复合抗菌肽对山羊瘤胃菌群结构的影响
陈 芸,刘 旗,邓俊良,杨颜铱,高 爽,陈 憧,姚淑华
(四川农业大学 动物医学院,动物疫病与人类健康四川省重点实验室/环境公害与动物疾病四川省高校重点实验室,四川 温江 611130)
复合抗菌肽对山羊瘤胃菌群结构的影响
陈 芸,刘 旗,邓俊良*,杨颜铱,高 爽,陈 憧,姚淑华
(四川农业大学 动物医学院,动物疫病与人类健康四川省重点实验室/环境公害与动物疾病四川省高校重点实验室,四川 温江 611130)
试验旨在探讨复合抗菌肽对山羊瘤胃菌群结构的影响,以期为抗菌肽应用于反刍动物提供理论依据。选取24只4月龄川中黑山羊,分为4组,每组6只,正常精料组(A),正常精料+抗菌肽组(C),双倍精料组(D),双倍精料+抗菌肽组(E)分别饲喂精料300、300、600和600 g·d-1·只-1,C与E组饲喂3 g·d-1·只-1复合抗菌肽。于21 d晨饲前每组随机选取3只采集瘤胃液,提取样品总DNA,扩增16S rRNA V4区,扩增产物采用Miseq平台测序。结果表明:1)共获得高质量序列629 634条,97%相似度下聚类后共获得13 227个OTU。2)所得OTU经物种注释后,门水平上,除D组最优势菌门为厚壁菌门(Firmicutes)(34.30%)外,其余各组最优势菌门均为拟杆菌门(Bacteroidetes)(38.52%~43.68%),且添加复合抗菌肽显著降低变形菌门(Proteobacteria)与螺旋菌门(Spirochaetes)含量(P<0.05);增加精料量显著增加厚壁菌门与螺旋菌门含量(P<0.05),显著降低拟杆菌门与变形菌门含量(P<0.05)。3)属水平上,普雷沃菌属(Prevotella)为所有样品中最优势菌属(25.54%~33.88%),添加抗菌肽后,琥珀酸菌属(Succiniclasticum)、瘤胃球菌属(Ruminococcus)、假丁酸弧菌属(Pseudobutyrivibrio)、脱硫弧菌属(Desulfovibrio)显著或极显著增加(P<0.05或P<0.01),琥珀酸弧菌属(Succinivibrio)极显著降低(P<0.01),且不受精料量影响,但月形单胞菌属(Selenomonas)与密螺旋体(Treponema)的变化趋势与精料量相关,正常精料组显著降低(P<0.05),而双倍精料组无显著变化(P>0.05);增加精料量显著增加普雷沃菌属、密螺旋体等含量(P<0.05),降低琥珀酸弧菌属等含量(P<0.05)。4)各样品在 alpha 多样性Chao、ACE、Shannon和Simpson指数上,差异不显著(P>0.05)。表明添加复合抗菌肽降低变形菌门和螺旋菌门的相对含量,提高部分降解纤维菌属的含量;增加精料量可增加厚壁菌门和螺旋菌门相对含量,降低拟杆菌门和变形菌门的相对含量,增加普雷沃菌属相对含量,降低琥珀酸弧菌属相对含量;瘤胃细菌多样性并不受复合抗菌肽及精料量的影响。
山羊;瘤胃细菌;菌群结构;高通量测序;复合抗菌肽;精料
反刍动物瘤胃生态系统是一个相当复杂的微生态系统,与瘤胃内营养物质的消化吸收及宿主健康极其相关[1],饲粮在瘤胃中消化降解,转化为挥发性脂肪酸,为宿主提供主要能量[2]。瘤胃微生物包括细菌、原虫、真菌和古菌等,其中,瘤胃细菌是瘤胃微生态重要组成部分,数量约为1×1011个·mL-1[3],在瘤胃生态系统中具有重要生物学意义,包括降解植物纤维、蛋白质、淀粉等[4-5]。随着反刍动物营养代谢研究的不断深入,反刍动物瘤胃内菌群结构的变化与宿主之间复杂的关系成为瘤胃营养代谢研究的热点。
抗菌肽(antimicrobial peptides)是一类存在于动植物及无脊椎动物组织和细胞内的防御性多肽物质,具有广谱杀菌、抗真菌、提高机体免疫力等作用[6],并且不易产生耐药性,是替代抗生素的新选择[7]。目前,已有研究结果表明抗菌肽作为饲料添加剂,可提高动物生长性能[8-9]、机体免疫力[10]、抗氧化功能[11]等。另有研究表明,抗菌肽可调节肠道菌群结构,降低肠道内有害菌数量、促进有益菌生长,改善肠道健康,促进动物生长[12-14]。目前,抗菌肽作为饲料添加剂用于反刍动物的研究还未见报道。本试验利用MiSeq高通量测序技术探讨不同精料下复合抗菌肽对山羊瘤胃细菌菌群结构的影响,为今后复合抗菌肽应用于反刍动物生产中提供理论依据。
1 材料与方法
1.1 试验药品及日粮组成
复合抗菌肽“态康利保”由猪防御素(37个氨基酸)和苍蝇抗菌肽(40个氨基酸)复合而成,各占50%,由四川华德生物工程有限公司提供,包装规格:每袋500 g。基础日粮组成如表1所示。
1.2试验设计与试验动物管理
表1基础日粮组成及营养水平
Table1Composition and nutrient levels of the concentrate

