3041 C2H4+ OH = CH2O + CH3,
R40 HCO + O = CO + OH,
R41 HCO + O2= CO + HO2,
R34 CO + O2= CO2+ O,
R35 CO + OH = CO2+ H.
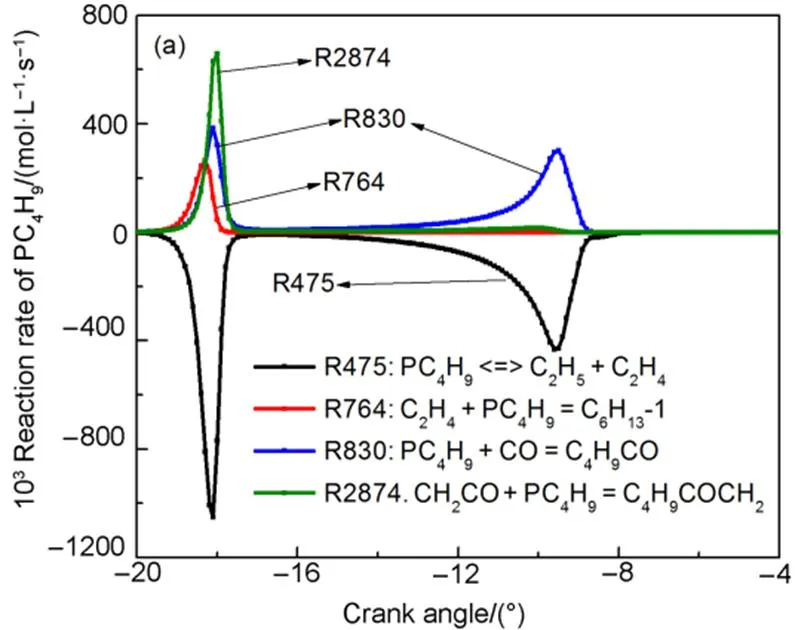
Based on the path flux analysis above, new species, which include PC4H9(reaction R475), CH3O2H (reaction R2982), and C4H7CO (reaction R2213), are added. Reaction rate analysis method was adopted in order to obtain important reactions of those species. As shown in Fig.2, the important reactions of PC4H9are R475, R764, R830, and R2874, the important reactions of CH3O2H are R123, R127, and R133. The important reactions of C4H7CO are R2213 and R2332.
Based on the preceding discussion, a reduced mechanism consists of 296 reactions and 109 species was obtained. Although the majority of reactions were added into the reduced mechanism by path flux analysis, a completely reduced mechanism of a biodiesel fuel surrogate cannot be formed. Further improvement of the mechanism can be achieved using temperature sensitivity analysis.
2.2 Temperature sensitivity analysis
Further improvement of the mechanism is required so that the reduced mechanism can have the same scope as the detailed mechanism. Temperature has a significant influence on the combustion process, so temperature sensitivity analysis method is adopted. Those reactions, which have a strong influence on the temperature will be analyzed, by changing initial pressure, speed, equivalence ratios, and initial temperature of the cylinder. The operating conditions are presented in Table 3.
