Progress in Characterization Techniques in Spintronic Enhanced Photocatalytic Hydrogen Evolution
ZHANG Wen-yan, GAO Wei , ZHANG Xu-qiang, LI Zhen, LYU Gong-xuan
(1. StateKeyLaboratoryforOxoSynthesisandSelectiveOxidation, LanzhouInstituteofChemicalPhysics, ChineseAcademyofScience, Lanzhou 730000, China; 2. UniversityofChineseAcademyofScience, Beijing 10080, China; 3. CollegeofMaterialEngineering, JinlingInstituteoftechnology, Nanjing 211169, China)
ProgressinCharacterizationTechniquesinSpintronicEnhancedPhotocatalyticHydrogenEvolution
ZHANG Wen-yan1,2,3, GAO Wei1,2, ZHANG Xu-qiang1, LI Zhen1,2, LYU Gong-xuan1
(1.StateKeyLaboratoryforOxoSynthesisandSelectiveOxidation,LanzhouInstituteofChemicalPhysics,ChineseAcademyofScience,Lanzhou730000,China; 2.UniversityofChineseAcademyofScience,Beijing10080,China; 3.CollegeofMaterialEngineering,JinlingInstituteoftechnology,Nanjing211169,China)
In recent years, the fossil fuel crisis has triggered the worldwide demand for new clean energy. Hydrogen is a desirable candidate due to its high combustive enthalpy and zero-pollution characteristics. A promising approach for the hydrogen production is splitting H2from water by solar driven photo-catalytic hydrogen evolution reaction (HER). The development of photo-catalytic HER is inhibited by many factors: especiallythe the large energy loss and the electron-hole recombination during electron transportation, and high over-potential of proton reduction and water oxidation.Spintronic science sheds new lights on solving these obstacles by triggering the high efficient spin transfer and electron tunneling, as well aselectrons spin filtering to decrease the over-potential of the reaction and suppress the yield of by-products.The progresses in characterization techniques have contributed greatly to the unveiling of the scientific "secrets" in spintronic enhanced HER research. Yet,few work hasbeen carried out to sum up the setechniques and to analyze the potential challenges that inhibit their future development. Given that, this review focuses on these topics and provides an expectation for its development trends.
photocatalytic;hydrogen evolution;characterization;spin detection;spintronics enhanced photocatalytic HER
In recent years,the worldwide fossil fuel crisis makes it necessary to exploreclean and sustainable energy carriers[1-44]. Due to its high combustion enthalpy and zero-pollution characteristics, hydrogen is recognized as an ideal sustainable energy carrier, in the future, to be used in cars, in houses, forportable power, and in many other applications[44-77]. Since the report of hydrogen evolution reaction (HER) over TiO2electrode in 1972[78], great progress have been realized in obtaining hydrogen from water by solar driven photocatalytic reaction[75-82]. A series of model shave been constructed for photocatalytic HER[80-88],including (1) light harvest by photocatalysts, (2) charge excitation (electrons and holes), (3) charge migrating to photocatalysts surface to reduce protons or to oxidize water, (4) bulk charge recombination, and (5) surface charge recombination[80-84,89-96].
Though solar driven photocatalytic HER is a potential and sustainable approach for renewable hydrogen generation, some problems still exist, such as: limited charge transporting efficiency[40,87-88,97-99,100-102], high over-potential of HER and oxygen evolution reaction (OER) half reactions in water splitting[55,103-105], and low photocatalytic stability of some photocatalysts[106-108]. Recent progress of spintronics sheds new lights on solving these obstacles. On the one hand, triggering spin transfer and electron tunneling in photo-catalysts can effectively enhance the efficiency of electron transporting and turnover frequency (TOF) value, due to lossless electron transportation and low electron-lattice interaction in photo-catalysts matrix[100,108-109]. It is amazing that the spin electron mobility could reach up to 18 000 cm2/Vs in some topological transfer media[100,108, 110-113]. On the other hand,spin filtering photoelectrons can lower the overpotential of solar driven photo-catalytic HER[105,109]and inhibit the yield of by-products (e.g., singlet H2O2)[105,114-115], resulting inhigher catalytic activity and robust catalyticstability.
Bridging spintronic science and photo-catalytic research demands a series of sophisticated characterization techniques, including sensitive spin detection devices, advanced optical measurements, energy analyze equipment, electrochemistry characterization devices, sensitive gas detection equipment, size and morphology detection, composition and elements characterization, as well as work function detection. These characterizations provide abundant information for the unveiling of theeffects of spintronic on enhancing solar driven photo-catalytic HER. Nevertheless, only few work on the progress of these important techniques in the detection of electron spin and transfer has been systematically reviewed.In this review, the classification, designs for devices and detection mechanisms of present characterization techniques for related research fields are systematically summarized, and the significant role of detecting techniques in the future development of spintronic enhanced HER research is given. The challenges and developing trends are discussed in this review to lead to more exploration in this research field.
1 ClassificationsoftechniquesfordetectingspintronicenhancedHER
A series of characterization techniques have been developed for the detection of the relationship among HER efficiency, photo-electronic performance, spin transport and spin filtering[53,116-122],as shown in Table 1. These techniques include the common devices which have already been commercialized, and some self-designed novel devices[53,118-132].

Table1 Characterizationtechniquesforspintronicenhancedphoto-catalyticresearch
2 Commercialized techniques
2.1 Spin detection
2.1.1 Electron spin resonance
ESR characterization provides essential and direct spin information of unpaired electrons. The mechanism of ESR detection based on the interaction between the external magnetic field and electron spin. An electron has two spin quantum number,ms=+1/2,ms=-1/2. When placing an electron in the magnetic field (B0), the electron magnetic moment aligns itself either parallel (ms=-1/2) or anti-parallel (ms=+1/2) to the field, each alignment having a specific energy due to the Zeeman effect:
E=msgeμBBo
geis theg-factor of the electron,ge=2.002 3 for the free electron,μBis the Bohr magneton.
As illustrated in Fig.1, the Zeeman effect results in the energy level splitting, and the energy gap between the lower and the upper state is ΔE=geμBBo.

Fig.1 (a)Energy level scheme of spin state (b) Diagram of ESR spectra
Evidently, the increaseof ΔEis directly in proportion to the magnetic field strengthBo. The unpaired electron can absorb energyhνand move from the lower to upper state, or emit a photon and move from upper to lower state. Both the two processes obey the fundamental equation of ESR spectroscopy:
hν=ΔE=geμBBo
ESR devices are designed and constructed based on the above equation. Usually, ESR measurements are conducted by varying the magnetic field (Bo) while holding the frequency of incident photon (ν) constant. When an unpaired electron is placed in the magnetic field (Bo), the increase ofBowould enlarge the value of ΔE(the energy gap betweenms=+1/2 andms=-1/2 states, Fig.1). When ΔEincreases to match the energy of incident microwaves (hν), the electron would absorb energy and jump betweenms=+1/2 andms=-1/2 spin states. The Maxwell-Boltzmann distribution of electrons yieldnet absorption of energy. Such net absorption can be detected and converted into a spectrum. Fig.1 (a) and (b) illustrate,respectively, the simulated absorption ESR spectrum and the first derivative of absorption ESR spectrum.
ESR is an effective technique to detect unpaired electron both in solid and liquid environments. It has been applied to detect the OH· radicals generated in water splitting to reveal the OER mechanism. Also, Lu et al. used to apply the technique to investigate heavy atom induced spin polarization in the photo-catalytic water splitting reaction. These investigations show a high potential of the technique for bridging the spintronic researches and photo-catalytic reactions.
2.1.2 Physical property measurement system
Physical property measurement system is a common and commercialized complex system designed for the detection of magnetic, electronic and thermal properties of materials. It can provide strong and tunable magnetic field, as well as tunable temperature control system for the measurement. More interestingly, PPMS system provides a high convenience for researches to design novel experiments. Fig.2 shows the sample chamber and sample mount of PPMS. The mount and chamber design are both very convenient for researchers to plug in or remove samples, and detect physical properties under magnetic/electronic/thermal fields and under microwave/laser irradiation.