原料Ingredients含量Content/%营养Nutrients2)水平Levels玉米Corngrain51消化能DE/(MJ·kg-1)13.34麦麸Barleygrain23干物质DM/%84.27菜籽饼Rapeseedcake10粗蛋白质CP/%16.66豆粕Soybeanmeal10粗纤维CF/%4.17鱼粉Fishmeal3中性洗涤纤维NDF/%13.72食盐NaCl1酸性洗涤纤维ADF/%6.91预混料Premix1)2合计Total100
1)预混料为每kg饲粮提供:铁(硫酸亚铁)30 mg,Cu(硫酸铜)10 mg,Zn(硫酸锌)50 mg,Mn(硫酸锰)60 mg,VA 2 937 IU,VD 343 IU,VE 30 IU;2)计算值
1) Premix provides the following to per kg of the diet: Fe( as ferrous sulfate) 30 mg, Cu( as copper sulfate) 10 mg, Zn( as zinc sulfate) 50 mg, Mn( as manganese sulfate) 60 mg, VA 2 937 IU, VD 343 IU, VE 30 IU; 2) Nutrient levels were calculated values
选择24只4月龄、体质量相近(16.17±0.72)kg的健康雄性(未阉割)川中黑山羊,随机分成4组,每组6只。A组,正常精料组(300 g·d-1精料);C组,正常精料+抗菌肽(300 g·d-1精料+3.0 g·d-1抗菌肽);D组:双倍精料组(600 g·d-1精料);E组:双倍精料+抗菌肽(600 g·d-1精料+3.0 g·d-1抗菌肽)。括号中饲喂量为每只山羊每天饲喂量。每天8:00和18:00各饲喂精料一次,精料全部采食后,饲喂足够新鲜青草,自由采食,单栏群养,全天自由饮水。羊舍温度20 ℃。预饲10 d后开始正式试验。
1.3 样品采集及基因组DNA的提取
于正式试验第20天,每组随机选取3只羊在晨饲前用真空泵胃管抽吸法[15]采集瘤胃液(100 mL·只-1),4层100目纱布过滤,111.8g离心5 min,分装,-70 ℃中保存。各样品取2 mL 瘤胃液用于总DNA提取(试剂盒为天根生化科技公司产品),将所提取的总基因组DNA于-20 ℃保存备用。
1.4 16S rDNA基因的扩增及MiSeq测序
细菌基因组文库构建及上机测序均由上海派森诺科技有限公司完成。以总DNA为模板,对细菌16S rRNA V4区进行PCR扩增,建立DNA文库,所采用的细菌通用引物为:520F(5’-GCACCTAAYTGGGYDTAAAGNG-3’)和802R(5’- TACNVGGGTATCTAATCC-3’), PCR产物进行2%琼脂糖凝胶电泳,并用Axygen 凝胶回收试剂盒回收目的片段。对文库质检和定量,将合格的文库利用MiSeq Reagent Kit V3(600 cycles)进行2×300 bp的双端测序。
1.5 数据分析
测序原始数据以Fastq格式保存,利用FLASH软件(v1.2.7)筛选(按照引物和Index信息,识别分配入对应样本,并去除嵌合体等疑问序列)而获得每个样本的有效序列,再运用QIIME 1.8.0识别疑问序列(要求序列长度≥ 150 bp,且不允许存在模糊碱基N,剔除5’端引物错配碱基数>1的序列以及含有连续相同碱基数>8的序列),筛选得到高质量序列。调用Uclust序列比对工具,按97%的序列相似度进行归类和可操作分类单元(Operational taxonomic units OUT)划分。将每个OTU中丰度最高的序列作为该OTU的代表序列,并与Greengenes数据库(Release 13.8,http://greengenes.secondgenome.com/)的模板序列相比对,获取每个OTU所对应的分类学信息,并获得每个样本在各分类水平的具体组成。使用QIIME软件绘制稀疏曲线,并分别对每个样本计算Alpha多样性指数(Chao1、ACE、Shannon、Simpson),并通过R软件进行菌群结构分析。采用Excel 2003整理数据,SPSS Statistics 20软件进行配对样本t检验,P<0.05为差异显著,P<0.01为差异极显著,结果以平均值±标准差表示。
2 结果与分析
2.1 测序深度及多样性分析
本试验利用Illumina MiSeq平台对细菌V4区测序,12个样品共获得高质量序列629 634条,平均每条序列的长度为225 bp。将序列聚类后(97%相似度下)共获得13 227个OUT, A组平均获得1 211个OTU,C组平均获得1 192个OTU,D组平均获得947个OTU,E组平均获得1 058个OTU。由样品稀释曲线(图1)所示,在本试验的测序深度下,各样品稀疏曲线最终均趋于平缓,表明本试验测序深度下可以覆盖各样品的大多数微生物。