Figs.3 to 6 show the results of temperature sensitivity analysis of different operating conditions.
Figs.3 to 6 show that 17 reactions have a strong influence on cylinder temperature, and the reactions are given in Table 4. Reactions R24, R29, R624, R627, R741, R828, R849, R3022, R3023, and R3084 have contained in the reduced mechanism. Thus, seven reactions were added into the reduced mechanism.
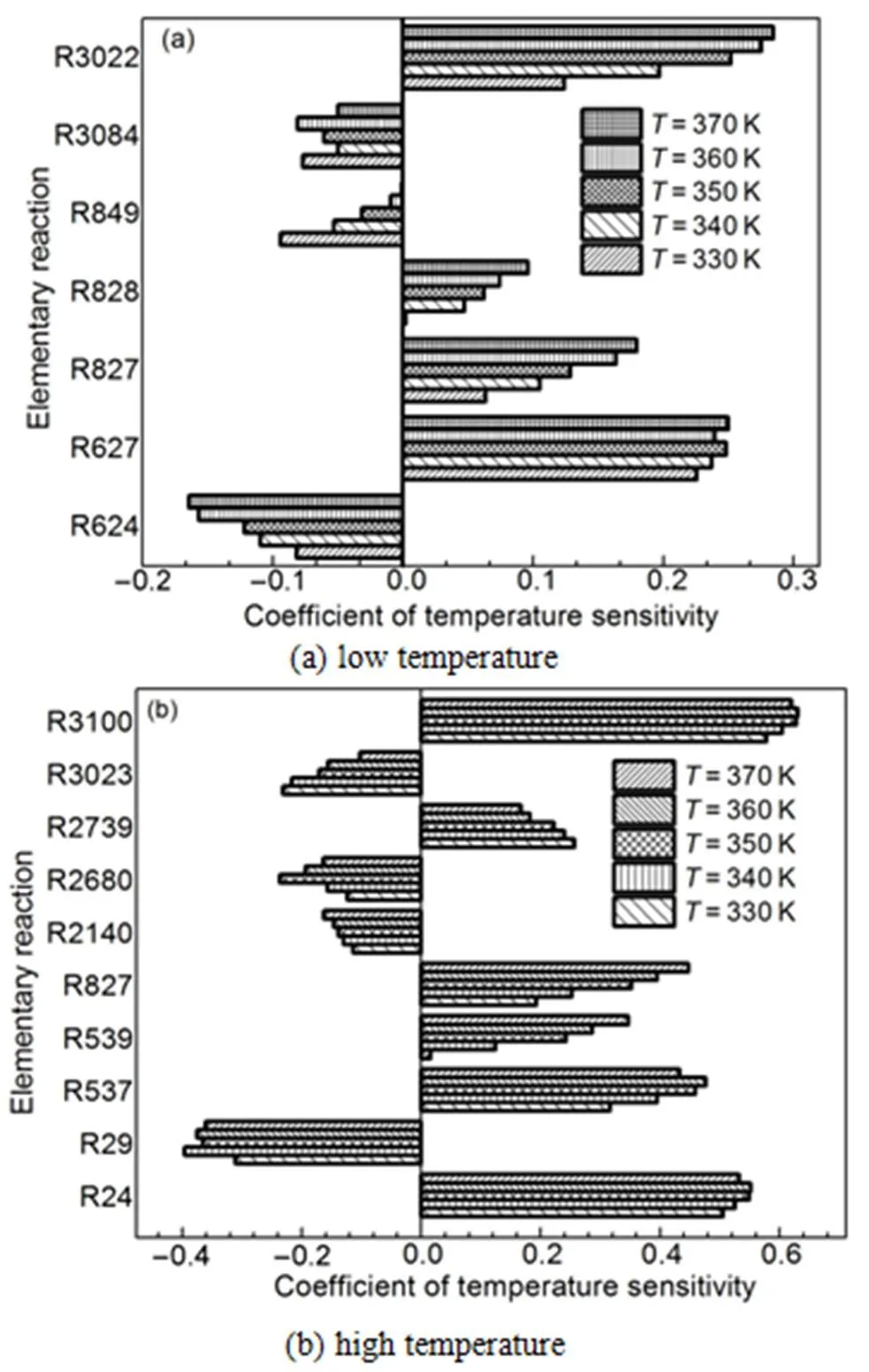
Fig.3 Temperature sensitivity analysis of different temperatures.
Based on the reaction R3100, a new species of C4H9CHO is added into the reduced mechanism during the temperature sensitivity analysis. The main pathway of consumption and generation is illustrated in Fig.7. Relevant elementary reactions are R3100 and R2992.
R3100 C2H5+ C4H9CHO = C7H15O-3,
R2992 C4H9CHO + OH = C4H9CO + H2O.
A completely simplified mechanism contains 113 species, and 306 elementary reactions were obtained by adopting the sensitivity analysis and path flux analysis methods. The reaction path of MD andC7H16are shown in Fig.8.
3 Validation of reduced mechanism
To validate the simplified mechanism under HCCI engine combustion conditions, ignition delay time of the simplified m echanism is compared with that of thedetailed mechanism, and the simulation results of the simplified mechanism are compared with the experimental results.

