Mismatch Negativity in Han Chinese Patients with Schizophrenia: A Meta-Analysis
Yanbing XIONG, Xianbin LI, Lei ZHAO, Chuanyue WANG*
•SYSTEMATIC REVIEW AND META-ANALYSIS•
Mismatch Negativity in Han Chinese Patients with Schizophrenia: A Meta-Analysis
Yanbing XIONG#, Xianbin LI#, Lei ZHAO, Chuanyue WANG*
schizophrenia; Chinese Han; mismatch negativity; meta-analysis
1. Introduction
It is well documented that cognitive impairment is a common feature of schizophrenia. A variety of cognitive abnormalities in brain function and structure are detected in patients with schizophrenia.[1-3]Hence,the implementation of validated biomarkers would improve the accurate diagnosis of cognitive dysfunction in schizophrenia patients. Mismatch negativity (MMN)is obtained by subtracting the event-related potential (ERP) to a deviant stimulus in a sequence ofstandard stimuli, elicited by auditory oddball paradigms.MMN is the negative component, typically peaking at about 250 ms from change to onset, which reflects the ability of actively and accurately predicting forthcoming sensations in the brain.[4]MMN is most commonly evoked using auditory stimuli that reflect information processing and encoding in the auditory cortex. Unlike the P300 test,in which the subject is actively reacting to the presence of target stimuli, the processes involved in MMN generation are assumed to initiate involuntary auditory change detection.[5]Thus, the mismatch negativity component could be considered as a hallmark of auditory prediction errors[6]and is well described as the electrophysiological response.[7]
MMN amplitude has been consistently shown to be attenuated in schizophrenia compared to healthy controls. In large-scale multi-site clinical studies from the US,[8]highly significant MMN (Cohen's d, d=0.96)amplitude reductions were observed in SZ patients, it is so far probably the most promising neurophysiological biomarkers to be used to guide assignment of patients to clinical interventions. Systematic reviews and metaanalysis reveal that reliability coefficients and effect sizes of deficits in schizophrenia for MMN are stable in distinguishing healthy controls from those with schizophrenia.[9-10]Furthermore, impaired MMN is closely associated with N-methyl D-aspartate receptor hypo-functioning which exist as race-related biological differences.[11-12]However, it is remains unclear whether Han Chinese individuals with schizophrenia show altered MMN responses and therefore we performed a meta-analysis of peer-reviewed MMN studies that had specifically targeted Han Chinese schizophrenia patients to examine the pooled effect size. This may contribute to the view of MMN as a translatable biomarker for diagnosing schizophrenia in China.
2. Methods
2.1 Criteria for considering studies
Study types considered for meta-analysis included all relevant parallel control studies on MMN with individuals who had a diagnosis of schizophrenia and healthy controls. Types of outcome measures included: (a) The study should include at least one psychiatrically healthy control group and individuals with schizophrenia. (b) Studies that have been carried out with a Han Chinese sample. (c) The MMN amplitude must be reported as a difference wave. (d) MMN amplitude must be extracted from auditory oddball paradigms, which have been clearly introduced. Types of outcome measures excluded: (a) Conference paper,academic dissertation, reviews or papers that didn't provide data; (b) Redundant publications; (c) other paradigms, e.g. paradigm (visual), or those which have not been clearly introduced.
2.2 Search methods for identification of studies
A literature search for papers published before May 8, 2017 was conducted using the following electronic databases: CNKI, Wanfang DATA, VIP and Pubmed. Keywords included synonyms of"schizophrenia" or "psychosis", "Chinese" or "China","mismatch negativity" or "MMN" in PubMed (and the Chinese equivalents in Chinese databases). A set of search terms were (("schizophrenia"[MeSH Terms] OR "schizophrenia"[All Fields]) OR ("psychotic disorders"[MeSH Terms] OR ("psychotic"[All Fields] AND"disorders"[All Fields]) OR "psychotic disorders"[All Fields] OR "psychosis"[All Fields])) AND ((mismatch[All Fields] AND negativity[All Fields]) OR MMN[All Fields])AND (("asian continental ancestry group"[MeSH Terms]OR ("asian"[All Fields] AND "continental"[All Fields]AND "ancestry"[All Fields] AND "group"[All Fields])OR "asian continental ancestry group"[All Fields] OR"chinese"[All Fields]) OR ("china"[MeSH Terms] OR"china"[All Fields])) were utilized.
2.3 Outcome measures
The outcome measures were MMN amplitude between the schizophrenia group and healthy control group.The effect size between the two groups was calculated using the difference value of MMN amplitude. The larger the effect size, the more serious the MMN amplitude deficits in schizophrenia, and the greater the difference between the two groups.
2.4 Data collection and analysis
2.4.1 Selection of trials
Two researchers(XYB and ZL) independently conducted a full-text review, extracted data cross checks, and consulted a third author (LXB) if there were any inconsistencies.
2.4.2 Data collection
Both authors independently extracted the data from the included trials. Any disagreement was discussed,the decisions documented. The remaining problems were arbitrated by the third reviewer (LXB).