图2-A显示,本次试验各样品聚类在一起,PCA获得主成分1(PC1)的贡献率为51.62%, 主成分2(PC2)的贡献率为26.16%;门水平分类上的丰度热图(图2-B)显示各组样品的主要菌群来自螺旋菌门Spirochaetes、厚壁菌门Firmicutes、浮霉菌门Planctomycetes、无壁菌门Tenericutes、放线菌门Actinobacteria、BacteriaTM7、黏胶球形菌门Lentisphaerae、疣微菌门Verrucomicrobia、纤维杆菌门Fibrobacteres、绿弯菌门Chloroflexi、Thermi、Elusimicrobia、Bacteria LD1、变形菌门Proteobacteria、酸杆菌门Acidobacteria、梭杆菌门Fusobacteria、衣原体Chlamydiae、Bacteria WPS-2、互养菌门Synergistetes、拟杆菌门Bacteroidetes、Armatimonadetes、蓝细菌门Cyanobacteria、BacteriaSR1和广古菌门Euryarchaeota。
Alpha多样性常用于反映微生物群落的丰富度和均匀度,常用的度量指数主要包括侧重于体现物种丰富度的Chao指数和ACE指数,以及兼顾群落均匀度的Shannon指数和Simpson指数。

图1 稀释曲线Fig.1 Rarefaction curves
各组多样性指数见表2,其中C组与A组无显著差异(P>0.05),E组各指数高于D组,但差异不显著(P>0.05),D(E)组各指数低于A(C)组,但差异亦不显著(P>0.05)。由此可见,瘤胃细菌多样性并不受本试验下复合抗菌肽及精料量改变的影响。
2.2菌群结构分析
在门水平上共获得23个细菌门类,同时还获得古菌界下广古菌门。各样品的优势菌门均为拟杆菌门、厚壁菌门、变形菌门(表3),其他门类相对含量较低,如:无壁菌门、螺旋体门等。A组、C组和E组最优势菌门为拟杆菌门(40.87%、43.68%、38.52%),其次为厚壁菌门(27.19%、29.65%、31.91%),而D组中最优势菌门为厚壁菌门(35.29%),其次为拟杆菌门(34.30%)。组间差异显著或极显著菌群统计如表3所示,与相应对照组相比,C、E两组中变形菌门显著降低(P<0.05),螺旋菌门极显著降低(P<0.01),说明添加抗菌肽后,可显著降低变形菌门、螺旋菌门的相对含量。另外,与A组相比,D组中拟杆菌门、变形菌门的含量显著降低(P<0.05),厚壁菌门、螺旋菌门含量显著增加(P<0.05)。

图2 瘤胃样品细菌 OTU 的主成分分析图(A)及其门水平分类上的热图(B)Fig.2 PCA profile(A) and heatmap in phylum(B) of the samples from the rumen
表2Alpha多样性指数对比
Table2Comparison of alpha diversity indices
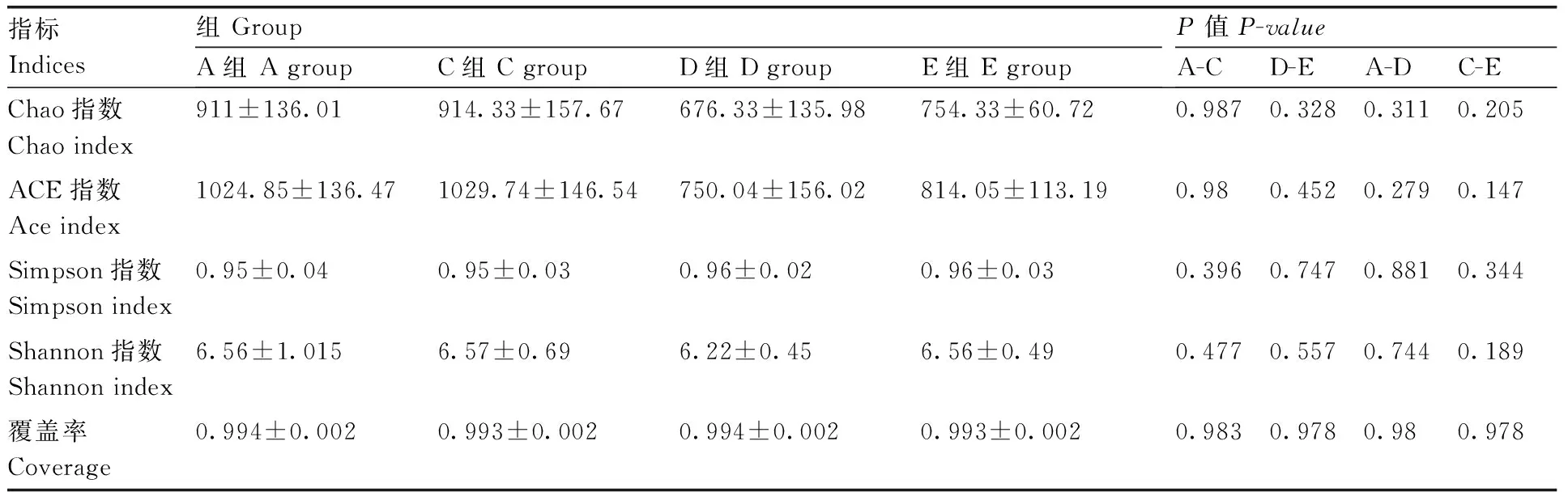
指标Indices组GroupA组AgroupC组CgroupD组DgroupE组EgroupP值P-valueA-CD-EA-DC-EChao指数Chaoindex911±136.01914.33±157.67676.33±135.98754.33±60.720.9870.3280.3110.205ACE指数Aceindex1024.85±136.471029.74±146.54750.04±156.02814.05±113.190.980.4520.2790.147Simpson指数Simpsonindex0.95±0.040.95±0.030.96±0.020.96±0.030.3960.7470.8810.344Shannon指数Shannonindex6.56±1.0156.57±0.696.22±0.456.56±0.490.4770.5570.7440.189覆盖率Coverage0.994±0.0020.993±0.0020.994±0.0020.993±0.0020.9830.9780.980.978
在属水平上,所有样品共检测到35个属,各样品属水平菌群结构见图3,各组中均含有大量未知属,所占的比例高达40.27%、39.96%、36.58%与38.41%,这充分显示出瘤胃内还有很多有价值的生物种群信息需要深入挖掘。