Considering that spin polarization could result in various magnetic, electronic and thermal properties of photo-catalysts, accurate detection of these properties can offer a large number of information to reveal their spin state variation and build reasonable relationship between their spin state and photo-catalytic properties. Equipped with advanced software and standard hardware, PPMS can provide fully automaticcharacterization for a series of physical properties including the resistivity, magneto resistance,magneto-electric coupling, differential resistance, ferroelectric property, dielectric property, Hall coefficient, volt ampere characteristic, critical current, AC susceptibility, hysteresis loop, specific heat, thermal magnetic curve, thermoelectric effect, Sebek coefficient, and thermal conductivity. It is expected that owing to its high accuracy and convenience, PPMS plays an important role in constructing interdisciplinary research of spintronics and photo-catalytic research.

Fig.2 (a) Sample chamber and (b) Sample mount of PPMS system
2.1.3 Vibrating sample magnetometer
VSM is an effective instrument to measures magnetic properties of photo-catalysts as a function of magnetic field, temperature, and time. As illustrated in Fig.3 (a) and (b), modern VSM devices are mainly composed of an electromagnet, a detection coil, a sample holder, a lock in amplifier and a Gauss meter. When placing a sample inside a uniform magnetic field, a dipole moment proportional to the applied field is induced in the sample. If the sample is made to undergo a sinusoidal motion, an electrical signal will be induced in the suitable located stationary pick-coils. This signal is proportional to the magnetic moment, vibration amplitude and vibration frequency, thus provids magnetic information of the samples.

Fig.3 (a) Schematic model and (b) Device image of VSM equipment
VSM test has some special advantages. Firstly, it has few requirement on the morphology and crystallization of samples, so it is widely applicable to detect powders, solids, liquids, single crystals, and thin film. Secondly, it has enough sensitivity to detect all kinds of magnetic materials, including diamagnetic materials, paramagnetic materials, ferromagnetic materials, ferrimagnetic materials, anti-ferromagnetic materials, anisotropic materials and magnetic-optical materials. More importantly, as the magnetic property originates from the spin polarization of electrons, VSM is effective to detect the magnetic property of spintronic material such as giant magneto resistance, colossal magneto resistance, and exchange biased and spin-valve.
2.2 Gas detection and electrochemical detection
Gas detection and electrochemical detection are two important measurement techniques for the photo-catalytic water splitting. Gas chromatography is applied to characterize the generation of H2and O2. Electrochemical workstation is widely applied to characterize the electronic and chemical properties of photocatalysts. A precise and versatile instrument for the electrochemical detection is the electrochemical workstation, which not only can record the photo-current, HER overpotential and other related photo-catalytic data, but can also reveal the spin polarization information of spintronics enhanced HRE by assembling the anodes with chiral molecules to induce chiral-induced spin selectivity effect on water splitting.
2.3 Morphology and size detection
Size and morphology have important effects on energy level, crystallization, lattice stress, surface energy, and formation of defects of photo-catalysts, thus the size and morphology of photo-catalysts are closed related to their spintronic and photocatalytic properties. TEM, STEM, SEM and AFM are inevitably the four keycommon techniques to display the morphology and size of photo-catalysts.
MRFM is a newly developed imaging technique especially suitable for the spin detection. As shown in Fig.4(a),MRFM devices are composed of a microwave coil, a magnetic tip, a resonant slice, a cantilever and an interferometer. The mechanism of MRFM is to detect magnetic force between a ferromagnetic tip and spins in a sample. The problem of single spin detection is that the force from a single spin is too small to be detected.

Fig.4 Scheme of (a) MRFM devices 38 and (b) MRFM device with one single spin detection sensitivity [120-121]
With the development of ultrasensitive cantilever-based force sensors, the problem is solved and now MRFM can detect magnetic force of one single spin[120-121]. The magnetic resonance force microscopy has a very high detection sensitivity, i.e., up to 10 billion times better than a medical magnetic resonance imaging(MRI) used in hospitals.Yacoby et al. developed the MRFM device, and the device is mainly composed of an excitation laser, a scanning diamond platform, a MW coil and a sensor NV, as illustrated in Fig. 4 (b)[120-121].Using the device accurate, real-space, and quantitative magnetic-field images of single molecule imaging at room temperature can be obtained. Considering its single-spin detection sensitivity, it is reasonable to prospect that the MRFM device will provide more useful spin information on the unveiling of interdisciplinary mechanism between spintronic science and photocatalytic reaction.
2.4 Work function detection
Work function (Φ) of a material is the energy difference between Fermi energy and vacuum level. As shown in Fig.5(a), the work function of metals is the energy difference between Fermi energy and vacuum level. The Fermi energy of a semiconductor is theenergy within the band gap (between the conductive band and valance band), so the work function of a semiconductor is somewhat more complex than that of metal, as illustrated in Fig.5(b). In that case, Fermi distribution should be considered since there are no allowed electronic states within the band gap. Fermi distribution is a statistical function, which gives the probability to find an electron in a given electronic state.

Fig.5 Scheme of work function in (a) metal and (b) n-type semiconductor(VBM: valence bands maximum, CBM: conductive bands minimum)
The work function detection is important both for spintronic research and photo-catalytic water splitting. For one thing, as illustrated in Fig. 6,the electron tunneling spin injection and transfer orientation in materials depend highly on the energy level matching of each component[133]. In order to promote the spin injection and spin transfer in photo-catalystic materials, it is necessary to realize suitable energy level matching according to their work function parameter. For another thing, the work function of materials is very sensitive to their surface conditions, so the work function detection can reveal many surface properties, including catalytic activity, reconstruction of surfaces, doping and band-bending of semiconductors, charge trapping in dielectrics and corrosion. These parameters provide abundance information for scientists to analyze photo-catalytic mechanism of water splitting reaction.

Fig.6 (a) Scheme of spintronic material Co/Al2O3/Si multi-layers (b) Dependence of Spin conductance on component variation of Co/Al2O3/Si multi-layers[133]
Work function can be measured by ultraviolet photoelectron spectroscopy [UPS,also known as photoemission spectroscopy (PES)], Kelvin probe force microscopy, and photoelectron microscopy. In this section, we briefly summarize the characteristics and mechanism of UPS and Kelvin probe method.
2.4.1 Ultraviolet photoelectron spectroscopy
UPS has a similar measure mechanism with another detection technique, the XPS. The only difference between UPS and XPS detection is the wavelength of ionizing radiation. UPS applies ultraviolet photons as the irradiation source to induce photoelectric effect, while XPS uses photons higher than 1 keV to excite the photoelectric effect. Ultraviolet irradiations of UPS are produced using a gas discharge lamp which typically filled with helium. He photons emitted by helium gas are of 21.2 eV (He I) and 40.8 eV (He II). UPS not only can detect work function but also can measure the valence band of photo-catalysts.
2.4.2 Kelvin probe force microscopy
KPFM is very sensitive to detect the work function of materials at atomic or molecular scales. KPFM has been considered to be a unique and ideal method to characterize the electronic/electrical properties of metal/semiconductor surfaces, considering that the work function relates to many surface properties, including catalytic activity, reconstruction of surfaces, doping and band-bending of semiconductors, charge trapping in dielectrics and corrosion. Besides, KPFM can provide detection information with high accuracy, as it can be applied in ultra high vacuum environment to avoid contaminates. In addition, with KPFM, researchers are able to recognized surface potential distribution of nano-scaled materials via the two-dimensional or three-dimensional work function images (Fig. 7)[123]. These two-dimensional or three-dimensional images give abundant information on the composition and electronic state of material surface.Therefore,KPFM is an effective characterization technique for exploring intrinsic mechanisms and optimizing properties for photo-catalysts and spintronic devices design.