Table 3 HCCI Engine operating conditions.

Fig.4 Temperature sensitivity analysis of different pressures.

Fig.5 Temperature sensitivity analysis of different equivalence ratios.
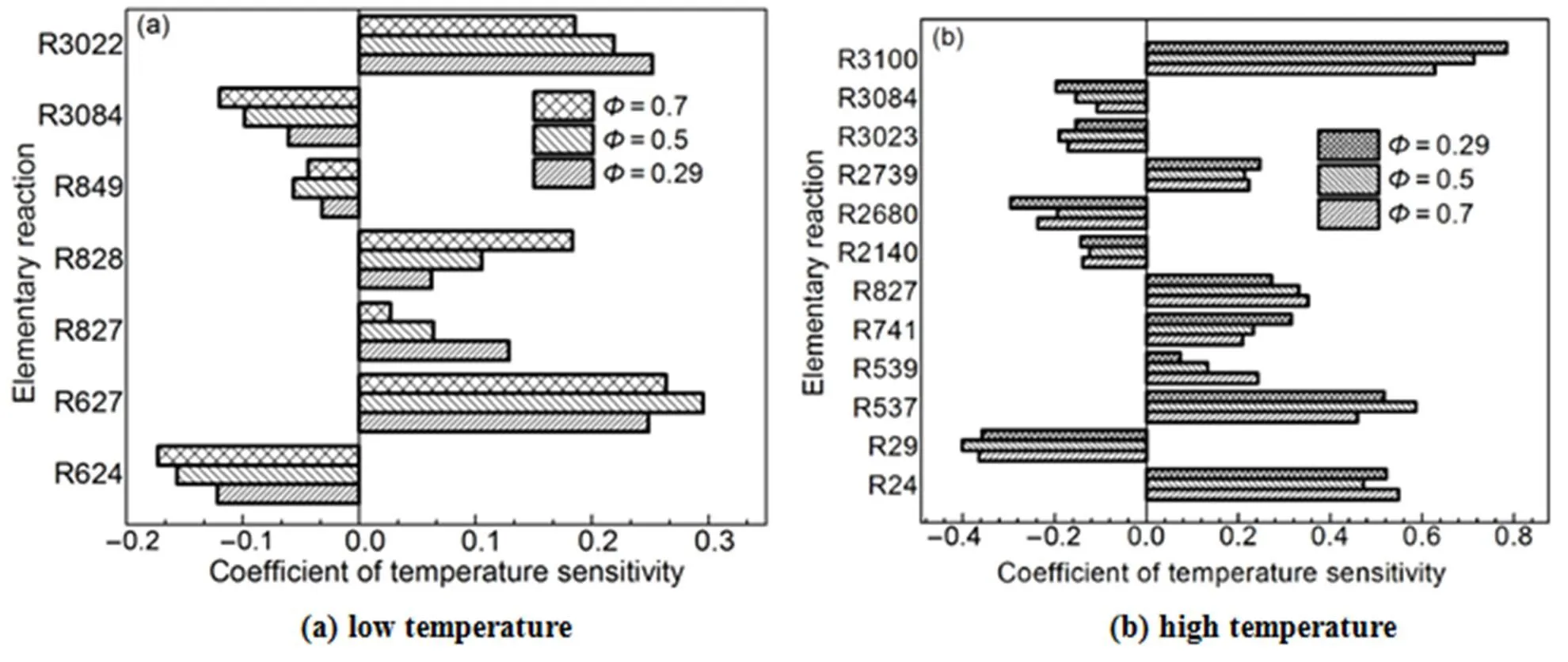
Fig.6 Temperature sensitivity analysis of different speeds.
3.1 Validation of ignition delay time
A closed homogeneous batch reactor of CHEMKIN-PRO is adopted to validate ignition delay ties. The simulation is implemented for zero-dimensional combustion modeling, adiabatic and concentrations are uniform in the chamber, and the influence of flow is ignored, moreover, the reactor has a constant volume. When the simulations are conducted, ignition delay times are defined as the times when the mixture needs to reach a temperature of 400 K above the initial temperature23.
Fig.9 shows that the ignition delay time of-heptane of the simplified mechanism and the detailed mechanism at different pressures and different equivalent ratios. Both simulation results are compared with experimental data24in the shock tube. The results show that the simplified mechanism is sensitive to temperature variations, and coincides with the detailed mechanism in the negative temperature coefficient region.
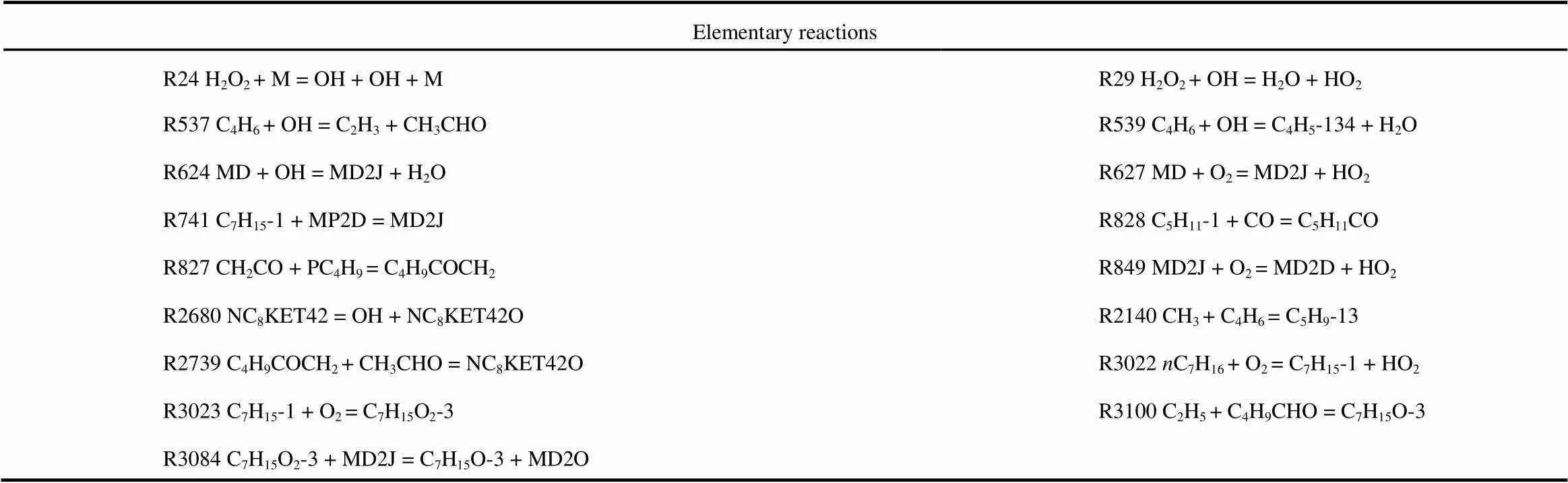
Table 4 Temperature sensitivity analysis results.