组间差异显著或极显著菌群统计结果见表3,各样品中普雷沃菌属Prevotella为所有样品中最优势菌属;由表3可知,添加抗菌肽后琥珀酸菌属Succiniclasticum、瘤胃球菌属Ruminococcus、假丁酸弧菌属Pseudobutyrivibrio、脱硫弧菌属Desulfovibrio显著或极显著增加(P<0.05或P<0.01),琥珀酸弧菌属Succinivibrio极显著降低(P<0.01),且不受精料量影响,但添加抗菌肽后月形单胞菌属Selenomonas与密螺旋体Treponema的变化趋势与精料量相关,正常精料组显著降低(P<0.05),而双倍精料组无显著变化(P>0.05),伯克氏菌属Burkholderia与慢生根瘤菌属Bradyrhizobium的变化也与精料量相关,但含量过少。另外,D组与A组相比,组间相对丰度差异达显著水平(P<0.05)的共有4个属,普雷沃菌属、假丁酸弧菌属、密螺旋体的相对丰度显著增加,伯克氏菌属显著降低;差异极显著(P<0.01)的有2个属,琥珀酸弧菌属极显著降低,慢生根瘤菌属极显著增加。
表3两组间差异显著和极显著的菌属
Table3The phylum and genus that were significantly different or extremely significantly different between groups

分类Classification组间有显著差异的菌群结构Significantlydifferentbacteriacommunitystructurebetweengroups/%ACDEP值P-valueA-CD-EA-DC-E拟杆菌门Bacteroidetes40.87±2.1943.68±3.5334.30±3.6738.52±2.950.4830.0580.0260.104普雷沃氏菌Prevotella25.54±2.6528.71±4.7731.57±3.9033.88±3.620.4670.5380.0480.349厚壁菌门Firmicutes27.19±1.7729.65±3.3235.29±1.5331.91±1.980.3870.6770.0160.472月形单胞菌属Selenomonas2.95±0.161.75±0.442.75±0.652.98±0.190.0420.6250.2940.018琥珀酸菌属Succiniclasticum1.01±0.091.80±0.080.85±0.261.96±0.130.0120.0040.2890.168瘤胃球菌属Ruminococcus0.44±0.050.75±0.100.25±0.020.54±0.040.0250.0180.0570.023假丁酸弧菌属Pseudobu-tyrivibrio0.10±0.010.24±0.020.31±0.040.60±0.020.0140.020.0120.004变形菌门Proteobacteria19.23±2.887.73±2.4512.54±2.557.19±1.310.0460.0420.0420.799琥珀酸弧菌属Succinivibrio7.56±0.691.00±0.124.85±0.451.20±0.380.0030.0010.0010.004脱硫弧菌属Desulfovibrio0.13±0.031.19±0.220.08±0.010.65±0.080.0140.0090.0690.088伯克氏菌属Burkholderia0.06±0.010.06±0.030.02±0.020.07±0.020.9490.0350.0170.982慢生根瘤菌属Bradyrhizobium0.05±0.0140.02±0.0020.41±0.050.09±0.130.0680.0480.0040.427螺旋菌门Spirochaetes1.25±0.170.41±0.083.02±0.431.43±0.390.0070.0010.0230.058密螺旋体Treponema1.22±0.150.38±0.112.35±0.361.67±0.240.0110.1790.0130.023绿弯菌门Chloroflexi0.20±0.160.03±0.0050.11±0.030.07±0.010.2250.070.5020.032广古菌门Euryarchaeota0.07±0.010.13±0.020.07±0.0010.047±0.0070.0790.0510.9120.027

图3 属水平上瘤胃细菌菌群结构Fig.3 Composition of rumen bacteria at genus level
3 讨论
3.1 多样性分析
