小视野高分辨DWI对胰腺实性病变的鉴别诊断价值
李晶 马超 边云 王馨蕊 史张 王莉 邵成伟 陈士跃 陆建平
小视野高分辨DWI对胰腺实性病变的鉴别诊断价值
李晶 马超 边云 王馨蕊 史张 王莉 邵成伟 陈士跃 陆建平
目的探讨小视野高分辨扩散加权成像(rFOV DWI)对胰腺实性占位的鉴别诊断价值。方法收集139例胰腺实性占位患者,其中胰腺导管腺癌(PDAC)105例,神经内分泌肿瘤(NET)16例,肿块型慢性胰腺炎(MFCP)7例,实性假乳头状瘤(SPT)11例。招募38名健康成年志愿者作为对照组。行包括单次激发平面回波成像(ss-EPI)DWI、rFOV DWI(b值为0和600 s/mm2)的MRI检查。采用四分法从解剖结构的可视性、胰腺病灶对比度、运动及磁敏感伪影3方面评估rFOV DWI及ss-EPI DWI图像质量,通过工作站自带软件测量感兴趣区(ROI)的表观扩散系数(ADC)值。比较两种DWI的图像质量及ADC值在各胰腺疾病及正常胰腺间的差异。绘制ADC 值的受试者工作特征(ROC)曲线,评价PDAC与其他胰腺良性肿块及正常胰腺的差异。结果b值为0和600 s/mm2的rFOV DWI在显示胰腺解剖结构、病灶对比度、伪影评分均优于ss-EPI DWI(b=0 s/mm2时为2.99±0.51比2.79±0.64、2.37±0.48比1.81±0.63、3.17±0.56比2.91±0.60;b=600 s/mm2时为3.63±0.50比3.32±0.56、3.45±0.50比3.01±0.49、3.74±0.44比3.12±0.37),差异均有统计学意义(P值均<0.001)。PDAC、NET、MFCP、SPT、正常胰腺rFOV DWI获得的ADC值分别为(1.38±0.17)×10-3、(1.22±0.35)×10-3、(1.29±0.13)×10-3、(1.04±0.38)×10-3、(1.86±0.15)×10-3mm2/s;ss-EPI DWI的ADC值分别为(1.73±0.24)×10-3、(1.63±0.39)×10-3、(1.58±0.19)×10-3、(1.25±0.26)×10-3、(2.04±0.20)×10-3mm2/s,各组间的差异及同组内两ADC值间的差异均有统计学意义(P值均<0.001)。MFCP与PDAC、NET与MFCP、MFCP与SPT间rFOW DWI的ADC值的差异及MFCP与PDAC、PDAC与NET、SPT与MFCP间ss-EPI DWI的ADC值差异无统计学意义,而其他两两组间差异均有统计学意义(P值均<0.05)。PADC与正常胰腺rFOV DWI和ss-EPI DWI的ROC曲线下面积(AUC)分别为0.983(95%CI0.944~0.998)和0.889(95%CI0.822~0.936),差异有统计学意义(P=0.0004),而PDAC与所有良实性病变的rFOV DWI和ss-EPI DWI的ADC值的AUC分别为0.799(95%CI0.719~0.864)和0.755(95%CI0.672~0.827),差异无统计学意义。结论rFOV DWI显著提高DWI图像质量,并且对胰腺导管腺癌的诊断效能更佳。
胰腺肿瘤; 磁共振成像; 诊断,鉴别
磁共振扩散加权成像(diffusion-weighted imaging, DWI)通过定量计算出表观扩散系数(apparent diffusion coefficient, ADC)从而反映组织内水分子扩散快慢。临床DWI应用中使用最为广泛的是单次激发平面回波成像(single-shot echo-planar-imaging, ss-EPI)序列,其具有成像速度快、对运动不敏感等优点,但与常规MRI结构图像(如T1、T2图像)相比,DWI因受到偏共振和磁敏感效应的影响而导致图像分辨率偏低。小视野(reduced field-of-view, rFOV)技术可以实现高分辨DWI,最初应用于脊髓成像[1]。有研究报道,rFOV在胰腺成像中能提高DWI图像质量并减少伪影,但研究的样本量较小且未见rFOV DWI对不同类型胰腺实性占位诊断的应用价值报道[2-4]。本研究通过rFOV DWI与ss-EPI DWI图像质量及ADC值的对比研究,明确rFOV DWI 在胰腺实性占位诊断中的应用价值。