2.4.3 Data synthesis
Meta-analysis: Unbiased Cohen's effect size (d) for each study was calculated by MMN amplitude as previously described. Stata 11.0 software was used to calculate size of effect from each study, and the average (d+)means d < 0.2 is a negligible effect size, d > 0.2 is a small effect size, d > 0.5 is a medium effect size, d >0.8 is a large effect size.[13]
Heterogeneity: Study heterogeneity was quantified for the outcome analysis using the I2statistic alongside the Chi2'P' value with I2> 50% indicating significant heterogeneity. When heterogeneity is present,sensitivity analyses was conducted to assess potential influences of any one single study on the pooled MD and associated P-values,[14]and we conducted a subgroup analyses by the course of schizophrenia, to explore whether MMN deficits were associated with the course of illness in schizophrenia.
The quality assessment of meta-analysis outcomes:Newcastle-Ottawa Scale was used for assessing the methodological quality of each study. The tool is used for assessing the quality of case-control and cohort studies. It is judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for casecontrol or cohort studies respectively. The total score was 10. The higher quality studies are given higher scores.[15]
Assessment of publication biases: To evaluate reporting bias, effect size data were inspected visually using funnel plots. The theory underlying this approach is that small studies have greater standard errors.[16]This was investigated using a funnel plot that is symmetrical if there is no bias. The degree of funnel-plot asymmetry was evaluated using the Egger regression test.[17]To provide an estimation of the possible effect of unpublished studies, effect sizes were corrected using the "trim and fill" method.[18]
3. Results
3.1 Results of the search