通过alpha多样性分析来反映微生物群落的丰度和多样性。由表2可知,添加抗菌肽对瘤胃细菌多样性无显著影响,而增加精料量对瘤胃细菌多样性亦无显著影响,但毛盛勇等[16]应用454高通量测序技术研究高精料对奶牛瘤胃微生物区系的影响,发现高精料(精粗比70∶30)饲粮较对照组(精粗比40∶60)下瘤胃菌群丰富度(ACE、Chao)及多样性(Shannon)指数显著降低(P<0.05)。比较本试验所得A、D两组alpha多样性指数, 虽然反映样品中物种丰富度(Chao、ACE)和均匀度(Shannon)指数组间差异均不显著,但在数值上,A 组的各项指数均大于 D 组,说明A组多样性高于D组,而差异不显著的原因可能是由于组内标准差过大。
另外,本试验所得A组多样性指数,低于王继文等[15]利用同样测序平台研究波尔山羊瘤胃细菌所得Chao指数(911 vs 2687)和Shannon指数(6.56 vs 7.26);但高于曾燕等[17]利用同样测序平台研究蒙古山羊瘤胃细菌所得(曾燕等用箱图表示并未给出具体数值)。瘤胃微生物的多样性是宿主和瘤胃微生物之间强烈选择和协同进化的结果,宿主动物的进化历程的差异可能决定其瘤胃细菌的差异[18],不同的品种在进化历程中经历不同的自然选择,因此,推测引起这种差异的主要原因是品种不同。这表明川中黑山羊瘤胃细菌丰富度和多样性高于蒙古山羊而低于波尔山羊。
3.2复合抗菌肽对瘤胃细菌菌群结构的影响
瘤胃微生物中,瘤胃细菌种类最为繁多,数量最为巨大,是瘤胃微生物中最主要的功能群,其菌群结构的变化影响瘤胃发酵功能[19]。有研究表明抗菌肽可以杀灭动物肠道中的有害菌,提高有益菌的数量,调节肠道菌群平衡, 增强消化吸收功能,促进动物生长[20-21]。Peng等[22]证实日粮中添加重组猪β-防御素2(recombinant porcine β-defensin 2)可以提高断奶仔猪生长性能,减少盲肠内大肠埃希菌(Escherichiacoli)、梭状芽胞杆菌(Clostridiumspp.)等病原体的数量,降低仔猪腹泻率。本试验利用MiSeq测序技术研究不同精料量下添加复合抗菌肽对瘤胃细菌菌群结构的影响,结果表明,门水平上,添加复合抗菌肽后并不影响瘤胃内最优势菌门,最优势菌门仍为拟杆菌门,其次为厚壁菌门,但变形菌门和螺旋菌门显著降低,且与精料量的改变无关;在属水平上,添加抗菌肽后部分利于降解纤维的菌属(琥珀酸菌属、瘤胃球菌属、假丁酸弧菌属)[23]含量增加,琥珀酸弧菌属(降解半纤维)[23]极显著降低,且不受精料量影响,但月形单胞菌属(降解淀粉)[23]与密螺旋体(降解半纤维)[23]的变化趋势与精料量相关,正常精料组显著降低,而双倍精料组无显著变化,伯克氏菌属与慢生根瘤菌属的变化与精料量相关,但含量过少,对瘤胃发酵功能影响不大。由此可见,添加复合抗菌肽并不影响瘤胃内优势菌属,仅增加一些相对含量较少的降解纤维菌属,降低了与降解淀粉相关的月形单胞菌属,因此推测复合抗菌肽并不影响瘤胃正常的发酵功能。
3.3不同精料量对瘤胃菌群结构的影响
饲粮结构是决定瘤胃发酵的主要因素,而改变饲粮精粗比,以研究瘤胃微生物区系的反应也是阐明反刍动物瘤胃微生物区系特点的重要手段[24-25]。
当精料量从300 g·d-1改变为600 g·d-1时,门水平上,拟杆菌门、变形菌门含量显著降低,厚壁菌门含量显著增加,这与其他学者研究结果一致。林波等[26]研究饲粮精粗比对泌乳水牛瘤胃细菌区系的影响,结果亦表明随着精料量的增加厚壁菌门相对含量显著增加,而拟杆菌门相对含量显著降低。Wetzels等[27]报道称,60%精料水平组瘤胃壁上的厚壁菌门含量高于30%精料水平组。属水平上,增加精料量后普雷沃菌属的相对丰度显著增加,琥珀酸弧菌属(降解半纤维)的相对丰度极显著降低。普雷沃菌属是广泛存在于瘤胃且含量最多的一类菌属,具有降解并利用淀粉和植物细胞壁多糖的能力[28],增加精料量同时增加了淀粉的含量,因此,增加精料量后普雷沃菌属含量也相对增加。这与Pitta等[29]研究结果一致,其研究表明,相比全粗料饲喂组,50∶50精粗比饲粮组普雷沃菌属含量较高。但与Mao等[30]研究结果不一致,Mao等称普雷沃菌属、密螺旋体属等属的比例在高精料饲粮组中较低,其原因可能是本试验与该研究的动物健康状态有差别,该研究精料比例达到70%,奶牛瘤胃处于亚急性酸中毒状态。此外,也有可能与物种不同有关。
4 结论
1) 复方抗菌肽(3 g·d-1·只-1)可降低变形菌门和螺旋菌门的相对含量,提高部分降解纤维菌属的含量。
2) 本试验下增加精料量可增加厚壁菌门和螺旋菌门相对含量,降低拟杆菌门和变形菌门的相对含量,增加普雷沃菌属相对含量,降低琥珀酸弧菌属相对含量。
[1] SINGH K M, PANDYA P R, TRIPATHI A K, et al. Study of rumen metagenome community using qPCR under different diets[J].MetaGene, 2014, 2(1):191-199.