资料与方法
一、病例资料
研究为前瞻性设计,经长海医院伦理委员会批准,所有参与者均签署知情同意书。收集2014年2月至2016年1月间经CT或者B超检查怀疑胰腺实性占位的住院患者139例。所有患者检查前均未行放疗或化疗,并在长海医院行MR检查后1周内手术治疗,有明确的病理检查结果。同期招募38名健康志愿者作为对照组。
139例胰腺实性占位患者中男性86例,女性53例,平均年龄(57±12)岁。其中胰腺导管腺癌(pancreatic ductal adenocarcinomas,PDAC)105例,男性69例,女性36例,平均年龄(61±9)岁;神经内分泌肿瘤(neuroendocrine tumor,NET)16例,男、女各8例,平均年龄(51±8)岁;肿块型慢性胰腺炎(mass-forming chronic pancreatitis,MFCP)7例,均为男性,平均年龄(51±9)岁;实性假乳头状瘤(solid pseudopapillary tumor,SPT)11例,男性2例,女性9例,平均年龄(32±9)岁。38名健康志愿者中男性26名,女性12名,年龄(49±11)岁。
二、MRI扫描方法
MRI检查均在同一台3-T磁共振仪上完成。所有受检者均于检查前禁食6 h以上并接受呼吸训练。被检者采取仰卧位、脚先进体位,行包括胰腺横断T1加权像(T1WI)、T2加权像(T2WI)、磁共振胰胆管造影(MRCP)、动态增强、ss-EPI DWI、rFOV DWI在内的胰腺MRI扫描。横断面呼吸触发T2WI序列:重复时间(TR)6 316 ms,回波时间(TE)72 ms,层厚5 mm,视野(FOV)380 mm×400 mm,矩阵320×192;肝脏容积加速采集(liver acquisition with volume acceleration,LAVA)序列:TR 2.5 ms,TE 1.1 ms,层厚5 mm,FOV 440 mm×418 mm,矩阵256×180;厚层块2D-MRCP序列:TR 7 000 ms,TE 1 221 ms,层厚54 mm,FOV 300 mm×300 mm,矩阵288×288。ss-EPI WDI序列:扩散梯度因子(b)值为0、600 s/mm2,TR 6 000 ms,TE 58.6 ms,层厚 5 mm,FOV 380 mm×400 mm,矩阵128×96;rFOV DWI序列:b值为0、600 s/mm2,TR 5 000 ms,TE 58.6 ms,层厚5 mm,FOV 160 mm×80 mm,矩阵128×64。rFOV DWI扫描层数为6层。患者组最后还要经静脉注射钆喷酸葡胺注射液0.2~0.3 ml/kg体重行动态增强扫描。
三、图像分析及数据测量
1.DWI图像质量评估:由一名腹部疾病诊断经验丰富的放射科医师对b值为0和600 s/mm2的DWI图像质量进行评分。四分法标准如下:(1)解剖结构的可视性:1分,肉眼无法观察解剖结构致图像无法用于诊断;2分,大体显示解剖结构但胰腺边缘模糊;3分,较清晰显示解剖结构且胰腺边缘清晰;4分,胰管结构清晰可见。(2) 胰腺病灶对比度:1分,病灶未见显示;2分,病灶与周围组织有微弱对比;3分,病灶与周围组织有中等对比度或有强对比但病灶边缘模糊;4分,病灶与周围组织有明显的对比度且病灶边缘清晰。(3)运动及磁敏感伪影:1分,伪影严重致图像无法用于诊断;2分,中度伪影;3分,轻度伪影;4分,无伪影。3个分值之和为DWI图像质量总分[5]。
2.ADC值:ADC值的测量在工作站自带软件(Function 6.3.1e, GE AW VolumeShare 2,GE Healthcare, Milwaukee,WI, USA)上进行。数据测量时避开胰胆管、血管及胰腺边缘来选取圆形或椭圆形感兴趣区(ROI)测量ADC平均值。ROI的面积分别为20~287 mm2(ss-EPI DWI)和20~238 mm2(rFOV DWI)。
四、统计学分析
结 果
一、图像质量分析
b值为0和600 s/mm2的rFOV DWI在显示胰腺解剖结构、病灶对比度均优于ss-EPI DWI,且伪影也明显少于后者,差异具有统计学意义(表1,图1、图2)。