This initial search strategy identified 63 articles on MMN in Han Chinese patients with schizophrenia, 56 of which were published in Chinese and 7 of which were in English. 18 studies met the criteria mentioned above after duplicate checking via EndNote 7X. 11 unique articles were selected for inclusion in the metaanalysis. 8 of these studies were in Chinese and 3 were in English (Fig. 1). 11 studies were included and the final total study population (total 824) consisted of 432 schizophrenia patients and 392 healthy controls. The combined demographic information from these studies is presented in Table 1.
3.2 Quality assessment
According to the Newcastle-Ottawa Scale, all the included studies have clear diagnostic criteria for schizophrenia, and the healthy controls group andschizophrenia group had no statistical difference in age and gender, and all the healthy controls had no family history of mental illness. Therefore, the two groups are comparable. In comparable education, there were 4 studies that didn't match between groups (Lin 2012[19],Yuan 2009[20], Zhao 2012[21], Zhou 2013[22]). All the included studies didn't mention the non-response rate for both groups. The total quality of all the studies was more than 6, suggesting that the quality of included studies was high.[15] See Table 2.
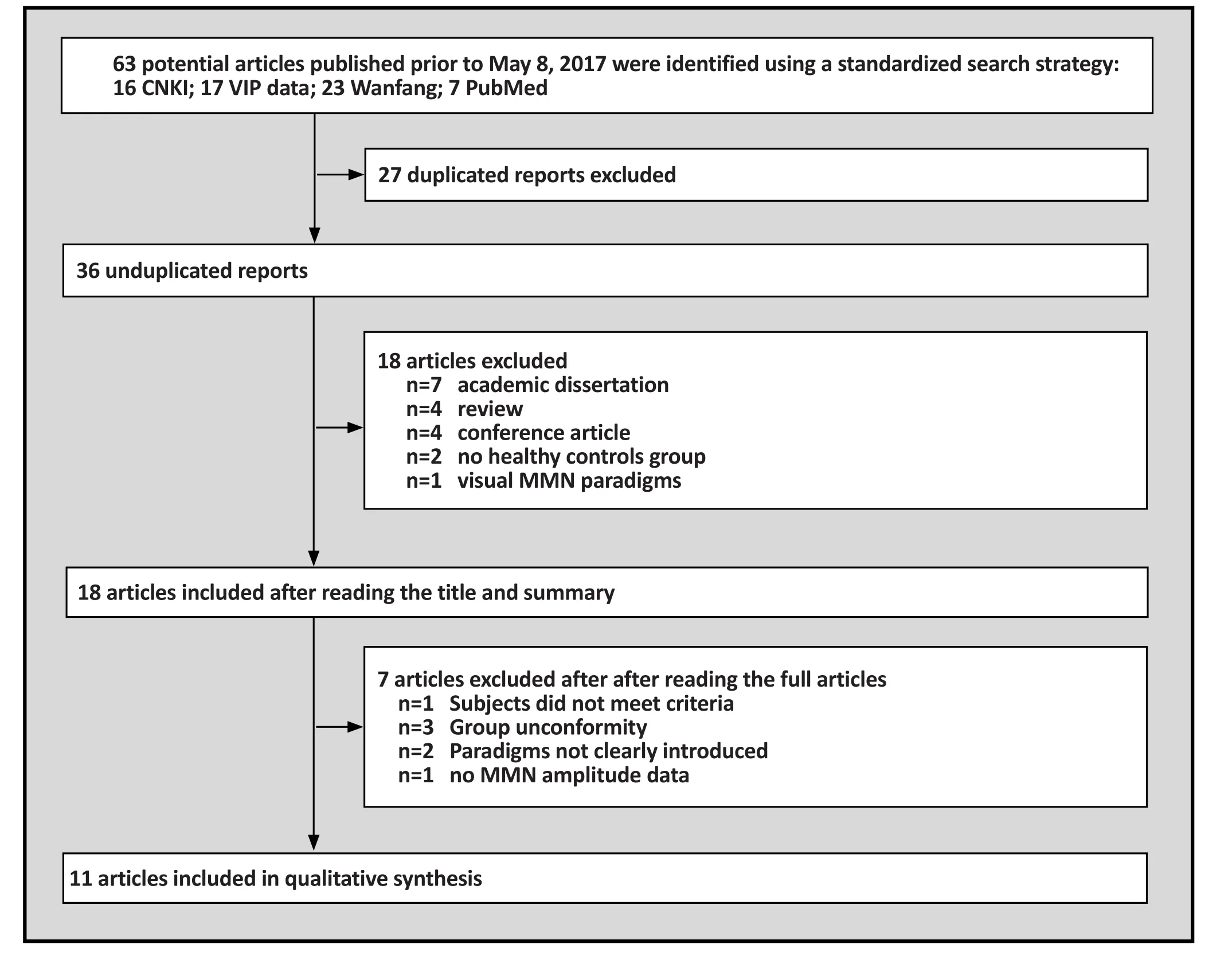
Figure 1. Flowchart of literature search and exclusion process
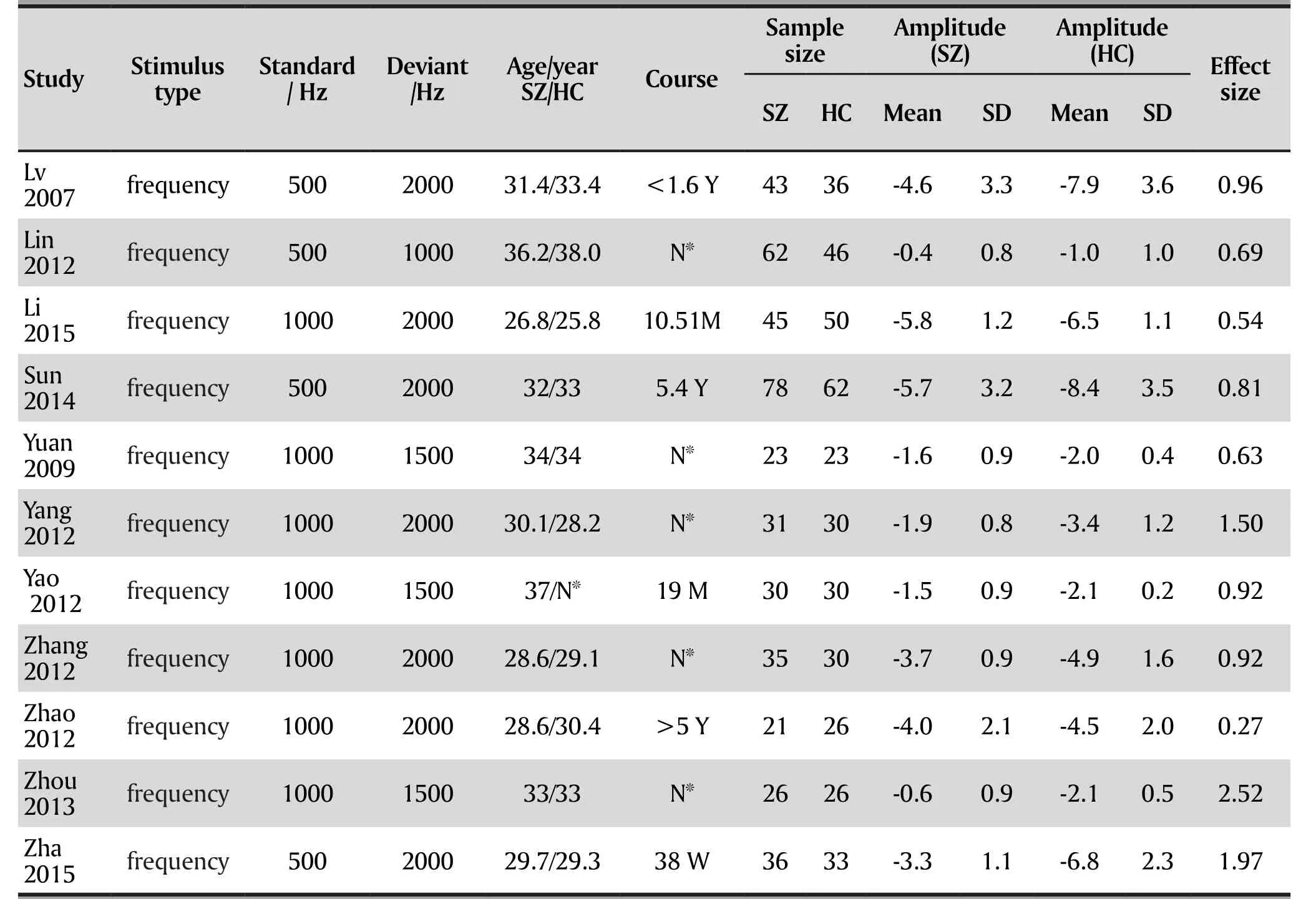
Table 1. Characteristics of included studies
3.3 Meta analysis
3.3.1 MMN Amplitude effect size
Heterogeneity was identified across all studies determined by Q-statistics and Galbraith index(I2=75.3%, df=10, p<0.001), indicating significant heterogeneity. Fig. 2 shows the effect size and 95%confidence interval for each investigation. The pooled effect size (random model) of the MMN differences between schizophrenia patients and healthy controls was 1.004 with 95%CI ranging from 0.703 to 1.305.