[2] AGARWAL U. Role of volatile fatty acids in regulating nitrogen utilization and urea nitrogen recycling in ruminants [D]. Maryland: University of Maryland, College Park, 2013.
[3] 申军士, 毛胜勇, 朱伟云. 反刍动物瘤胃高效产氨菌菌群结构、功能及其调控[J]. 动物营养学报, 2015, 27(8):2323-2327.
SHEN J S, MAO S Y, ZHU W Y. Ruminal hyper ammonia producing bacteria in ruminants: Community structure, function and its manipulation[J].ChineseJournalofAnimalNutrition, 2015, 27(8):2323-2327. (in Chinese with English abstract)
[4] ISAACSON H R, HINDS F C, BRYANT M P, et al. Efficiency of energy utilization by mixed rumen bacteria in continuous culture[J].JournalofDairyScience, 1975, 58(11):1645-1659.
[5] RUSSELL J B, SNIFFEN C J, SOEST P J V. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria[J].JournalofDairyScience, 1983, 66(66):763-775.
[6] BROWN K L, HANCOCK R E. Cationic host defense (antimicrobial) peptides[J].CurrentOpinioninImmunology, 2006, 18(1):24-30.
[7] FJELL C D, HISS J A, HANCOCK R E, et al. Designing antimicrobial peptides: form follows function[J].NatureReviewsDrugDiscovery, 2011, 11(1):37-51.
[8] 李方方, 苏航, 张勇, 等. 饲粮添加复合抗菌肽与包被氧化锌对断奶仔猪生长性能及血清生化指标的影响[J]. 动物营养学报, 2015, 27(9):2811-2819.
LI F F, SU H, ZHANG Y, et al. Effects of dietary anti-microbial peptides and coated zinc oxide on growth performance and serum biochemical indices of weaning piglets[J].ChineseJournalofAnimalNutrition, 2015, 27(9):2811-2819. (in Chinese with English abstract)
[9] WU S, ZHANG F, HUANG Z, et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged withEscherichiacoli[J].Peptides, 2012, 35(2):225-230.
[10] 田春雷, 袁威, 任志华, 等. 复合抗菌肽“态康利保”对断奶仔猪红细胞免疫功能的影响[J]. 中国兽医学报, 2015, 35(5):795-798.
TIAN C L, YUAN W, REN Z H, et al. The effect of the composite antibacterial peptide “Taikanglibao” on the immune function of erythrocytes in weaning piglets[J].ChineseJournalofVeterinaryScience, 2015, 35(5):795-798. (in Chinese with English abstract)
[11] 但启雄, 袁威, 李刚, 等. 复合抗菌肽对断奶仔猪血清抗氧化功能的影响[J]. 中国兽医学报, 2015, 35(5):804-808.
DAN Q X, YUAN W, LI G, et al. Effects of compound antibacterial peptide on antioxidant function of serum in weaned piglets[J].ChineseJournalofVeterinaryScience, 2015, 35(5):804-808. (in Chinese with English abstract)
[12] TANG Z R, YIN Y L, ZHANG Y M, et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d[J].BritishJournalofNutrition, 2008, 101(7):998-1005.
[13] 潘行正, 黄正明, 李永新. 抗菌肽制剂对母猪死产率和仔猪成活率的影响[J]. 现代农业科技, 2010 (12):285-286.