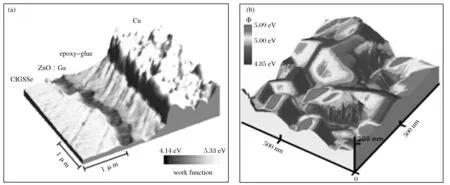
Fig.7 (a) Cross-sectional KPFM image of Cu In Ga S Se solar cell (b) Three-dimensional KPFM image of Co Ga Se solar cell[123]
3 Self-designed novel devices
3.1 Chiral-inducedspinselectivitydeviceforspindetection
In order to detectCISSeffect, a new type of device has been designed to realize the effective measurement for charge transfer andCISSinduced spin polarization. Fig. 8 illustrates a typicalCISSdevice constructed for the spin detection.The assembly consists of dye decorated deoxyribonucleic acid(DNA) oligomers, a Ag film, an AlOx film and a ferromagnetic Ni layer[53,118-119]. Under light irradiation, the electrons are excited from dye molecules. These photo-generated electrons then migrate through the DNA molecules and arrive at the Ag substrates (Metal 2). The photo electrons, which spin parallel to the net spin of ferromagnetic Ni layer, could tunnel through the AlOx layer and migrate from the Ag substrates (Metal 2) tothe ferromagnetic Ni layer (Metal 1).The spin detection sensitivity ofCISSdevice can be enhanced by reducing the thickness of silver films and replacing the Ni layer with more spin specific substrate[119].

Fig.8 (a1, b1) Scheme of CISS device (red circles are dye molecules, metal 1 is Ni, AlOx is a dielectric layer, and metal 2 is Ag), (a2, b2) energy level diagram for measuring spin detection; (c) SEM image of the device and its electrical connection scheme[118]
3.2 Nano-scaledspin-mechanicaldeviceforspindetection
Since spin is a quantum property characterized by the angular momentum, the spin flip of electrons results in a tiny variation of angular momentum, which can be converted to the tiny variation of mechanical torque. This is the basic mechanism of the spin-mechanical device for spin detection. Besides, the sizes of spin-mechanical devices are usually at the nano scale to enhance the detection sensitivity for tracing spin flip. Fig.9 (a1, a2) and Fig.9 (b) illustrate two typical nano-scaled torque-shaped spin-mechanical device for the spin detection[124-125]. Both of them are composed of nano-scaled materials, such as nano-sheets and nano-wires, to form the torsion oscillator[124-125]. The nano-scaled torsion oscillators can flip sensitively in respond to the flip of spin electrons. Moreover, the direction of spin flip (up or down) can be determined by observing the flip direction of the torsion oscillator.

Fig.9 (a1) SEM image and (a2) Scheme of a single-crystal silicon torsion oscillator[124-125]
3.3 Nano-scaledspin-mechanicaldeviceforspindetection
Taking advantages of the tunnel barrier penetration [Fig. 10 (a)], the constructed tunnel contacts are recognized as the viable and robust method to detect spin polarizations. Fig. 10(b-c) illustrates several tunnel contacts devices composed of ferromagnetic material (FM), tunnel barrier (TB) and non-magnetic material (NM), with a nano-voltmeter to measure voltage and a galvanometer to record currents[126-132]. Tunnel contacts mainly include 2-terminal (2T) [Fig. 10 (c)], nonlocal (NL) measurement [Fig. 10 (d)] and 3-terminal (3T) [Fig. 10 (e)], and measure the spin signal based on the mechanism of local magneto-resistance effect, Hanle effect, and non-local spin transport/diffusion[126-132]respectively. Tunnel contact devices are useful tools for the analysis of spintronic phenomenon in photo-catalysts. By choosing suitable tunnel contact devices, researchers can measure the spin transport, spin lifetime and diffusion length, as well as spin state of spin-polarized surface and Quantum spin Hall (QSH) edge[101,105,126-132]. For example, Parkin et al. utilized the 3T measurement [Fig. 10 (e)] to study the spin transportation in SrTiO3, an efficient photo-catalyst for solar driven HER,and found that the short spin lifetime in SrTiO3originated from the Ti3+defects of the formed SrTiO3[86]. Based on their investigation, the Ti3+concentration in SrTiO3lattice can be controlled if one makes effort on SrTiO3-based spin photocatalysts for water splitting.

Fig.10 Schemes of (a) tunnel barrier penetration (b)Spin current through a FM/TB/NM junction (c) Local magneto-resistance detection device (2T) (d) Scheme of non-local spin transport detection device (NL), (e) Three-terminal spin transport detection device (3T), (f) 3T spin detection device for detecting spin transfer in SrTiO3[86, 126-133]
4 ChallengesandbrightfutureofdevelopmentofcharacterizationforspintronicenhancedHER
Through the above discussions we know that the progresses of characterization techniques have greatly promoted the development of spintronic enhanced photo-catalytic HER research, from the aspects of mechanism discovery, catalysts design, and HER efficiency improvement. Consequently, development of characte-rization displays bright future in the spintronic enhanced HER research and other interesting catalytic problems[134-147].
Nevertheless, the contributions to spintronic enhanced photo-catalytic HER are highly limited due to the lack of real-timedetection, low convenience, limited detection sensitivity, as well as high detection cost. As the spin flipping takes place in a very short time, in order to obtain precise data of spintronics effect on HER, establishing areal-time detection system with high sensitivity is a must done work, otherwise it is difficult to pursue some ultra-fastreaction phenomena which are meaningful in the unveiling of the secrets in spintronic enhanced HER.Another Achilles′ Heel is the complexity and high cost of some common and commercialized devices (such as PPMS, ESR and PEEM). Considering that, it is necessary to accelerate the development of self-designed novel devices, for their complexity and cost could be manipulated more conveniently than those common commercialized devices.
[1] Xie G C, K Zhang, B D Guo, et al. Graphene-based materials for hydrogen generation from light-driven water splitting[J]. Adv Mater, 2013, 25(28): 3820-3839.
[2] Lu Q, Li C L, Wang F, et al. Synthesis of novel flower-like Cu2O photocatalysts for hydrogen evolution under visible light[J]. J Mol Catal (China), 2016, 30(6): 557-565.[卢强,李曹龙,王飞,等.新型花状Cu2O制备及其可见光分解水产氢性能[J].分子催化, 2016, 30(6): 557-565.]
[3] Zhang X Q, Tian B, Zhen W L, et al.Construction of Möbius-strip-like graphene for highly efficient charge transfer and high active hydrogen evolution[J]. J Catal, 2017, 354: 259-269.
[4] Wu C H, Fang Y F, Zhao P, et al. Preparation of Ag-BiVO4composite and its photocatalytic oxidation mechanism[J]. J Mol Catal (China) 2015, 29(4): 369-381.[吴春红, 方艳芬, 赵萍,等.Ag-BiVO4复合光催化剂的制备及其可见光光催化机理的研究[J]. 分子催化,2015, 29(4):369-381.]
[5] Tian B, Gao W, Zhang X Q, et al. Water splitting over core-shell structural nanorod CdS@Cr2O3catalyst by inhibition of H2-O2recombination via removing nascent formed oxygen using perfluorodecalin[J]. Appl Catal B, 2018, 221: 618-625.
[6] Li C L, Lei Z Q, Wang Q Z, et al. Synthesis of TiO2( B) nanobeltsphotocatalyst for water splitting to H2[J]. J Mol Catal (China), 2015, 29(4): 382-389.[李曹龙, 雷自强, 王其召,等. TiO2(B)纳米带光催化剂的制备及分解水产氢性能[J]. 分子催化,2015,29(4):382-389.]
[7] Zhen W L, Ning X F, Yang B J, et al. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill[J]. Appl Catal B, 2018, 221: 243-257.