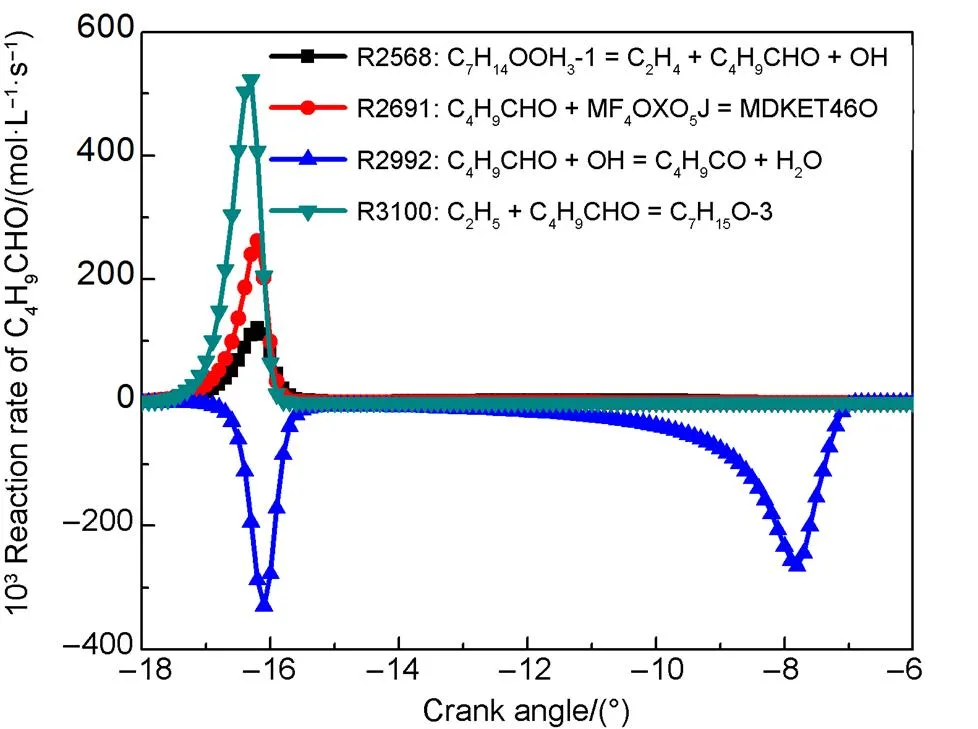
Fig.7 Reaction rate analysis of C4H9CHO.

Fig.8 Reaction path of MD and nC7H16.
These graphs show that the simplified mechanism coincides with the experimental data of ignition delay time, in-cylinder pressure and the emission. The simplified mechanism coincides with the detailed mechanism in the variation tendency of ignition delay times when the pressure, temperature, and equivalent ratio vary. Therefore, the simplified mechanism is reasonable.
3.2 Comparison of simplified mechanism and detailed mechanism
The simplified mechanism is further validated through comparison with the detailed mechanism under different conditions. In the calculation process, the engine parameters are identical to those in Table 1. Zero-dimensional and single-area models were adopted. The details are presented in Table 5.
3.2.1 Comparing computed data at different initial temperatures
Fig.13 shows the in-cylinder pressure and in-cylinder temperature simulation results of the simplified mechanism at different initial temperatures, and compared with those of the detailed mechanism. The results show that the simplified mechanism coincides with the detailed mechanism in the firing process. Firing can occur ahead of time and the cylinder temperature rises as the initial temperature increases.
Fig.14 shows the main species mole fraction simulation results of the simplified mechanism at different initial temperature, and compared with those of the detailed mechanism. The mole fraction of main species of the simplified mechanism is slight lower than that of the detailed mechanism. However, the simplified and detailed mechanisms have the same tendency; thus, the simplified mechanism is reasonable.
3.2.2 Comparison of computed data at different initial pressures
Fig.15 shows the in-cylinder temperature and in-cylinder pressure simulation results of the simplified mechanism at different initial pressures, compared with those of thedetailed mechanism. The simplified mechanism shows good agreement with the detailed mechanism. The cylinder pressure rise and firing can occur ahead of time, and temperature seldom changes when the initialpressure increases.

Fig.9 Ignition delay chart of n-heptane.
(a)= 0.5; (b)= 05; (c)= 1.0; (d)= 1.0.