PAN X Z, HUANG Z M, LI Y X. Effect of antimicrobial peptides on the death rate and survival rate of piglets[J].ModernAgriculturalScienceTechnology, 2010 (12):285-286. (in Chinese with English abstract)
[14] 刘莉如, 杨开伦, 滑静, 等. 抗菌肽对蛋用仔公鸡生长性能、免疫指标及空肠组织相关细胞因子基因mRNA表达的影响[J]. 动物营养学报, 2012, 24(7):1345-1351. DOI: 10.3969/j.issn.1006-267x.2012.07.020
LIU L R, YANG K L, HUA J, et al. Antimicrobial peptides: Effects on growth performance, immune indices and mRNA expression of related cytokine genes in jejunum of young roosters for egg production[J].ChineseJournalofAnimalNutrition,2012, 24(7):1345-1351. (in Chinese with English abstract)
[15] 王继文, 王立志, 闫天海, 等. 山羊瘤胃与粪便微生物多样性[J]. 动物营养学报, 2015, 27(8): 2559-2571.
WANG J W, WANG L Z, YAN T H, et al. Diversity of ruminal and fecal microbiota of goat[J].ChineseJournalofAnimalNutrition, 2015, 27(8):2559-2571. (in Chinese with English abstract)
[16] 毛胜勇, 张瑞阳, 王东升, 等. 应用454高通量测序研究高精料对奶牛瘤胃微生物区系的影响 [C]//李爱科,李绍钰. 第七届中国饲料营养学术研讨会论文集. 北京:中国农业大学出版社, 2014: 463-463.
MAO S Y, ZHANG R Y, WANG D S, et al. Application of 454 high throughput sequencing to study the effect of high concentrate on rumen microflora in dairy cows [C]// LI A K, LI S Y. Proceedings of the seventh Chinese feed nutrition seminar. Beijing: China Agricultural University Press, 2014: 463-463. in Chinese)
[17] 曾燕, 简平, 倪学勤, 等. Illumina MiSeq测序平台测定蒙古羊瘤胃液相和固相菌群多样性[J]. 动物营养学报, 2015, 27(10): 3256-3262.
ZENG Y, JIAN P, NING X Q, et al. Bacteria community diversity of liquid and solid phases of ruminal contents of mongolian sheep analyzed by Illumina MiSeq platform[J].ChineseJournalofAnimalNutrition, 2015, 27(10): 3256-3262. (in Chinese with English abstract)
[18] 裴彩霞, 毛胜勇, 朱伟云. 山羊瘤胃产甲烷古菌多样性及与其他动物瘤胃的比较[J]. 畜牧兽医学报, 2012, 43(6): 909-914.
PEI C X, MAO S Y, ZHU W Y. Molecular diversity of rumen methanogens from the goat and its comparison with other ruminants[J].ActaVeterinariaEtZootechnicaSinica, 2012, 43(6):909-914. (in Chinese with English abstract)
[19] SHI P J, MENG K, ZHOU Z G, et al. The host species affects the microbial community in the goat rumen[J].LettersinAppliedMicrobiology, 2008, 46(1):132.
[20] 侯振平, 印遇龙, 王文杰, 等. 乳铁蛋白素B和天蚕素P1对投喂大肠杆菌断奶仔猪生长及肠道微生物区系的影响[J]. 动物营养学报, 2011, 23(9): 1536-1544.
HOU Z P, YIN Y L, WANG W J, et al. Effects of lactoferricin B and cecropin P1 on growth and gut microflora in weaned piglets challenged with enterotoxigenicEscherichiacoli[J].ChineseJournalofAnimalNutrition, 2011, 23(9):1536-1544. (in Chinese with English abstract)
[21] TANG Z R. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d[J].BritishJournalofNutrition, 2009, 101(7):998-1005.
[22] PENG Z, WANG A, XIE L, et al. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets[J].ScientificReports, 2016, 6:26790.
[23] 冯仰廉,卢德勋,陆治年,等. 反刍动物营养学 [M]. 北京:科学出版社, 2006.
[24] 娜仁花, 董红敏, 陈永杏, 等. 日粮精粗比对瘤胃发酵特性的影响[J]. 中国畜牧杂志, 2011, 47(9):49-54.
NA R H, DONG H M, CHEN Y X, et al. Effect of dietary forage to concentrate ratio on rumen fermentation characteristics[J].ChineseJournalofAnimalScience, 2011, 47(9):49-54. (in Chinese)
[25] PETRI R M, FORSTER R J, YANG W, et al. Characterization of rumen bacterial diversity and fermentation parameters in concentrate fed cattle with and without forage[J].JournalofAppliedMicrobiology, 2012, 112(6):1152.
[26] 林波, 梁辛, 李丽莉, 等. 饲粮精粗比对泌乳水牛瘤胃细菌和甲烷菌区系的影响[J]. 动物营养学报, 2016, 28(10):3101-3109.