[8] Ma L, Kang X X, Hu S Z, et al. Preparation of Fe,P Co-doped graphitic carbon nitride with enhanced visible- light photocatalytic activity[J]. J Mol Catal (China), 2015, 29(4): 359-368.[马琳, 康晓雪, 胡绍争,等. Fe-P 共掺杂石墨相氮化碳催化剂可见光下催化性能研究[J]. 分子催化,2015,29(4):359-368.]
[9] Li L, Huang Y P, Zhang A Q, et al. Synthesis and visible-light photocatalysis performance research of BiVO4/Bi6O6(OH)3(NO3)3composite photocatalyst[J]. J Mol Catal (China) 2016, 30(5): 470-479.[李灵, 黄应平, 张爱清,等. BiVO4/Bi6O6(OH)3(NO3)3复合光催化剂的制备及光催化性能研究[J]. 分子催化,2016,30(5):470-479.]
[10] Hu X, Lu G X. Acetic acid steam reforming to hydrogen over Co-Ce/Al2O3and Co-La/Al2O3catalysts-The promotion effect of Ce and La addition[J]. Catal Commun, 2010, 12(1): 50-53.
[11] Cao Y Y, Huang S B, Yin G Z. Study on the photocatalytic activities of n-p type CeO2/BiOBr composite prepared at different calcination temperatures[J]. J Mol Catal (China), 2016, 30(2): 159-168.[曹亚亚, 黄少斌, 尹佳芝, 不同煅烧温度制备的n-p型CeO2/BiOBr光催化性能研究[J]. 分子催化,2016,30(2):159-168.]
[12] Xie Y Z, Wang X, Liu S Q, et al. Preparation and visible-light photocatalytic activity of TiO2composite catalyst modified by soy protein[J]. J Mol Catal (China), 2016, 30(4): 372-382.[谢艳招, 王鑫, 刘顺琴,等. 大豆蛋白改性TiO2的制备及其可见光催化性能[J]. 分子催化,2016,30(4):372-382.]
[13] Jiao Z B, Chen T, Xiong J Y, et al. Visible-light-driven photoelectrochemical and photocatalytic performances of Cr-doped SrTiO3/TiO2heterostructured nanotube arrays[J]. Sci Rep, 2013, 3(9): 2720.
[14] Sun S N, Li C H, Yang W W, et al. Photocatalytic removal of NO from flue gas by TiO2loaded on semi-coke prepared by sol-gel Method[J]. J Mol Catal (China), 2015, 29(2):188-196.[孙圣楠, 李春虎, 杨微微,等. 溶胶-凝胶法制备TiO2负载活化半焦光催化烟气脱硝[J]. 分子催化,2015,29(2):188-196.]
[15] Zhang X J, Jin Z L, Li Y X, et al. Visible-light-induced hydrogen production over Pt-Eosin Y catalysts with high surface area silica gel as matrix[J]. J Power Source, 2007, 166(1): 74-79.
[16] Zhang J Q, Li L, Liu D, et al. Preparation of three-dimensionally ordered macroporouscomposite ZrO2-TiO2and its photocatalytic degradationof organic pollutants under multiple modes[J]. J Mol Catal (China), 2015, 29(4): 348-358.[张剑琦, 李莉, 柳迪,等. 3DOM TiO2-ZrO2复合材料制备与多模式光催化降解有机污染物[J]. 分子催化,2015,29(4):348-358.]
[17] Li Y X, Tang L F, Peng S Q, et al. Phosphate-assisted hydrothermal synthesis of hexagonal CdS for efficient photocatalytic hydrogen evolution[J]. Cryste Eng Comm, 2012, 14(20): 6974-6982.
[18] Wang X, Bai S, Bao Z.Hydroxylation of benzene to phenol by photocatalysis on NiOx/MesoHangjin 2# clay[J]. J Mol Catal (China), 2015, 29(3): 266-274.[王旭,萨嘎拉, 照日格图. NiOx/介孔杭锦2#土的制备及其对苯羟基化光催化性能研究[J]. 分子催化,2015,29(3):266-274.]
[19] Wang W P, Lu G X. Advances in catalytic generation of hydrogen from ethanol[J]. Prog Chem, 2003, 15(1): 74-78.[王卫平, 吕功煊. 乙醇催化制氢研究进展[J]. 化学进展, 2003, 15(1): 74-78.]
[20] Zhang L N, Deng Y Q, Shi F.Preparation of Fe-doped TiO2for the selective oxidation ofaromatic alcohols with oxygen under visible light irradiation[J]. J Mol Catal (China), 2015, 29(2): 179-187.[张丽娜, 邓友全, 石峰. Fe掺杂改性TiO2的制备及其可见光催化醇氧化性能研究[J]. 分子催化,2015,29(2):179-187.]
[21] Li Z K, Hu X, Zhang L J, et al. Steam reforming of acetic acid over Ni/ZrO2catalysts: Effects of nickel loading and particle size on product distribution and coke formation[J]. Appl Catal A, 2012, 417-418(1): 281-289.
[22] Tain C S, Liu Y T, Sheng W L, et al. Preparation of TiO2nanofibers templated with mesoporous SiO2spheres and photocatalytic synthesis of ammonia[J]. J Mol Catal (China), 2016, 30(6): 566-574.[田长水, 刘雅婷, 盛文龙,等. 介孔SiO2球为模板制备TiO2纳米纤维及光催化合成氨[J]. 分子催化,2016,30(6):566-574.]
[23] Ahmad H, Kamarudin S K, Minggu L J, et al. Hydrogen from photo-catalytic water splitting process: A review[R]. Renew Sustain Energy, 2015, 43: 599-610.
[24] Yi R, bai S, Bao Z. Preparation of Pd/MCM-41 and its photocatalyticperformance for benzene hydroxylation[J]. J Mol Catal (China), 2016, 30(6): 583-593.[意如, 萨嘎拉,照日格图. Pd/MCM-41催化剂的制备及其光催化苯羟基化的研究[J].分子催化,2016,30(6):583-593.]
[25] Ma Z Y, Li X B, Deng L J, et al. Preparation and visible-light-driven photocatalytic performance of TiO2/Bi2WO6nano-heterostructure[J]. J Mol Catal (China), 2016, 6(30): 575-582.[马占营, 李小博, 邓玲娟, 等. TiO2/Bi2WO6纳米异质结的制备及其可见光光催化性能[J]. 分子催化, 2016, 6(30): 575-582.]
[26] He P, Chen Y, Fu W F. Study of visible-light driven preparation of Fe/g-C3N4composite catalyst with simultaneous hydrogen evolution[J]. J Mol Catal (China), 2016, 30(3): 269-275.[何平, 陈勇, 傅文甫. 可见光驱动制备Fe/g-C3N4复合催化剂及其产氢研究[J].分子催化,2016, 30(3): 269-275.]
[27] Lu G X, Gao H X, Suo J S. Catalytic oxidation of cyclohexane into cyclohexanol and cyclohexanone over a TiO2/TS-1 system by dioxygen under UV irradiation [J]. Chem Commun, 1994, 21(21): 2423-2424.
[28] Shen Z, Zhong J Y, Wang L Y, et al. In-situ FTIR and SSNMR study of photocatalytic degradation of 2-CEES and DMMP on zirconium-doped TiO2[J]. J Mol Catal (China), 2016, 30(3): 260-268.[沈忠, 钟近艺, 王泠沄,等. 锆掺杂TiO2光催化降解2-CEES和DMMP 的原位红外与固体核磁研究[J].分子催化,2016,30(3):260-268.]
[29] Ni K, Chen L, Lu G X. Synthesis of silver nanowires with different aspect ratios as alcohol-tolerant catalysts for oxygen electro reduction[J]. Electrochem Commun, 2008, 10(7): 1027-1030.