3.3.2 Sensitivity analysis
Q-test revealed that heterogeneity has been identified across all studies (Stata11.0, I2=75.3%, df=10,p<0.001). Sensitivity analysis showed 2 studies(Zha 2015[23], Zhou 2013[22], et al) had significant heterogeneity from the others. When we excluded these 2 studies, Q-test was not statistically significant(I2=33.1%, df=7, p=0.153), However, the d+ value was reduced to 0.79 (0.60, 0.99), Fig. 3. It is indicated that these studies are the reason for heterogeneity.
3.3.3 Subgroup analysis
In order to assess if there is a relationship between the course of disease and MMN impairment across studies,we performed subgroup analysis. The schizophrenia patients were separated into groups with two disease stages: one group was over 3 years and another was below 3 years. Effect sizes by group are depicted in Fig. 4. 5 studies were excluded due unknown course of illness records (Zhang 2012[24], Yang 2012[25], Lin 2012[19],Yuan 2009[20], Zhou 2013[22]). 6 studies were included
in the subgroup analysis. Among individuals who had been diagnosed with schizophrenia for more than three years, the results were heterogeneous across groups(I2=89.3%, df=2, p<0.001) and the pooled effect size was 0.95(0.68, 1.21), and less than three years had the pooled effect size of 0.77. For the patients who had schizophrenia for less than three years, the results were homogenous (I2=5.9%, df=2, p=0.345) and the pooled effect size was 0.77 (95%CI: 0.51, 1.04).
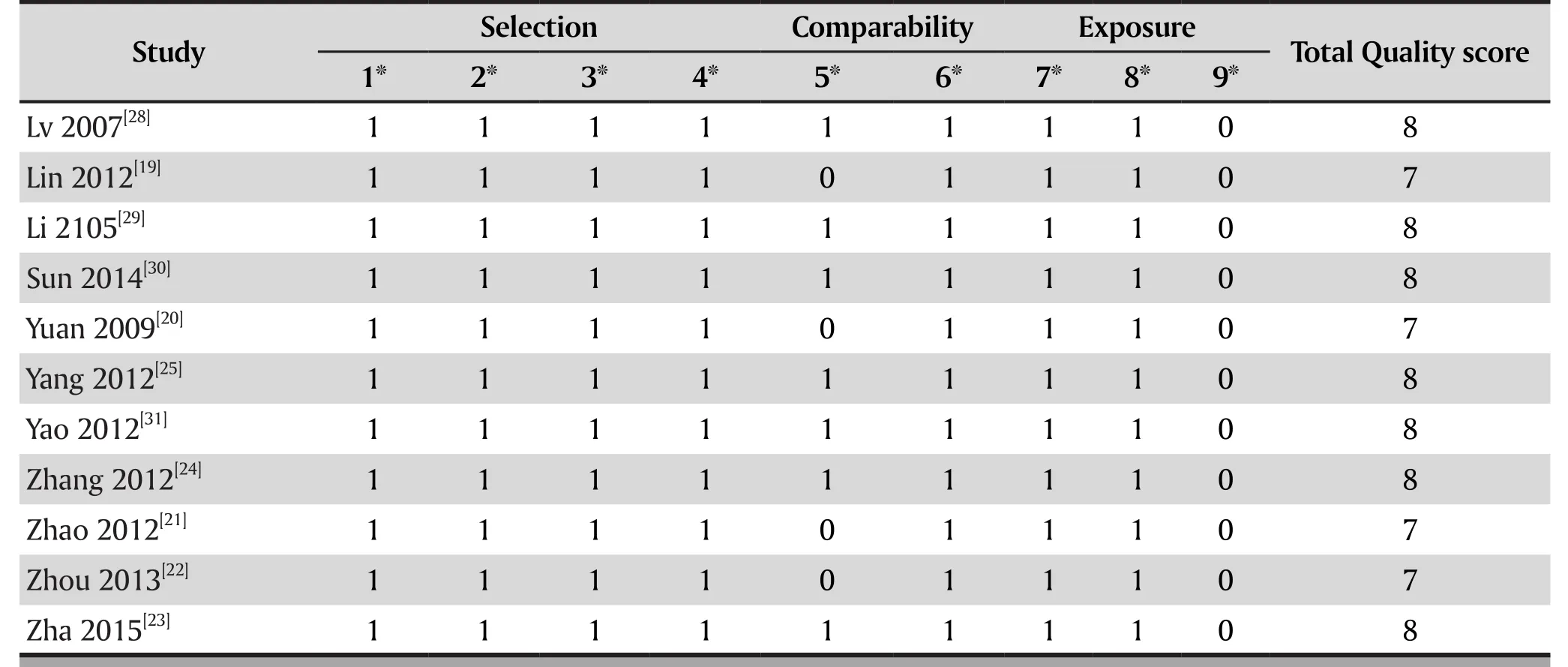
Table 2. The methodological assessment of case control trials by Newcastle-Ottawa Scale
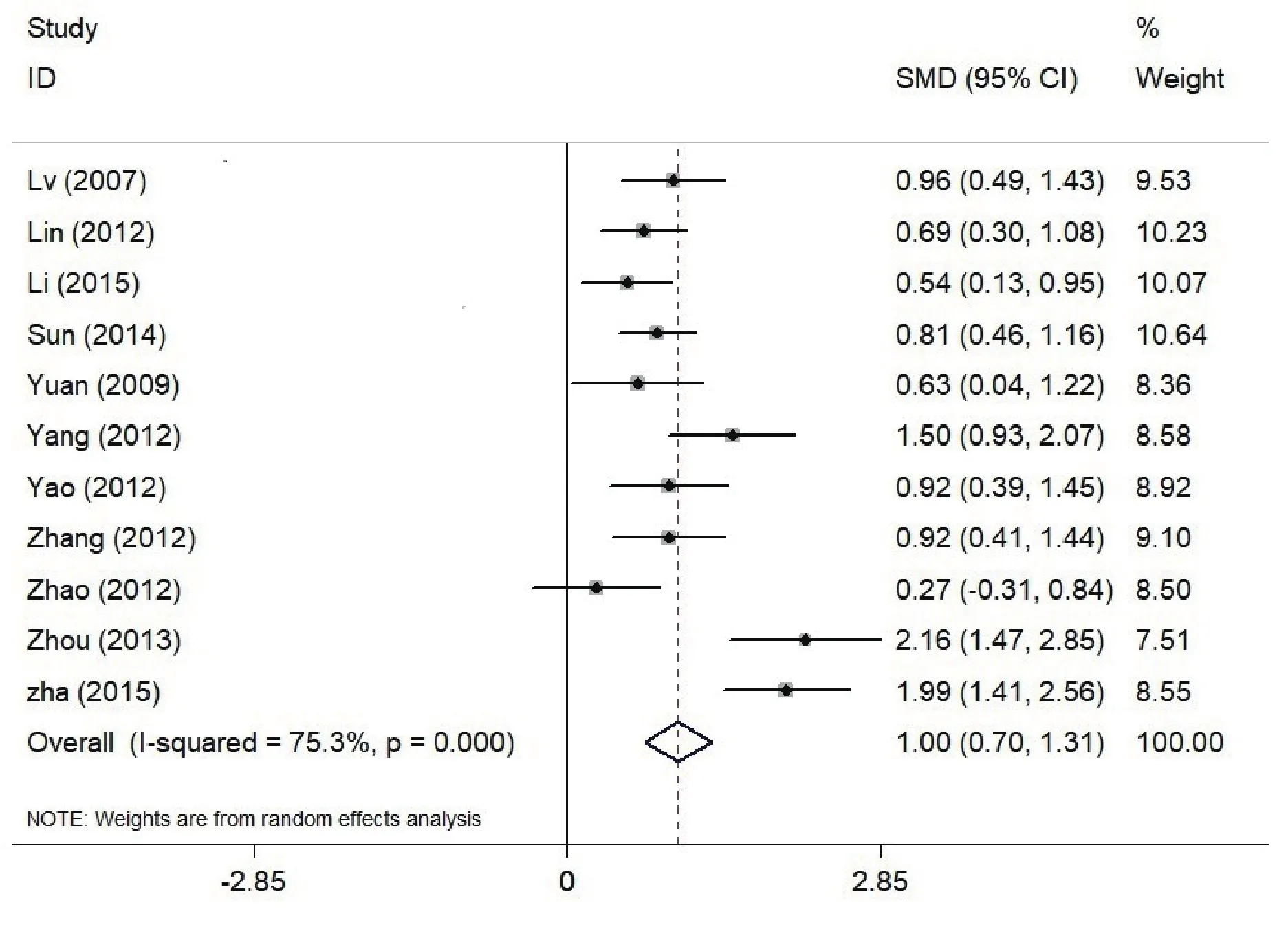
Figure 2. Effects of MMN amplitude in Patients with Schizophrenia (SZ) vs Healthy Controls (HC); The x axis represents effect size
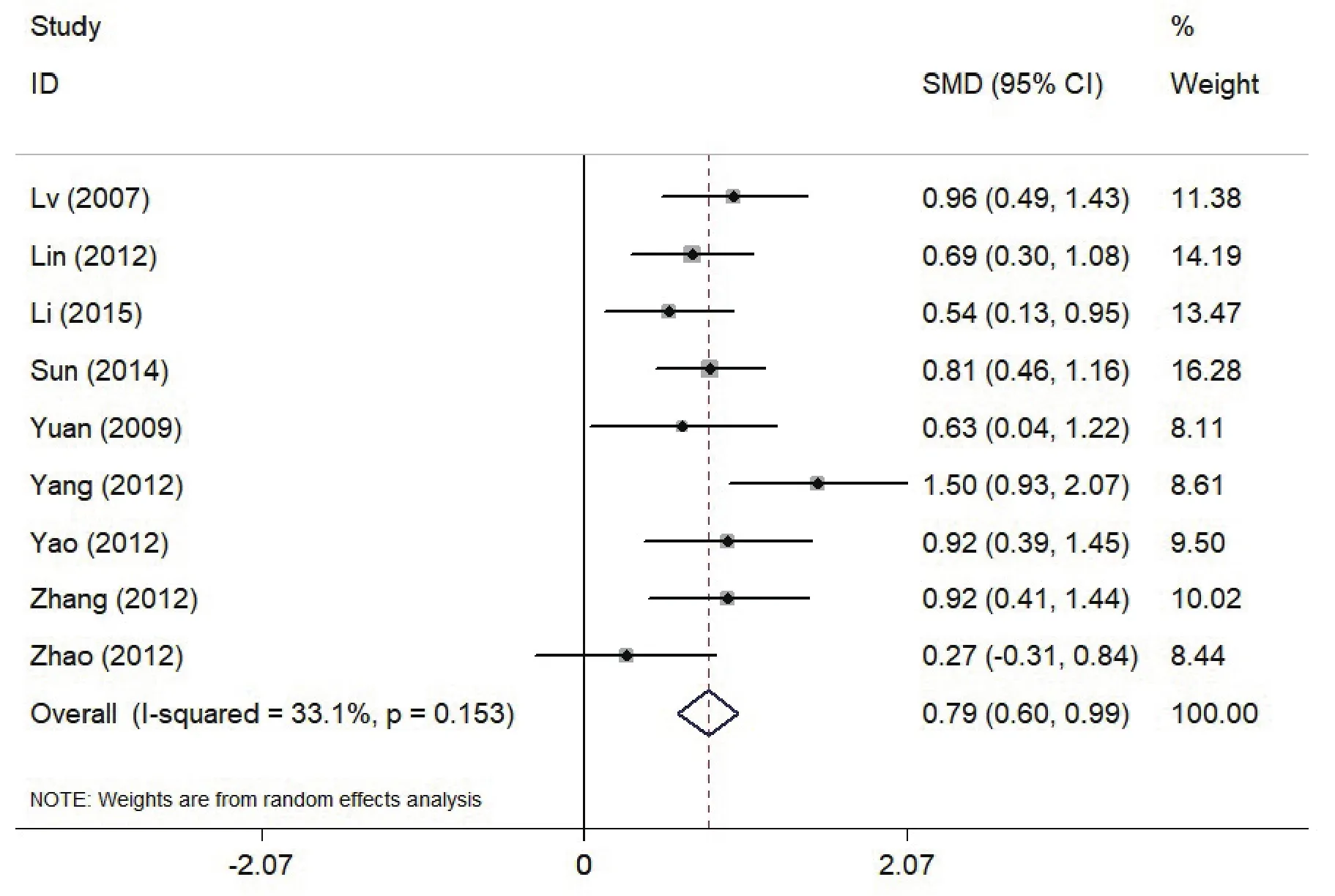
Figure 3. Effects of MMN amplitude in Patients with Schizophrenia (SZ) vs Healthy Controls (HC) when excluding two studies; The x axis represents effect size
3.3.4 Publication bias
Plotting MMN amplitude effect size from each study in a funnel plot revealed symmetry, suggesting that the sample could not be affected by reporting bias (Egger regression test: t = 1.83; p = 0.101; 95% CI, −1.17 to 11.08).
4. Discussion
4.1 Main findings
From this paper, 11 studies were included for performing meta-analysis of MMN in Han Chinese patients with schizophrenia. MMN amplitude also decreased in Han Chinese patients with schizophrenia,and the pooled effect size was large (1.004), close to 0.95-0.96, which are the effects sizes seen in other published meta-analyses.[8-9]This result demonstrates that the effect size of amplitude in Han Chinese schizophrenia is similar to non-Han Chinese schizophrenia. It also suggested MMN could be a reliable biomarker for monitoring cognitive function alteration in Han Chinese patients with schizophrenia, as it is not affected by ethnic factors. However, the heterogeneity test revealed that there exists heterogeneity across all studies. 2 studies (Zha 2015[23], Zhou 2013[22], et al)showed significant heterogeneity from the others, after excluding these studies, the average value of effect size(d+) decreased to 0.79, demonstrating that these 2 studies raised the overall effect sizes.