LIN B, LIANG X, LI L L, et al. Dietary forage to concentrate ratio affects ruminal bacterial and methanogen community composition of water buffaloes[J].ChineseJournalofAnimalNutrition,2016, 28(10):3101-3109. (in Chinese with English abstract)
[27] WETZELS S U, MANN E, METZLER-ZEBELI B U, et al. Pyrosequencing reveals shifts in the bacterial epimural community relative to dietary concentrate amount in goats[J].JournalofDairyScience, 2015, 98(8):5572-5587.
[28] DODD D. Degradation of xylan by rumen Prevotella SPP [D]. Illinois: Urbana-Champaign, 2010.
[29] PITTA D W, KUMAR S, VEICCHARELLI B, et al. Bacterial diversity associated with feeding dry forage at different dietary concentrations in the rumen contents of Mehshana buffalo (Bubalusbubalis) using 16S pyrotags[J].Anaerobe, 2014, 25(1):31-41.
[30] MAO S Y, ZHANG R Y, WANG D S, et al. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing[J].Anaerobe, 2013, 24(12):12-19.
(责任编辑卢福庄)
Effectsofcompositeantimicrobialpeptideonrumenbacteriacommunitystructureofgoat
CHEN Yun, LIU Qi, DENG Junliang*, YANG Yanyi, GAO Shuang, CHEN Chong, YAO Shuhua
(1.CollegeofVeterinaryMedicine,SichuanAgricultureUniversity;KeyLaboratoryofAnimalDiseaseandHumanHealthofSichuanProvince;KeyLaboratoryofEnvironmentalHazardsandAnimalDiseasesofSichuanProvinceCollegesandUniversities,Wenjiang611130,China)
The aim of this study was to evaluate the effects of composite antimicrobial peptide (AMP) on rumen bacteria community structure by MiSeq high throughput sequencing technology, which could provide theoretical basis for the application of antimicrobial peptide in ruminant. Twenty-four male goats (4-month-old) were randomly divided into 4 groups according to the similar weight. Four groups were normal concentrate group A (concentrate 300 g·d-1·goat-1), normal concentrate+AMP group C (concentrate 300 g·d-1·goat-1+ AMP 3 g·d-1·goat-1), double concentrate group D (concentrate 600 g·d-1·goat-1), double concentrate + AMP group E (concentrate 600 g·d-1·goat-1+ AMP 3 g·d-1·goat-1). The rumen fluid samples were collected after 20 d, and then the total DNA were extraced for amplification 16S rRNA(520F-802R), sequencing by MiSeq Illumina250. Results showed as follows: 1) A total of 629 634 valid 16S rDNA sequences and 13 227 operational taxonomic unit (OTU) across 12 samples were obtained. 2) At phylum level, Bacteroidetes was the most abundant phyla in A, C and E groups (38.52%-43.68%), except for the D group (Firmicutes was the most abundant phyla, 34.30%), and Proteobacteria and Spirochaetes were significantly decreased when adding AMP (P<0.05); Firmicutes and Spirochaetes were significantly increased in D group compared with A group(P<0.05), while Bacteroidetes and Proteobacteria were significantly decreased (P<0.05). 3) At genus level,Prevotellawas the most abundant genus in all samples (25.54%-33.88%),Succiniclasticum,Ruminococcus,PseudobutyrivibrioandDesulfovibriowere significantly increased when adding AMP (P<0.05 orP<0.01), whileSuccinivibriowas extremely significantly decreased (P<0.01), and it was not affected by the amount of concentrate; But the change tendency ofSelenomonasandTreponemawere affected by the amount of concentrate when adding AMP, significantly decreased in C group compared with A group (P<0.05), but no significant difference between D and E groups (P>0.05).PrevotellaandTreponemawere significantly increased in D group compared with A group (P<0.05), whileSuccinivibriowas significantly decreased (P<0.05). 4) There were no significant differences (P>0.05) between groups on alpha diversity index (Chao, ACE, Shannon and Simpson). In conclusion, Proteobacteria and Spirochaetes were decreased when adding complex antibacterial peptide, while some genus of cellulolytic were increased; Firmicutes and Spirochaetes were increased when the amount of concentrate changed from 300 to 600 g·d-1·goat-1, while Bacteroidetes and Proteobacteria were decreased, andPrevotellawas increased, whileSuccinivibriowas decreased. Moreover alpha diversity index was not affected by complex antibacterial peptide or the amount of concentrate changed from 300 to 600 g·d-1·goat-1.
goat; rumen bacteria; community structure; high-throughput sequencing; composite antimicrobial peptide; concentrate
陈芸,刘旗,邓俊良,等. 复合抗菌肽对山羊瘤胃菌群结构的影响[J].浙江农业学报,2017,29(11): 1800-1808.
10.3969/j.issn.1004-1524.2017.11.05
2017-03-20
“长江学者和创新团队发展计划”创新团队项目(IRT0848);四川农业大学双支计划(03572070)
陈芸(1992—),女,云南昭通人,硕士研究生,研究方向为中西兽医与临床。E-mail:609835279@qq. com
*通信作者,邓俊良, E-mail:dengjl213@126. com
S827;Q939
A
1004-1524(2017)11-1800-09