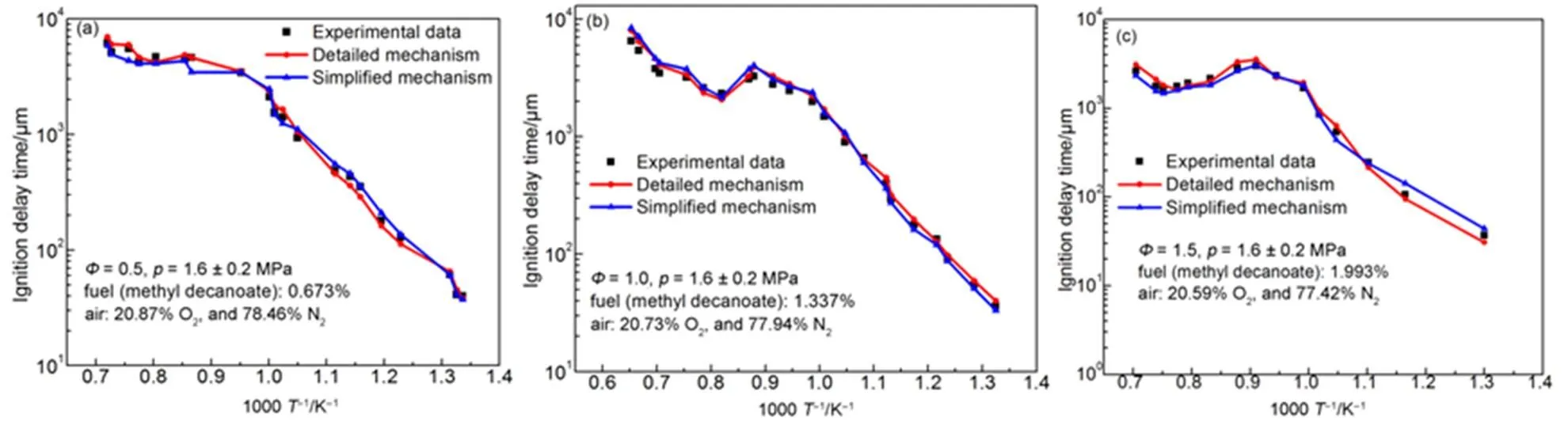
Fig.10 Ignition delay chart of methyl decanoate.

Fig.11 In-cylinder pressure chart of MD-NH.

Fig.12 Comparison of CO emission at different speed.
Table 5 HCCI engine operating conditions

Fig.13 Comparison of in-cylinder temperature and in-cylinder pressure at different initial temperatures.
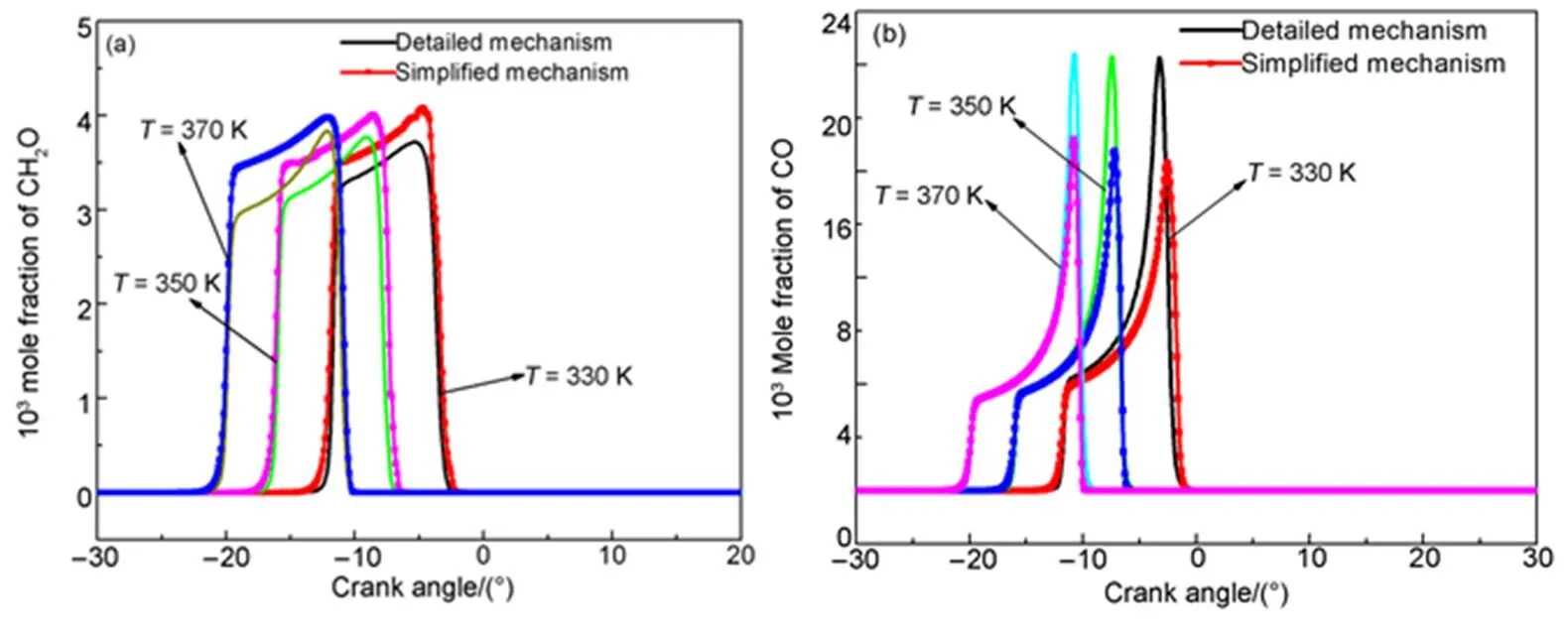
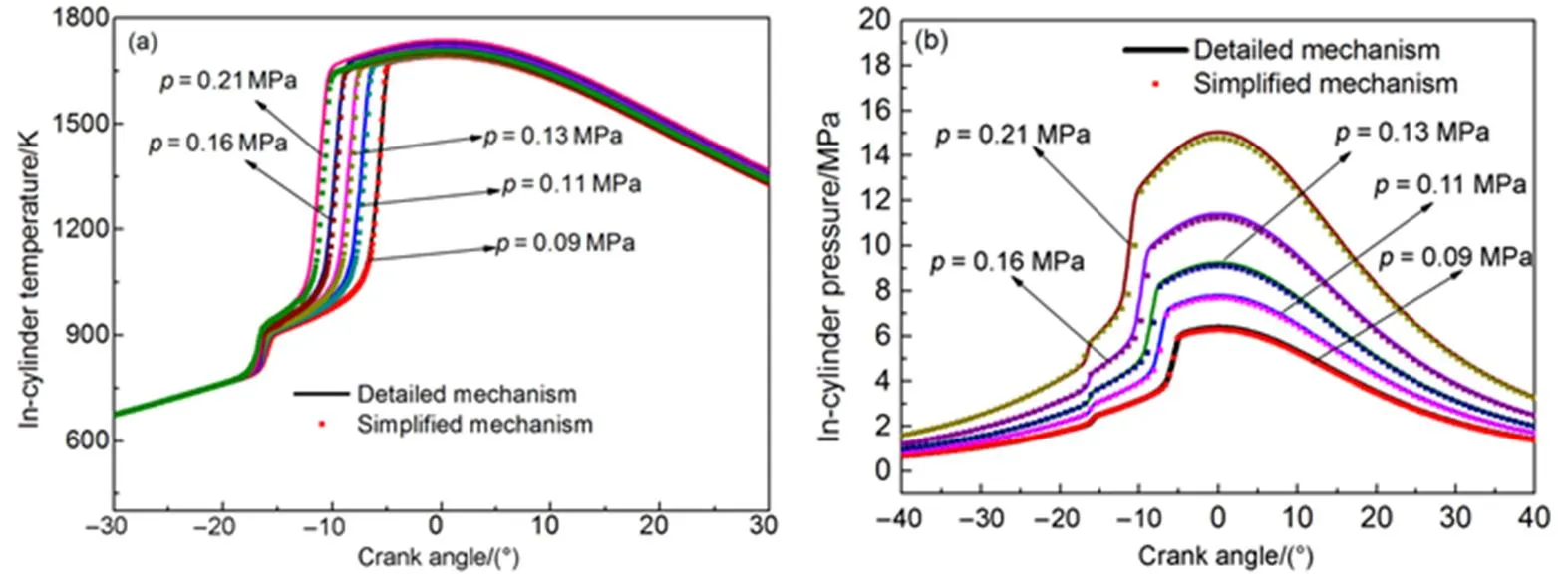
Fig.15 Comparison of in-cylinder temperature and in-cylinder pressure at different initial pressures.

3.2.3 Co mparison of computed data at different equivalence ratios
Fig.17 shows the in-cylinder temperature and in-cylinderpressure simulation results of the simplified mechanism at different equivalence ratios, compared with those of the detailed mechanism. The cylinder temperature rises when the equivalent ratio increases, and the simplified mechanism coincides with the detailed mechanism.
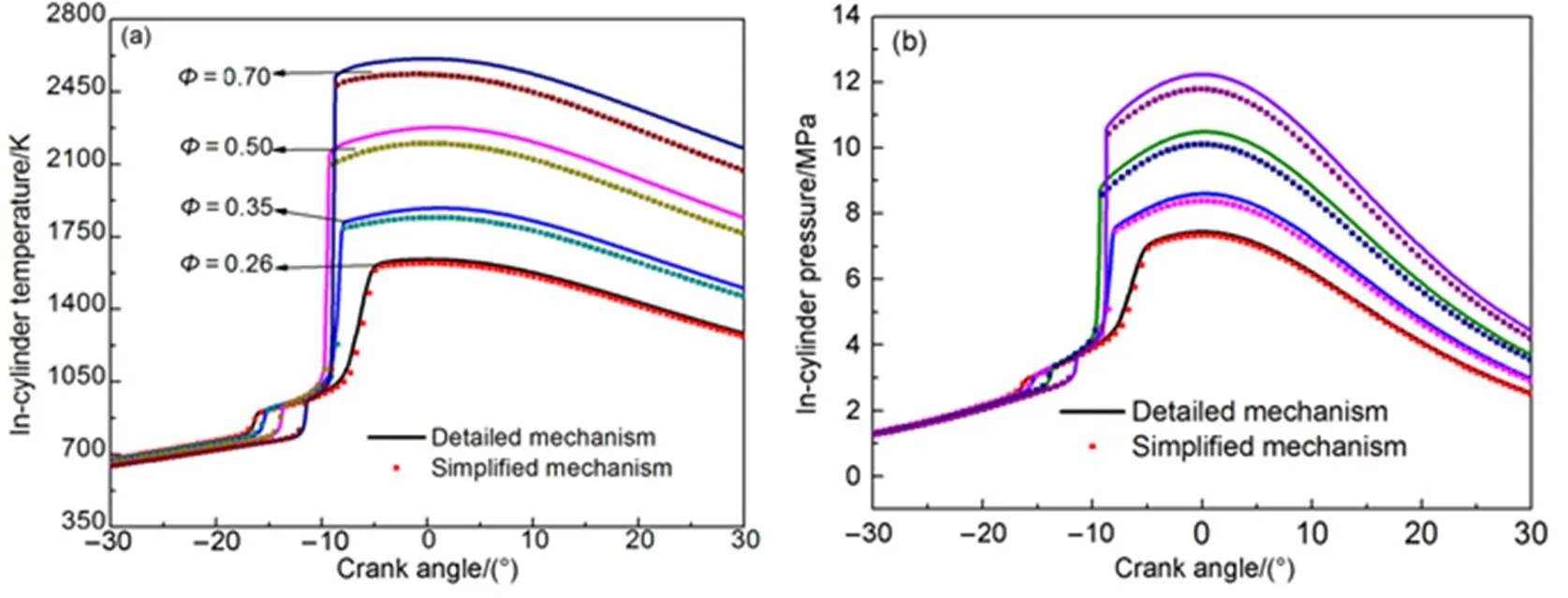
Fig.17 Comparison of temperature and pressure at different equivalence ratios.

From what we have discussed above, we can draw the conclusion that the simplified mechanism can reproduce the combustion process of the detailed mechanism at different initial pressures, different initial temperatures, and different equivalence ratios. Thus, the simplified mechanism is reasonable.
4 Conclusions
A simplified mechanism with 113 species and 306 reactions was obtained through temperature sensitivity analysis and path flux analysis methods. The simplified mechanism was validated by comparing with the detailed mechanism and the experimental data at different conditions, and both of them prove that the simplified mechanism is reasonable. Specific conclusions are as follows:
(1) Firing can occur ahead of time, cylinder temperature rises, and pressure changes occasionally while the initial temperature is increasing.
(2) Firing can occur ahead of time, cylinder pressure rises, and temperature changes occasionally while the initial pressure is increasing.
(3) Cylinder temperature and pressure rise while the equivalence ratio is increasing.
(4) The simplified mechanism agrees well with the detailed mechanism in temperature and pressure and varies the tendency of the main species in the cylinder. Therefore, the simplified mechanism is reasonable.
Supporting Information:available free of chargethe internet at http://www.whxb.pku.edu.cn.
(1) Tsolakis, A.; Megaritis, A.; Wyszynski, M. L.; Theinnoi, K.2007,(11), 2072. doi: 10.1016/j.energy.2007.05.016
(2) Tat, M. E.; Wang, P. S.; Van Gerpen, J. H.; Clemente, T. E.2007,(19), 865. doi: 10.1007/s11746-007-1109-6
(3) Mccormick, R. L.; Ross, J. D.; Graboski, M. S.1997,(4), 1144. doi: 10.1021/es9606438
(4) Westbrook, C. K.; Pitz, W. J.; Curran, H. J.2006,(21), 6912. doi: 10.1021/jp056362g
(5) Cai, F.; Zhang, B. B.; Lin, J.; Zhang, G. Y.; Fang, W. P.; Yang, L. F.2008,(10), 1817. [蔡 钒, 张彬彬, 林 静, 张国玉, 方维平, 杨乐夫. 物理化学学报, 2008,(10), 1817.] doi: 10.3866/PKU.WHXB20081014
(6) Fisher, E. M.; Pitz, W. J.; Curran, H. J.; Westbrook, C. K.2000,(2), 1579. doi: 10.1016/S0082-0784(00)80555-X
(7) Timothy, V.; Matthew, H.; Michael, H.2006,doi: 10.4271/2006-01-3302
(8) Gaïl, S.; Thomson, M. J.; Sarathy, S. M.; Syed, S. A.; Dagaut, P.; Diévart, P.; Marchese, A. J.; Dryer, F. L.2007,(1), 305. doi: 10.1016/j.proci.2006.08.051