二、ADC定量分析
PDAC、NET、MFCP、SPT、正常胰腺rFOV DWI获得的ADC值分别为(1.38±0.17)×10-3、(1.22±0.35)×10-3、(1.29±0.13)×10-3、(1.04±0.38)×10-3、(1.86±0.15)×10-3mm2/s;ss-EPI DWI的ADC值分别为(1.73±0.24)×10-3、(1.63±0.39)×10-3、(1.58±0.19)×10-3、(1.25±0.26)×10-3、(2.04±0.20)×10-3mm2/s,各组间的差异均有统计学意义(P值均<0.001)。此外,同组内rFOV DWI获得的ADC值均显著小于ss-EPI DWI的ADC值,差异均有统计学意义(P值均<0.001)。两两组比较时, rFOV DWI的ADC值在MFCP与PDAC(P=0.078)、NET与MFCP(P=0.937)、SPT与MFCP(P=0.09)的组间差异以及ss-EPI DWI的ADC值在MFCP与PDAC(P=0.104)、PDAC与NET(P=0.133)、SPT与MFCP(P=0.285)的组间差异无统计学意义,而其他两两组间差异均有统计学意义(P值均<0.05)。
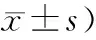
表1 rFOV DWI与ss-EPI DWI图像质量评分(分,

图1 胰头部中分化导管腺癌。1A为ss-EPI DWI(b=600 s/mm2),1B为rFOV DWI(b=600 s/mm2),1C为轴位T2WI,1D为轴位T1WI。rFOV DWI对病灶的显示边缘结构清晰,信号显示较ss-EPIDWI均匀
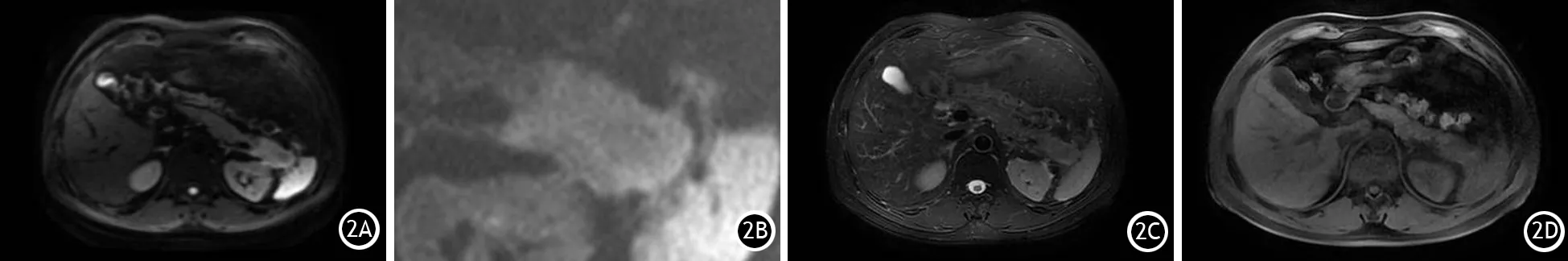
图2 胰尾部慢性肿块型胰腺炎。2A为ss-EPI DWI(b=600 s/mm2),2B为rFOV DWI(b=600 s/mm2),2C为轴位T2WI,2D为轴位T1WI。rFOV DWI对病灶的显示边缘结构清晰,信号显示较ss-EPIDWI均匀
PDAC与正常胰腺的rFOV DWI和ss-EPI DWI的ADC值的ROC曲线下面积(AUC)分别为0.983(95%CI0.944~0.998)和0.889(95%CI0.822~0.936),差异有统计学意义(P=0.0004,图3A)。
将NET、MFCP、SPT合并为良实性病变组,PDAC与良实性病变组的rFOV DWI和ss-EPI DWI的ADC值的AUC 分别为0.799(95%CI0.719~0.864)和0.755(95%CI0.672~0.827),差异无统计学意义(P=0.4355,图3B)。
讨 论
影响DWI分辨率的因素有两个,一个是FOV,另一个是采集矩阵。ss-EPI DWI使用的ss-EPI序列TE时间长且在相位编码方向的带宽窄,这有可能会产生磁敏感伪影和化学位移伪影,从而导致图像质量欠佳、信噪比较低以及空间分辨率较差。对于ss-EPI DWI序列来说,采集矩阵的大小直接决定了整体读出窗口的长度,采集矩阵越大,整体读出窗口的长度越长,ss-EPI DWI序列的伪影也越重,因此通过增加矩阵提高ss-EPI DWI分辨率是行不通的。此外,可以通过减小FOV实现图像的分辨率的提高,因为FOV小于成像物体的面积时,图像在相位编码的方向会产生卷褶伪影,所以ss-EPI DWI的FOV不能随意缩小。为保证FOV之外的物体不被激发或激发之后的信号对图像没有影响,本研究使用的rFOV DWI利用一种2D选择性激励的射频脉冲来激发小范围的感兴趣区以避免卷褶伪影的干扰,且随着FOV的增大,b=0 s/mm2时磁场的不均匀性也会越明显,所以rFOV DWI脂肪压制的效果优于ss-EPI DWI,有助于提高小病灶的检出率[1,6-8]。本研究采用的rFOV DWI的空间分辨率是ss-EPI DWI的4倍以上,可同时明显改善DWI图像的变形和伪影。

图3 ROC曲线分析。3A为rFOV DWI和ss-EPI DWI对PDAC与正常胰腺的鉴别诊断ROC分析;3B为rFOV DWI和ss-EPI DWI对PDAC与胰腺良性病变的鉴别诊断ROC分析