[30] Wang L L, Wang Y, Liao W P, et al. Ethanol electrocatalyticoxidation performance of carbon black-supported Pt-Sn bimetallic catalysts[J]. J Mol Catal (China) 2015, 29(1): 35-44. [王琳琳,王赟,廖卫平,等. 炭黑负载Pt-Sn双金属催化剂对乙醇的电催化氧化性能[J]. 分子催化,2015, 29(1): 35-44.]
[31] Lu G, Zhang S, Hou G Y, et al. Effect of pH regulators on structural performance and photocatalytic degradation of RhB over BiOCl samples[J]. J Mol Catal (China) 2016, 30(2): 169-176.[陆光, 张爽, 侯冠宇,等. pH 调节剂对BiOCl结构和光催化降解RhB的影响[J]. 分子催化,2016,30(2):169-176.]
[32] Cui E T, Lu G X. Enhanced surface electron transfer by fabricating a core/shell Ni@NiO cluster on TiO2and its role on high efficient hydrogen generation under visible light irradiation[J]. Int J Hydrogen Energy, 2014, 39(17): 8959-8968.
[33] Wang L P, Wang G Y. Progress in metal-organic frameworks based on the carboxyl ligands as the catalyst[J]. J Mol Catal (China) 2015, 29(3): 275-287. [王丽苹, 王公应. 羧基配体金属有机骨架材料作为催化剂的研究进展[J]. 分子催化,2015, 29(3): 275-287.]
[34] Kong C, Min S X, Lu G X. Robust Pt-Sn alloy decorated graphene nanohybrid cocatalyst for photocatalytic hydrogen evolution[J]. Chem Commun, 2014, 50(66): 9281-9283.
[35] Ren H Y, Liu Z J, Xu S, et al. Rod-like ceria supported Pt as catalysts for methanol oxidation[J]. J Mol Catal (China), 2015, 29(3) :173-178. [任红艳, 刘郑娟, 许珊,等. 棒状CeO2负载Pt催化剂的合成及其电化学性能研究[J]. 分子催化,2015,29(2) :173-178.]
[36] Guo M, Lu G X. The effect of impregnation strategy on structural characters and CO2methanation properties over MgO modified Ni/SiO2catalysts[J]. Catal Commun, 2014, 54(5): 55-60.
[37] Ou Y J, Li S P, Bi Y P. Performance of highly efficient hydrogen production by alkaline formaldehyde solutions over Pd/BiOCl at room temperature[J]. J Mol Catal (China), 2015, 29(5): 441-447. [欧玉静, 李少鹏, 毕迎普. Pd /BiOCl高效室温催化甲醛产氢性能研究[J]. 分子催化,2015, 29(5): 441-447.]
[38] Xiang Q J, Yu J G, Jaroniec M. Graphene-based semiconductor photocatalysts[J]. Chem Soc Rev, 2012, 41(2): 782-796.
[39] Ni J, Luo X F, Zhan Y, et al. Application and progress of the novel activated carbonin the field of catalysis[J]. J Mol Catal (China), 2016, 30(3): 282-296. [倪军, 罗小芳, 詹勇,等. 新型碳材料在催化领域中的应用及进展[J].分子催化,2016, 30(3): 282-296.]
[40] Zhou Q L, Li L, Yang C L, et al. The Photocatalytic and hydrogen production of Pt-doped three-dimensionally ordered macroporous composite ZrO2by photoreduction method[J]. J Mol Catal(China), 2017, 31(3): 236-246.[周黔龙, 李莉, 杨长龙, 等. 光还原Pt掺杂三维有序大孔ZrO2复合材料的光降解与光解水制氢[J].分子催化, 2017, 31(3): 236-246.]
[41] Huang Z X, Li Y F, Li Y X, et al. Effect of polyvinyl alcohol on performance of Ni nanoparticles prepared by photoreduction of Ni2+and Its dye-sensitized photocatalytic hydrogen production[J]. J Mol Catal (China), 2017, 31(2): 181-187. [黄振星, 李亚飞, 李越湘,等. 聚乙烯醇对光还原Ni2+制备纳米Ni性能及染料敏化制氢的影响[J]. 分子催化, 2017, 31(2): 181-187.]
[42] Zhen W L, Li B, Lu G X, et al. Enhancing catalytic activity and stability for CO2methanation on Ni@MOF-5 via control of active species dispersion[J]. Chem Commun, 2015, 51(9): 1728-1731.
[43] Liu P, Lu J C, Chen D K, et al. Research of hydrogen production by thermocatalytic decomposition of methane on carbonaceous and metal catalysts[J]. J Mol Catal (China), 2016, 30(5): 480-495. [刘攀, 陆继长, 陈定凯等, 碳质与金属催化剂热催化裂解甲烷产氢研究进展[J]. 分子催化,2016,30(5):480-495.]
[44] Bard A J, Fox M A. Artificial photosynthesis: solar splitting of water to hydrogen and oxygen[J]. Acc Chem Res, 1995, 28(3): 141-145.
[45] Zhang W Y, Kong C, Lu G X. Super-paramagnetic nano-Fe3O4/graphene for visible-light-driven hydrogen evolution[J]. Chem Commun, 2015, 51(50): 10158-10161.
[46] Kong C, Li Z, Lu G X. The dual functional roles of Ru as co-catalyst and stabilizer of dye for photocatalytic hydrogen evolution[J]. Int J Hydrogen Energy, 2015, 40: 5824-5830.
[47] Li Z, Kong C, Lu G X. Rhodium tin composite oxides co-catalyst for high efficient photocatalytic hydrogen evolution[J]. Int J Hydrogen Energy, 2015, 40(17): 9061-9068.
[48] Kong C, Li Z, Lu G X. Noble-metal-free NiSnxOydecorated graphenecocatalyst for highly efficient reduction of water to hydrogen[J]. Int J Hydrogen Energy, 2015, 40(31): 9634-9641.
[49] Jiao W J, Wu Y Q, Lu G X, et al. Inhibition of the excited-state Rose Bengal (RB) nonradiative process by introducing DMSO for highly efficient photocatalytic hydrogen evolution[J]. RSC Adv, 2016, 6(35): 29538-29544.
[50] Ren Y L,Tian X Z, Ma J Y, et al. Mn-Catalyzed reductive cleavage of aromatic carbon-oxygen bonds[J]. J Mol Catal (China), 2016, 30(5): 401-408. [任运来, 田欣哲, 马军营,等. 锰催化芳香碳-氧键的还原断裂[J]. 分子催化,2016, 30(5): 401-408.]
[51] Li Z, Kong C, Lu G X. Visible photocatalytic water splitting and photocatalytic two-electron oxygen formation over Cu- and Fe-doped g-C3N4[J]. J Phys Chem C, 2016, 120(1): 56-63.
[52] Zhao X X, Lu G X. Modulating and controlling active species dispersion over Ni-Co bimetallic catalysts for enhancement of hydrogen production of ethanol steam reforming[J]. Int J Hydrogen Energy, 2016, 41(5): 3349-3362.
[53] Tian B, Li Z, Zhen W L, et al. Uniformly sized (112) facet Co2P on graphene for highly effective photocatalytic hydrogen evolution[J]. J Phys Chem C, 2016, 120(12): 6409-6415.
[54] Zhen W L, Gao H B, Tian B, et al. Fabrication of low adsorption energy Ni-Mo cluster cocatalyst in metal-organic frameworks for visible photocatalytic hydrogen evolution[J]. ACS Appl Mater Interfaces, 2016, 8(17): 10808-10819.
[55] Liu J, Liu Y, Liu N Y, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway[J]. Science, 2015, 347(6225): 970-974.
[56] Guo Y P, Lu G X. Graphene supported Co-Mo-P catalyst for efficient photocatalyzed hydrogen generation[J]. Int J Hydrogen Energy, 2016, 41: 6706-6712.
[57] Li Z, Wu Y Q, Lu G X. Highly efficient hydrogen evolution over Co(OH)2nanoparticles modified g-C3N4co-sensitized by Eosin Y and Rose Bengal under visible light irradiation[J]. Appl Catal B, 2016, 188: 56-64.