Previous study showed that first-episode schizophrenia patients had an average effect size of 0.42[26], far less than chronic schizophrenia patients. Moreover, MMN attenuation in first-episode schizophrenia patients was significantly lower than in chronic schizophrenia patients. The MMN frequency appeared to be oppositely attenuated along with later disease stage. Thus, MMN amplitude might reflect disease progression.
In order to investigate whether MMN deficit was correlated with the progression of schizophrenia, we performed subgroup analysis by dividing recruited schizophrenia patients into two groups, depending on whether the onset of schizophrenia was more than three years. Usually, schizophrenia patients with three year disease duration or above are considered chronic patients.[27]Patients with schizophrenia over three years had the pooled effect size of 0.95, and patients with schizophrenia less than three years had the pooled effect size of 0.77. Therefore, MMN amplitudes were slightly lower in patients with schizophrenia below three years compared to those with schizophrenia over three years,suggesting that MMN amplitude may reflect disease progression. These findings suggest frequency MMN might be a state marker of schizophrenia.
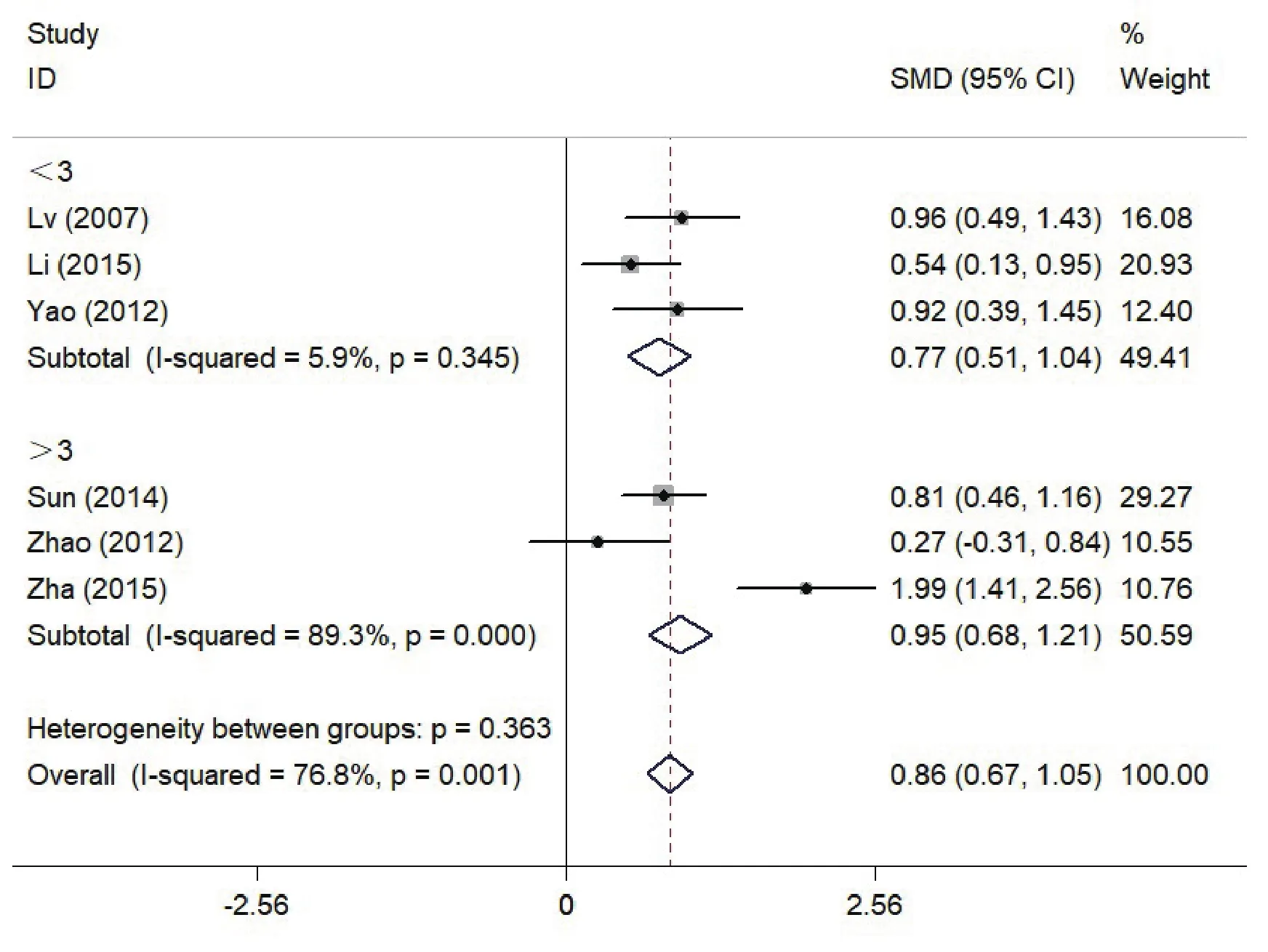
Figure 4. Effects of MMN amplitude in Patients with Schizophrenia (SZ) vs Healthy Controls (HC) by subgroup analysis with the course of schizophrenia when over 3 years; The x axis represents effect size
4.2 Limitations
Some methodological limitations of the current study should be taken into account. First, in terms of Chinese database retrieval, we did not search the databases from Hong Kong, Macao and Taiwan. Second, in terms of English database retrieval, we did not search database such as EMBASE and PsycInfo. This may have caused incomplete retrieval of studies. Third,we did not investigate the correlation between MMN amplitude and treatment, therefore, we do not know whether MMN can be used as an indicator of efficacy evaluation for antipsychotic drugs. Finally, the types of MMN in the included studies are all frequency MMN,which cannot be compared between different kinds of MMN effect values.