腹部DWI检查中b值常取值在500~1 000 s/mm2[9-13]。本研究在胰腺DWI成像使用b值为600 s/mm2可以保持较好的信噪比和图像质量[9]。基于ss-EPI DWI的研究可以有效地鉴别胰腺良恶性病变,因胰腺癌的ADC值显著低于良性病变[10-18]。本研究结果也显示胰腺癌的ADC值显著低于胰腺良性病变,其主要原因是胰腺癌基质纤维化含量、增多的肿瘤细胞限制了细胞外间隙水分子的运动,而肿瘤细胞内核质比的增加也限制了细胞内水分子的扩散运动[1]。但本研究的ss-EPI DWI及rFOV DWI的ADC值对MFCP与PDAC的无明显鉴别诊断价值,与Philipp等[19]报道结果一致。其可能原因一方面是MFCP入组例数少,另一方面是MFCP富含纤维化基质,且其有可能进展为胰腺癌。此外,PDAC的肿瘤组织内及其周围可因为主胰管或分支胰管的阻塞而伴发胰腺炎也可导致MFCP与PDAC的ADC值存在一定程度的重叠[20]。Fattahi等[15]的研究(b为600 s/mm2)则认为ADC值可以鉴别这两种疾病。也有研究认为高b值的DWI可以有效地鉴别诊断MFCP与PDAC[21]。目前ADC值对两者的鉴别诊断价值尚存在争议。
rFOV DWI较ss-EPI DWI对PADC的诊断准确性高的可能原因是rFOV DWI的体素体积(7.8 mm3)小于ss-EPI DWI的体素体积(61.8 mm3),从而有效减少肿瘤组织内各成分之间的部分容积效应,其ADC值更能反映病变的本质。
本研究尚存在一些不足。首先,本研究选取了相对较低的b值(600 s/mm2)来减少运动伪影、提高信噪比,但更高的b值才有可能更准确地反映组织的真实扩散[22-23]。此外,两个b值(0和600 s/mm2)的DWI虽然可减少检查时间,但多b值DWI成像可能得到更加准确的ADC值[24-25]。
总之,rFOV DWI对胰腺解剖结构的显示、对病灶的显示对比度明显优于ss-EPI DWI,其鉴别诊断胰腺导管腺癌与正常胰腺的效能更高。
[1] Zaharchuk G, Saritas EU, Andre JB, et al. Reduced field-of-view diffusion imaging of the human spinal cord: comparison with conventional single-shot echo-planar imaging[J]. AJNR Am J Neuroradiol, 2011, 32(5): 813-820. DOI: 10.3174/ajnr.A2418.
[2] Riffel P, Michaely HJ, Morelli JN, et al. Zoomed EPI-DWI of the pancreas using two-dimensional spatially-selective radiofrequency excitation pulses[J]. PLoS One, 2014, 9(3): e89468. DOI: 10.1371/journal.pone.0089468.
[3] Ma C, Li YJ, Pan CS, et al. High resolution diffusion weighted magnetic resonance imaging of the pancreas using reduced field of view single-shot echo-planar imaging at 3T[J]. Magn Reson Imaging, 2014, 32(2): 125-131. DOI: 10.1016/j.mri.2013.10.005.
[4] Kim H, Lee JM, Yoon JH, et al. Reduced field-of-view diffusion-weighted magnetic resonance imaging of the pancreas: comparison with conventional single-shot echo-planar imaging[J]. Korean J Radiol, 2015,16(6):1216-1225. DOI: 10.3348/kjr.2015.16.6.1216.