[58] Zhen W L, J T Ma, Lu G X. Small-sized Ni(111) particles in metal-organic frameworks with low over-potential for visible photocatalytic hydrogen generation[J]. Appl Catal B, 2016, 190: 12-25.
[59] Zhao X X, Lu G X. Improving catalytic activity and stability by in-situ regeneration of Ni-based catalyst for hydrogen production from ethanol steam reforming via controlling of active species dispersion[J]. Int J Hydrogen Energy, 2016, 41: 13993-14002.
[60] Hu X, Zhang L J, Lu G X. Steam reforming of acetic acid over CuZnCo catalyst for hydrogen generation: Synergistic effects of the metal species[J]. Int J Hydrogen Energy, 2016, 41(32): 13960-13969.
[61] Hu X, Dong D H, Shao X, et al. Steam reforming of acetic acid over cobalt catalysts: Effects of Zr, Mg and K addition[J]. Int J Hydrogen Energy, 2017, 42(8): 4793-4803.
[62] Tian B, Zhen W L, Gao H B, et al. Carboxyl-assisted synthesis of Co nanorods with high energy facet on graphene oxide sheets for efficient photocatalytic hydrogen evolution[J]. Appl Catal B, 2017, 203: 789-797.
[63] Li Z, Tian B, Zhen W L, et al. Inhibition of hydrogen and oxygen recombination using oxygen transfer reagent hemin chloride in Pt/TiO2dispersion for photocatalytic hydrogen generation[J]. Appl Catal B, 2017, 203: 408-415.
[64] Zhen W L, Gao F, Tian B, et al. Enhancing activity for carbon dioxidemethanation by encapsulating (111) facet Ni particle in metal-organic frameworks at low temperature[J]. J Catal, 2017, 348: 200-211.
[65] Tian B, Yang B J, Li J, et al. Water splitting by CdS/Pt/WO3-CeOxphotocatalysts with assisting of artificial blood perfluorodecalin[J]. J Catal, 2017, 350: 189-196.
[66] Hou Y D, Abrams B L, Vesborg P C K, et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution[J]. Nat Mater, 2011, 10(6): 434-438.
[67] Gao H B, Zhen W L, J T Ma, et al. High efficient solar hydrogen generation by modulation of Co-Ni sulfide (220) surface structure and adjusting adsorption hydrogen energy[J]. Appl Catal B, 2017, 206: 353-363.
[68] Zhang W Y, Yang S L, Li J, et al. Visible-to-ultraviolet upconvertion: Energy transfer, material matrix, and synthesis strategies[J]. Appl Catal B, 2017, 206: 89-103.
[69] Tian B, Li Z, Zhen W L, et al. Fe2S2nano-clusters catalyze water splitting by removing formed oxygen using aid of an artificial gill under visible light[J]. J Catal, 2017, 352: 572-578.
[70] Hao X Q, Jin Z L, Lu G X, et al. Peculiar synergetic effect of MoS2quantum dots and graphene on metal-organic frameworks for photocatalytic hydrogen evolution[J]. Appl Catal B, 2017, 210: 45-56.
[71] Wang M, Li Z, Wu Y Q, et al. Inhibition of hydrogen and oxygen reverse recombination reaction over Pt/TiO2by F-ions and its impact on the photocatalytic hydrogen formation[J]. J Catal, 2017, 353: 162-170.
[72] Ning X F, Li J, Yang B J, et al. Inhibition of photocorrosion of CdS via assembling with thin film TiO2and removing formed oxygen by artificial gill for visible light overall water splitting[J]. Appl Catal B, 2017, 212: 129-139.
[73] Gao W, Zhang W Y, Lu G X. A two-pronged strategy to enhance visible-light-driven overall water splitting via visible-to-ultraviolet upconversion coupling with hydrogen-oxygen recombination inhibition[J]. Appl Catal B, 2017, 212: 23-31.
[74] Lu G X, S B Li. Hydrogen production by H2S photodecomposition on ZnFe2O4catalyst[J]. Int J Hydrogen Energy, 1992, 17(10): 767-770.
[75] Li X Y, Lu G X, Li S B. Synthesis and characterization of fine particle ZnFe2O4powders by a low temperature method[J]. J Alloys Compounds, 1996, 235(2): 150-155.
[76] Lu G X, Li S B. Effects of surface etching on the structure and performance of Rh2O3/CdS catalyst[J]. J Photobiol Photochem A Chem, 1996, 97(1-2): 65-72.
[77] Clough A J, Yoo J W, Mecklenburg M H, et al. Two-dimensional metal-organic surfaces for efficient hydrogen evolution from water[J]. J Am ChemSoc, 2015, 137(1): 118-121.
[78] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37.
[79] Zhang J, Xu Q, Feng Z, et al. Importance of the relationship between surface phases and photocatalytic activity of TiO2[J]. Angew ChemInt Ed, 2008, 47(9): 1766-17699.
[80] Xiang Q J, Yu J G, Jaroniec M. Synergetic effect of MoS2and graphene as cocatalysts for enhanced photocatalytic H2production activity of TiO2nanoparticles[J]. J Am Chem Soc, 2012, 134(15): 6575-6578.
[81] Wen J, Xie J, Chen X, et al. A review on g-C3N4-based photocatalysts[J]. Appl Surf Sci, 2017, 391: 72-123.
[82] Ran J, Zhang J, Yu J, et al. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting[J]. Chem Soc Rev, 2014, 43(22): 7787-7812.
[83] Kudo S, Miseki Y. Heterogeneous photocatalyst materials for watersplitting[J]. Chem Soc Rev, 2009, 38(1): 253-278.
[84] Li X, Yu J G, Wageh S, et al. Graphene in photocatalysis: A review[J]. Small, 2016(48), 12: 6640-6696.
[85] Li X, Yu J G, Jaroniec M. Hierarchical photocatalysts[J]. Chem Soc Rev, 2016, 45(9): 2603-2636.
[86] Grätzel M. Photoelectrochemical cells[J]. Nature, 2001, 414: 338-344. DOI:
[87] Annabella S. Crystal growth: Anatase shows its reactive side[J]. Nat Mat, 2008, 7(8): 613-615.
[88] Subramanyam K, Sreelekha N, Reddy D A, et al. Chemical synthesis, structural, optical, magnetic characteristics and enhanced visible light active photocatalysis of Ni doped CuS nanoparticles[J]. Solid State Sci, 2017, 65: 68-78.
[89] Xiang Q, Cheng B, Yu J. Graphene-based photocatalysts for solar-fuel generation[J]. Angew ChemInt Ed, 2015, 54(39): 11350-11366.
[90] Zhang Z, Zhang Y J, Lu L H, et al. Graphitic carbon nitride nanosheet for photocatalytic hydrogen production: The impact of morphology and element composition[J]. Appl Surf Sci, 2017, 391: 369-375.
[91] Wang P, Lu Y G, Wang, X F. Co-modification of amorphous-Ti(IV) hole cocatalyst and Ni(OH)2electron cocatalyst for enhanced photocatalytic H2-production performance of TiO2[J]. Appl Surf Sci, 2017, 391: 259-266.
[92] Ni Z L, Sun Y J, Zhang Y X, et al. Fabrication, modification and application of (BiO)2CO3-based photocatalysts: A review[J]. Appl Surf Sci, 2016, 365: 314-335.
[93] Liu Y X, Wang Z L, Huang W X. Influences of TiO2phase structures on the structures and photocatalytic hydrogen production of CuOx/TiO2photocatalysts[J]. Appl Surf Sci, 2016, 389: 760-767.
[94] Mahmood J, Li F, Jung S M, et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nat Nanotechnol, 2017, 12(5): 441-446.