(9) Herbinet, O.; Glaude, P. A.; Warth, V.; Battin-Leclerc, F.2011,(7), 1288. doi: 10.1016/j.combustflame.2010.11.009
(10) Diévart, P.; Won, S. H.; Dooley, S.; Dryer, F. L.; Ju, Y. G.2012,(5), 1793. doi: 10.1016/j.combustflame.2012.01.002
(11) Seshadria, K.; Lu, T. F.; Herbinet, O.; Humer, S.; Niemann, U.; Pitz, W. J.; Seiser, R.; Law, C. K.. 2009,(1), 1067. doi: 10.1016/j.proci.2008.06.215
(12) Hakka, M. H.; Glaude, P.; Herbinet, O.; Battin-Leclerc, F.2009,(11), 2129. doi: 10.1016/j.combustflame.2009.06.003
(13) Brakora, J. L.; Ra, Y.; Reitz, R. D.; McFarlane, J.; Daw, C. S.2008,doi: 10.4271/2008-01-1378
(14) Herbinet, O.; William, J. P.; Charles, K. W.2008,(3), 507. doi: 10.1016/j.combustflame.2008.03.003
(15) Westbrook, C. K.; Naik, C. V.; Herbinet, O.; Pitz, W. J.; Mehl, M.; Sarathy, S. M.2011,(4), 742. doi: 10.1016/j.combustflame.2010.10.020
(16) Pei, Y. Q.; Zheng, Z. L.; Zhang, B.2014,(2), 217. [裴毅强, 郑朝蕾, 张 博, 物理化学学报, 2014,(2), 217.] doi: 10.3866/PKU.WHXB201312102
(17) Harun, M. I.; Kiat, N. H.; Suyin, G.; Tommaso, L.; Angelo, O.2013,, 388. doi: 10.1016/j.fuel.2012.10.015
(18) Luo, Z.; Plomer, M.; Lu, T. F.; Som, S.; Longman, D. E.; Sarathy, S. M.; Pitz, W. J.2012,, 143. doi: 10.1016/j.fuel.2012.04.028
(19) An, H.; Yang, W. M.; Maghbouli, A.; Li, J.; Chua, K. J.2014,51. doi: 10.1016/j.enconman.2014.02.012
(20) Sun, W.; Chen, Z.; Gou, X.; Ju, Y.2010,(7), 1298. doi: 10.1016/j.combustflame.2010.03.006
(21) Susnow, R. G.; Dean, A. M.; William, H. G.; Peczak, P.; Broadbelt, L. J.1997,(20), 3731. doi: 10.1021/jp9637690
(22) Zhang, B. An Investigation on Surrogate Mixture and its Chemical Kinetic Models of Bio-diesel Fuel. M.S. Dissertation, Chongqing University, Chongqing,2013. [张 博. 生物柴油替代混合物及其化学动力学模型研究[D]. 重庆: 重庆大学, 2013.]