[5] Kartalis N, Loizou L, Edsborg N, et al. Optimising diffusion-weighted MR imaging for demonstrating pancreatic cancer: a comparison of respiratory-triggered, free-breathing and breath-hold techniques[J]. Eur Radiol, 2012, 22(10): 2186-2192. DOI: 10.1007/s00330-012-2469-3.
[6] Wilm BJ, Svensson J, Henning A, et al. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging[J]. Magn Reson Med, 2007, 57(3): 625-630. DOI: 10.1002/mrm.21167.
[7] Jeong EK, Kim SE, Guo J, et al. High-resolution DTI with 2D interleaved multislice reduced FOV single-shot diffusion-weighted EPI (2D ss-rFOV-DWEPI)[J]. Magn Reson Med, 2005. 54(6): 1575-1579. DOI: 10.1002/mrm.20711.
[8] Karampinos DC, Van AT, Olivero WC, et al. High resolution reduced-FOV diffusion tensor imaging of the human pons with multi-shot variable density spiral at 3T[J]. Conf Proc IEEE Eng Med Biol Soc, 2008, 2008: 5761-5764. DOI: 10.1109/IEMBS.2008.4650523.
[9] Koc Z, Erbay G. Optimal b value in diffusion-weighted imaging for differentiation of abdominal lesions[J]. J Magn Reson Imaging, 2014, 40(3): 559-5566. DOI: 10.1002/jmri.24403.
[10] Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature[J]. Eur Urol, 2012, 61(2): 326-340. DOI: 10.1016/j.eururo.2011.09.019.
[11] Kartalis N, Lindholm TL, Aspelin P, et al. Diffusion-weighted magnetic resonance imaging of pancreas tumours[J]. Eur Radiol, 2009, 19(8): 1981-1990. DOI: 10.1007/s00330-009-1384-8.
[12] Matsuki M, Inada Y, Nakai G, et al. Diffusion-weighed MR imaging of pancreatic carcinoma[J]. Abdom Imaging, 2007, 32(4): 481-483. DOI: 10.1007/s00261-007-9192-6.
[13] Lee SS, Byun JH, Park BJ, et al. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses[J]. J Magn Reson Imaging, 2008, 28(4): 928-936. DOI: 10.1007/s00330-012-2687-8.
[14] Muraoka N, Uematsu H, Kimura H, et al. Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations[J]. J Magn Reson Imaging, 2008, 27(6): 1302-1308. DOI: 10.1002/jmri.21340.
[15] Fattahi R, Balci NC, Perman WH, et al. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas[J]. J Magn Reson Imaging, 2009, 29(2): 350-356. DOI: 10.1002/jmri.21651.
[16] Fukukura Y, Takumi K, Kamimura K, et al. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings[J]. Radiology, 2012, 263(3): 732-740. DOI: 10.1148/radiol.12111222.
[17] Wiggermann P, Grützmann R, Weissenböck A,et al. Apparent diffusion coefficient measurements of the pancreas, pancreas carcinoma, and mass-forming focal pancreatitis[J]. Acta Radiol, 2012, 53(2): 135-9. DOI: 10.1258/ar.2011.100252.
[18] Rosenkrantz AB, Matza BW, Sabach A, et al. Pancreatic cancer: lack of association between apparent diffusion coefficient values and adverse pathological features[J]. Clin Radiol, 2013, 68(4): e191-e197. DOI: 10.1016/j.crad.2012.11.006.
[19] Philipp W, Robert G, Angelika W, et al. Apparent diffusion coefficient measurements of the pancreas, pancreas carcinoma and mass-forming focal pancreatitis[J]. Acta Radiol, 2012: 1-5. DOI: 10.1258/ar.2011.100252. DOI: 10.1258/ar.2011.100252.
[20] Buetow PC, Parrino TV, Buck JL, et al. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status[J]. Am J Roentgenol, 1995, 165(5): 1175-1179. DOI: 10.2214/ajr.165.5.7572498.
[21] Takeuchi M, Matsuzaki K, Kubo H, et al. High-b-value diffusion-weighted magnetic resonance imaging of pancreatic cancer and mass-forming chronic pancreatitis: preliminary results[J]. Acta Radiol, 2008, 49(4): 383-386. DOI: 10.1080/02841850801895381.
[22] Ichikawa T, Erturk SM, Motosugi U, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results[J]. Am J Roentgenol, 2007, 188(2): 409-414. DOI: 10.2214/AJR.05.1918.