[95] Gao M M, Connora P K N, Ho G W. Plasmonicphotothermic directed broadband sunlight harnessing for seawater catalysis and desalination[J]. Energy Environ Sci, 2016, 9: 3151-3160.
[96] Strmcnik D, Lopes P P, Genorio B, et al. Design principles for hydrogen evolution reaction catalyst materials[J]. Nano Energy, 2016, 29: 29-36.
[97] Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting[J]. Chem Soc Rev 2009, 38(1): 253-278.
[98] Chen X B, Shen S H, Guo L J, et al.Semiconductor-based photocatalytic hydrogen generation[J]. Chem Rev, 2010, 110(11): 6503-6570.
[99] Tong H, Ouyang S X, Bi Y P, et al. Nano-photocatalytic materials: Possibilities and challenges[J]. Adv Mat, 2012, 24(2): 229-251.
[100] Zhang W Y, Lu G X. The enhancement of electron transportation and photo-catalytic activity for hydrogen generation by introducing spin-polarized current into dye-sensitized photo-catalyst [J]. Catal Sci Technol, 2016, 6(21): 7693-7697.
[101] Xiang Q J, Yu J G. Graphene-based photocatalysts for hydrogen generation[J]. J Phys Chem Lett, 2013, 4(5): 753-759.
[102] Min S X, Lu G X. Sites for high efficient photocatalytic hydrogen evolution on a limited-Layered MoS2cocatalystconfined on graphenesheets-The role of graphene[J]. J Phys Chem C, 2012, 116(48): 25415-25424.
[103] Michaeli K, Kantor-Uriel K, Naaman R, et al. The electron's spin and molecular chirality-how are they related and how do they affect life processes[J] Chem Soc Rev, 2016, 38(1): 188-229.
[104] Mtangi W, Kiran V, Fontanesi C, et al. Role of the electron spin polarization in water splitting[J]. J Phys Chem Lett, 2015, 6(24): 4916-4922.
[105] Wilbert R M, Franscesco T, Kiran V, et al. Control of electrons’ spin eliminates hydrogen peroxide formation during water splitting[J]. J Am Chem Soc, 2017, 139(7): 2794-2798.
[106] Li Q, Guo B D, Yu J G, et al. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphenenanosheets[J]. J Am Chem Soc, 2011, 133: 10878-10884.
[107] Liu J, Zhang Y, Lu L, et al. Self-regenerated solar-driven photocatalytic water-splitting by urea derived graphitic carbon nitride with platinum nanoparticles[J]. Chem Commun, 2012, 48(70): 8826-8828.
[108] Zhang X Q, Lu G X. The spin-orbit coupling induced spin flip and its role in the enhancement of the photocatalytic hydrogen evolution over iodinated graphene oxide[J]. Carbon, 2016, 108: 215-224.
[109] Li Z, Tian B, Zhang W Y, et al. Enhancing photoactivity for hydrogen generation by electron tunneling via flip-flop hopping over iodinated graphitic carbon nitride[J]. Appl Catal B, 2017, 204: 33-42.
[110] Song F Q, Wang B G, Wang B L, et al. High-mobility Sm-doped Bi2Se3ferromagnetic topological insulators and robust exchange coupling[J]. Adv Mater, 2015, 27(33): 4823-4829.
[111] Linder J, Grønsleth M S, Sudbø A. Tunneling currents in ferromagnetic systems with multiple broken symmetries[J]. Phys Rev B, 2007, 75(2): 024508.
[112] Wang X F, Pan X C, Gao M, et al. Evidence of both surface and bulk Dirac bands and anisotropic nonsaturating magnetoresistance in ZrSiS[J]. Adv Electron Mater, 2016, 2(10): 1600228.
[113] Zhang K, Pan H Y, Zhang M H,et al. Controllable synthesis and magnetotransport properties of Cd3As2Dirac semimetal nanostructures[J]. RSC Adv,2017, 7(29): 17689-17696.
[114] Torun E, Fang C M, Wijs G A D, et al. Role of magnetism in catalysis: RuO2(110) surface[J]. J Phys Chem C, 2013, 117(12): 6353-6357.
[115] Mtangi W, Kiran V, Fontanesi C, et al. Role of the electron spin polarization in water splitting[J]. J Phys Chem Lett, 2015, 6(24): 4916-4922.
[116] Farrell J R, Mirkin C A, Guzei I A, et al. The weak-link approach to the synthesis of inorganic macrocycles[J]. Angew Chem Int Ed, 1998, 37(4): 465-467.
[117] Holliday B J, Mirkin C A. Strategies for the construction of supramolecular compounds through coordination chemistry[J]. Angew ChemInt Ed, 2001, 40(11): 2022-2043.
[118] Spitler E L, Johnson C A, Haley M M. Renaissance of annulenechemistry[J]. Chem Rev, 2006, 106(12): 5344-5386.
[119] Gianneschi N C, Bertin P A , Nguyen S T, et al. A supramolecular approach to an allosteric catalyst[J]. J Am Chem Soc, 2003, 125(35): 10508-10509.
[120] John R P, Park M, Moon D, et al. A chiral pentadecanuclear metallamacrocycle with a sextuple twisted Möbiustopology[J]. J Am Chem Soc, 2007, 129(46): 14142-14143.
[121] Zhao N, Dong H, Yang S, et al. Observable topological effects in molecular devices with Möbius topology[J]. Phys Rev B. 2009, 79(12): 125440.
[122] Ballon D J , Voss H U. Classical Möbius-ring resonators exhibit Fermion-Boson rotational symmetry[J]. Phys Rev Lett, 2008, 101(24): 247701.
[123] Starostin E L,Heijden G H M Van Der. The shape of a Möbius strip[J]. Nat Mat, 2007, 6: 563-567.
[124] Yamashiro A, Shimoi Y, Harigaya K, et al. Novel electronic states in graphene ribbons-competing spin and charge orders[J]. Physica E, 2004, 22(1-3): 688-691.
[125] Zheng Y P , Xu L Q , Fan Z Y, et al. A molecular dynamics investigation of the mechanical properties of graphene nanochains[J]. J Mater Chem, 2012, 22(19): 9798-9805.
[126] Wang X L, Zheng X H, Ni M. Y, et al. Theoretical investigation of Möbius strips formed from graphene[J]. Appl Phy Lett, 2010, 97: 123103.
[127] Tanda S, Tsuneta T, Okajima Y, et al. Crystal topology: A Möbius strip of single crystals[J]. Nature, 2002, 417: 397-398.
[128] Kui S C , Huang J S , Sun R W, et al. Self-assembly of a highly stable, topologically interesting metallama- crocycle by bridging gold(i) ions with pyridyl-2,6-diphenyl2- and diphosphanes[J]. Angew Chem Int Ed, 2006, 45: 4663-4666.
[129] Jiang D, Dai S. Spin states of Zigzag-edged Möbiusgraphene nanoribbons from first principles[J]. J Phys Chem C, 2008, 112(14): 5348-5351.
[130] Guo Z L, Gong Z R, Dong H, et al. Spin-orbit coupling effects in two-dimensional circular quantum rings: elliptical deformation of confined electron density[J]. Phys Rev B, 2009, 80(19): 195319.
[131] Li Z, Ram-Mohan L R. Quantum mechanics on a Möbius ring: Energy levels, symmetry, optical transitions, and level splitting in a magnetic field[J]. Phys Rev B, 2012, 85: 195438.
[132] Li Z, Tian B, Zhang W Y, et al. Enhancing photoactivity for hydrogen generation by electron tunneling via flip-flop hopping over Möbius strip-like RGO[J]. Appl Catal B, 2017, 219: 501-510.
[133] Min B C, Motohashi K, Lodder C, et al. Tunable spin-tunnel contacts to silicon using low-work-function ferromagnets[J] Nat Mater, 2006, 5: 817-822.