(23) Zheng, Z. L.; Lv, Z. M.2015,(1), 59.doi: 10.1016/j.apenergy.2015.01.062
(24) Ciezki, H. K.; Adomei, G.1993,(4), 421. doi: 10.1016/0010-2180(93)90142-P
(25) Wang, W. J.; Oehlschlaeger, M. A.2012,(2), 476. doi: 10.1016/j.combustflame.2011.07.019
(26) Cheng, X. B.; Chen, D. L.; Ju, H. L.2008,, 46. [成晓北, 陈德良, 鞠洪玲. 汽车技术, 2008, No. 1, 46.]
适用于HCCI的生物柴油替代混合物简化机理的构建及验证
肖 杰1张 博2郑朝蕾1,*
(1重庆大学低品位能源利用技术及系统教育部重点实验室,重庆 400044;2北京汽车动力总成有限公司,北京 101100)
在正庚烷以及葵酸甲酯混合物详细机理的基础上, 提出了一个适用于均质压燃发动机的生物柴油简化机理模型。该模型包含113种物质以及306个反应, 通过路径分析以及温度敏感性分析方法得到。采用实验所得的燃料着火延迟时间、发动机缸内压力以及CO的排放来验证该简化机理的有效性。结果显示简化机理模型同正庚烷以及葵酸甲酯的着火延迟时间保持一致, 并且能有效模拟缸内的压力变化过程以及CO的排放。为了进一步验证简化机理的有效性, 将简化机理计算结果同详细机理进行对比。结果显示简化机理的缸内压力、缸内温度以及主要物质的变化趋势同详细机理保持一致。因此, 简化机理是合理的。
HCCI;生物柴油;葵酸甲酯;正庚烷;简化机理
O643
10.3866/PKU.WHXB201704273
January 12, 2017;
April 5, 2017;
April 27, 2017.
. Email: zhengzhaolei@cqu.edu.cn; Tel: +86-23-6510 2473; Fax: +86-23-6510 2473.
The project was supported by the National Natural Science Foundation of China Program (51376202).
国家自然科学基金资助项目(51376202)
- 物理化学学报的其它文章
- First-Principles Study: the Structural Stability and Sulfur Anion Redox of Li1−xNiO2−ySy
- Efficient Synthesis of Sulfur and Nitrogen Co-Doped Porous Carbon by Microwave-Assisted Pyrolysis of Ionic Liquid
- OL负载Cu催化剂及其催化氧化CO及乙酸乙酯性能
- Combined Effects of the Hole and Twin Boundary on the Deformation of Ag Nanowires: a Molecular Dynamics Simulation Study
- Cobalt@cobalt Carbide Supported on Nitrogen and Sulfur Co-Doped Carbon: an Efficient Non-Precious Metal Electrocatalyst for Oxygen Reduction Reaction
- S掺杂促进Fe/N/C催化剂氧还原活性的实验与理论研究