[23] Huang WC, Sheng J, Chen SY, et al. Differentiation between pancreatic carcinoma and mass-forming chronic pancreatitis: usefulness of high b value diffusion-weighted imaging[J]. J Dig Dis, 2011, 12(5): 401-408. DOI: 10.1111/j.1751-2980.2011.00517.x.
[24] Klauss M, Lemke A, Grünberg K, et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma[J]. Invest Radiol, 2011, 46(1): 57-63. DOI: 10.1097/RLI.0b013e3181fb3bf2.
[25] Lemke A, Laun FB, Klauss M, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters[J]. Invest Radiol, 2009, 44(12): 769-775. DOI:10.1097/RLI.0b013e3181b62271.
ValueofreducedfieldofviewDWIindifferentiatingsolidpancreaticfocallesions
LiJing,MaChao,BianYun,WangXinrui,ShiZhang,WangLi,ShaoChengwei,ChenShiyue,LuJianping.
DepartmentofRadiology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LuJianping,Email:cjr.lujianping@vip.163.com
ObjectiveTo study the value of reduced field-of-view (rFOV DWI) in differentiating patients with solid pancreatic focal lesions.Methods139 patients with solid pancreatic mass were enrolled, including 105 patients with pancreatic ductal acinar carcinoma (PDAC), 16 patients with neuroendocrine neoplasms, 7 patients with mass forming chronic pancreatitis (MFCP) and 11 patients with solid papillary tumor (SPT). 38 healthy adult volunteers served as controls, and underwent single stimulated echo planar imaging (ss-EPI)DWI and rFOV DWI(b value=0 and 600 s/mm2)MRI examination. Quartation method was used to evaluate the image quality of ss-EPI)DWI and rFOV DWI in the three terms of the visibility of anatomical structure, contrast of pancreatic lesions, motion and the susceptibility artifacts during MRI. Work station self-carried software was used to measure the ADC value of the region of interest (ROI).The image quality and ADC values of different pancreatic diseases and normal pancreas were compared. ROC curve for ADC value was drawn to evaluate the difference among PDAC, other benign pancreatic masses and normal pancreas.ResultsAt b value of 0 and 600 s/mm2, rFOV DWI was superior to ss-EPI DWI in terms of showing pancreatic anatomic structure, the contrast of the lesion and the score evaluation for susceptibility artifacts(b=0 s/mm22.99±0.51vs2.79±0.64, 2.37±0.48vs1.81±0.63, 3.17±0.56vs2.91±0.60;b=600 s/mm23.63±0.50vs3.32±0.56, 3.45±0.50vs3.01±0.49, 3.74±0.44vs3.12±0.37), and the differences were statistically significant (P<0.001). ADC values of PDAC, NET, MFCP, SPT and normal pancreas were (1.38±0.17)×10-3,(1.22±0.35)×10-3,(1.29±0.13)×10-3,(1.04±0.38)×10-3and(1.86±0.15)×10-3mm2/s for rFOV DWI, and(1.73±0.24)×10-3,(1.63±0.39)×10-3,(1.58±0.19)×10-3,(1.25±0.26)×10-3and(2.04±0.20)×10-3mm2/s for ss-EPI DWI. The difference on ADC values among different groups and within one group were all statistically significant (P<0.001). There were no statistical significant differences on ADC values between MFCP and PDAC, between MFCP and SPT as well as on ss-EPI DWI ADC values between PDAC and NET, but statistical differences were found between other two groups (P<0.05). The area under the ROC curve of rFOV and ss-EPI DWI was 0.983 (95%CI0.944-0.998) and 0.889 (95%CI0.822-0.936), respectively, and the difference was statistically significant (P=0.0004), but rFOV DWI and ss-EPI DWI ADC values for PDAC and all benign solid diseases were 0.799 (95%CI0.719-0.864) and 0.755 (95%CI0.672-0.827), and the difference was not statistically significant.ConclusionsrFOV DWI could significantly enhance the quality of DWI images, and its diagnostic efficacy was much better than ss-EPI DWI.
Pancreatic neoplasms; Magnetic resonance imaging; Diagnosis, differential
FundprogramShanghai Natural Science Foundation(14ZR1408300);Medical Guidance Project of Shanghai Science and Technology Commission(14411960100)
10.3760/cma.j.issn.1674-1935.2017.06.009
200433 上海,第二军医大学长海医院放射科
陆建平,Email:cjr.lujianping@vip.163.com
上海市自然科学基金(14ZR1408300);上海市科委医学引导项目(14411960100)
2017-06-18)
吕芳萍)