[134] Shi X F, Hu X H, Fan B B, et al. Preparation and cyclohexane oxidation click catalysis of periodic mesoporousorganoilicas functionalized withV-Schiiff base[J]. J Mol Catal (China), 2015, 29(2): 126-134.[史秀峰,胡晓虹,范彬彬,等. 钒席夫碱官能化PMOs的制备及在环己烷氧化中的点击催化作用[J]. 分子催化, 2015, 29(2): 126-134.]
[135] Ren H Y, Liu Z J, Xu S, et al. Rod-like ceris supported Pt as catalysts for methanol oxidation [J]. J Mol Catal (China), 2015, 29(2): 173-178. [任红艳,刘郑娟,许珊,等. 棒状CeO2负载Pt催化剂的合成及其电化学性能研究[J]. 分子催化,2015, 29(2): 173-178.]
[136] Guo N,Chen S L,Liu J Q,et al. The mechanism of GD hydrolysis catalyzed by marcrocyclicployamino metal artificial enzyme [J]. J Mol Catal (China), 2015, 29(6): 575-585.[郭楠,陈世稆,刘景泉,等. 大环多胺金属模拟酶催化水解梭曼机理的研究[J]. 分子催化,2015, 29(6): 575-585.]
[137] Wang H L, Yu S Y, Peng J, et al. Quantum chemical study on TS-1 oxidation of thiophene and methythiophene reaction mechanism [J]. J Mol Catal (China), 2015, 29(5): 458-466.[王寒露,余思钰,彭晶,等.含缺陷位TS-1催化氧化噻吩及甲基噻吩反应机理的量子化学研究[J]. 分子催化,2015, 29(5): 458-466.]
[138] Wang L L, Wang Y, Liao W P, et al. Ethanol electrocatalytic oxidation performance of carbon black-supported Pt-Sn bimetallic catalysts [J]. J Mol Catal (China), 2015, 29(1): 35-44.[王琳琳,王赟,廖卫平,等. 碳黑负载Pt-Sn双金属催化剂对乙醇的电催化氧化性能[J]. 分子催化, 2015, 29(1): 35-44.]
[139] Liu H Y, Bai J, Wang J Z, et al. Ullmann-type coupling reaction catalyzed by SAPO-34 supported copper nanoparticles [J]. J Mol Catal (China), 2016, 30(4): 317-323.[李恒宇,白杰,王俊忠,等. SAPO-34分子筛载铜催化剂催化Ullmann偶联反应[J]. 分子催化,2016, 30(4): 317-323.]
[140] Wang Q Y, Tong Y C, Xu X J, et al. The influence of the Stone-wales defects in graphene on the Platinum catalyzed dissociation of oxygen [J]. J Mol Catal (China), 2016, 30(1): 80-87. [王清云,佟永纯,徐新建,等. 石墨烯中的Stone-wales缺陷对铂原子催化解离氧分子的影响[J]. 分子催化,2016, 30(1): 80-87.]
[141] Ren Y L, Wang W H, Tian Q Z, et al. Mn-catalyzed reductive cleavage of aromatic carbon-oxygen bonds [J]. J Mol Catal (China), 2016, 30(5): 401-408. [任运来,王文会,田欣哲,等. 锰催化芳香碳-氧键的还原断裂[J]. 分子催化,2016, 30(5): 401-408.]
[142] Yang J, Niu L H, Zhang Z J. Study electrocatalytic performance for oxygen reduction reaction of dopamine derived transition metal-nitrogen codoped carbon nanotube [J]. J Mol Catal (China), 2016, 30(5): 409-419. [杨建,牛丽红,张治军. 多巴胺为前驱体过渡金属与N共掺杂的碳纳米管催化剂ORR性能研究[J]. 分子催化,2016, 30(5): 409-419.]
[143] Tang X J, Zhang Z J, Li Z N, et al. Highly efficient copper-catalyzed N-Arylation of amine with arythalide usinghydrazine as ligand [J]. J Mol Catal (China), 2016, 30(5): 420-427. [唐旭静,张占金,李争宇,等. 腙为配体的高效铜催化的胺的N-芳基化反应[J]. 分子催化,30(5): 420-427.]
[144] Gao T, Song H Y, Chen J. Supported ionic liquid as novel catalyst for the prins reaction of olifins and formaldehyde [J]. J Mol Catal (China), 2016, 30(3): 199-206. [高腾,宋河远,陈静. 负载型离子液体催化芳香烯和甲醛Prins反应[J]. 分子催化,2016, 30(3): 199-206.]
[145] Chen X N, Gao B, Xie P, et al. Palladium-catalyzed oxidative carbonylation of arylazos via N=N bond cleavage[J]. J Mol Catal (China), 2016, 30(3): 207-213.[陈向宁,高宝,解攀,等. 钯催化芳基偶氮的氧化羰基化反应[J]. 分子催化,2016, 30(3): 207-213.]
[146] Fan H C, Xin J Y, Wan Y, et al. Methanobactin-mediated one-step synthesis of silver nanoparticles [J]. J Mol Catal (China), 2016, 30(3): 276-281. [范洪臣,辛家英,王艳,等. 甲烷氧化菌素介导一步法合成纳米银[J]. 分子催化,2016, 30(3): 276-281.]
[147] Ren X Y, Zheng L, Wang Z, et al. Rh-catalyzed hydroformylation of alkynes toα,β-unsaturated aldehydes [J]. J Mol Catal (China), 2016, 30(6): 497-504. [任新意,张磊,王正,等. 铑催化炔烃氢甲酰化反应合成α,β-不饱和醛[J]. 分子催化,2016, 30(6): 497-504.]
自旋电子学-光催化产氢交叉学科研究中的测试表征技术进展
张文妍1,2,3, 高 薇1,2, 张旭强1, 李 振1,2, 吕功煊1
(1. 中国科学院 兰州化学物理研究所 羰基合成与选择氧化国家重点实验室,甘肃 兰州 730000; 2. 中国科学院大学,北京 10080; 3. 金陵科技学院, 江苏 南京 211169)
清洁能源的研究和开发为解决化石燃料的日益枯竭问题带来了希望. 氢能燃烧热值高,产物零污染,是理想的清洁能源. 利用太阳能,通过光催化反应从水中制取氢气,是一条极有发展前景的制氢途径. 然而,太阳能光催化制氢的发展受到许多因素的限制,特别是光电子传输过程中的电子-空穴复合及能量损失导致的电子输运效率低以及高的产氢产氧过电位导致水分解过程的势垒增大. 自旋电子学的发展,为太阳能光催化制氢中的这些问题提供了解决之道. 通过将自旋电子学的思路及原理应用于太阳能光催化制氢,借助自旋输运及电子隧穿可有效提高电子的输运效率,光电子的自旋极化还可降低产氢产氧过电位并抑制副产物的生成. 测试表征技术的发展为揭示自旋电子学-太阳能光催化制氢交叉科学的内秉机理做出了重要贡献. 然而,目前尚无相关文籍对此类测试表征技术的发展进行总结和评述. 考虑到这些测试表征技术在自旋电子学-太阳能光催化制氢交叉科学研究中的重要作用,对它们进行归纳和总结,评述其发展面临的问题与挑战,探索并合理预测其未来的发展方向.
光催化;产氢;表征;自旋检测;自旋增益的光催化产氢
综述(219~236)
date: 2017-11-20;
date: 2017-12-04.
Foundation: The National Natural Science Foundation of China (Grant Nos. 21433007 and 21673262) and the 973 Program of Department of Sciencesand Technology China (Grant No. 2013CB632404)
Zhang Wen-yan(1985-), female, PhD, photocatalysis and new materials, E-mail: zhangwenyan8531@163.com
LYU Gong-xuan(1964-), PhD, Researcher, E-mail: gxlu@lzb.ac.cn, Tel: +86-931-4968178.
O643.32Documentcode: AArticleID:1006-3757(2017)04-0219-18
10.16495/j.1006-3757.2017.04.004