4.3 Implications
In summary, MMN amplitude deficits in Han Chinese schizophrenia are similar to the reports from western countries. Consistently, there also exists a large effect size of MMN between Chinese schizophrenia patients and healthy controls, which may be used as a biomarker of schizophrenia. At present, most of the studies on schizophrenia in China are caused by a single frequency deviation stimulus. In the future, a control study of MMN caused by different stimuli can be carried out.The current research focused on analyzing amplitude of MMN, in-depth analysis of MMN data, such as durationfrequency analysis, may be a new research approach in the future. It is also necessary to carry out multi-center MMN experiments to establish MMN norms for patients with schizophrenia in China.
Funding statement
This work was supported by grants from the National Natural Science foundation of China(81471365,81601169); Neuroscience research program of the Beijing science and technology plan (Z161100002616017); Beijing Hospital Authority "young researchers plan"(QML20161901);Foundation and clinical projects of Capital Medical University(16JL25); Excellent personnel project of the Beijing Municipal Committee Organization Department(2015000021469G193)
Conflict of interest statement
The authors report no conflict of interest related to this manuscript.
Authors' contributions
Yanbing Xiong and Lei Zhao were responsible for searching papers, data extraction, and the assessment of study quality. Yanbing Xiong performed the data analyses. Yanbing Xiong and Xianbin Li wrote the manuscript; Chuanyue Wang contributed to the conceptualization of the study and manuscript preparation.
1. Lam M, Collinson SL, Sim K, Mackay CE, James AC,Crow TJ. Asymmetry of lexico-semantic processing in schizophrenia changes with disease progression. Schizophr Res. 2012; 134(2-3): 125-130. doi: http://dx.doi.org/10.1016/j.schres.2011.10.020
2. Zhang F, Qiu L, Yuan L, Ma H, Ye R, Yu F, et al. Evidence for progressive brain abnormalities in early schizophrenia:a cross-sectional structural and functional connectivity study. Schizophr Res. 2014; 159(1): 31-35. doi: http://dx.doi.org/10.1016/j.schres.2014.07.050
3. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia.Arch Gen Psychiatry. 2007; 64(5): 521-529. doi: http://dx.doi.org/10.1001/archpsyc.64.5.521
4. Randeniya R, Oestreich LKL, Garrido MI. Sensory prediction errors in the continuum of psychosis. Schizophr Res. 2017;pii: S0920-9964(17)30206-2. doi: http://dx.doi.org/10.1016/j.schres.2017.04.019
5. Light GA, Swerdlow NR. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann N Y Acad Sci.2015; 1344: 105-119. doi: http://dx.doi.org/10.1111/nyas.12730
6. Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007; 118(12): 2544-2590. doi: http://dx.doi.org/10.1016/j.clinph.2007.04.026
7. Näätänen R, Shiga T, Asano S, Yabe H. Mismatch negativity(MMN) deficiency: a break-through biomarker in predicting psychosis onset. Int J Psychophysiol. 2015; 95(3): 338-344.doi: http://dx.doi.org/10.1016/j.ijpsycho.2014.12.012
8. Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia:characterization of demographic, clinical, cognitive,and functional correlates in COGS-2. Schizophr Res.2015; 163(1-3): 63-72. doi: http://dx.doi.org/10.1016/j.schres.2014.09.042
9. Erickson MA, Ruffle A, Gold JM. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biol Psychiatry.2016; 79(12): 980-987. doi: http://dx.doi.org/10.1016/j.biopsych.2015.08.025
10. Haigh SM, Coffman BA, Salisbury DF. Mismatch Negativity in First-Episode Schizophrenia: A Meta-Analysis. Clin EEG Neurosci. 2017; 48(1): 3-10. doi: http://dx.doi.org/10.1177/1550059416645980
11. Michie PT, Malmierca MS, Harms L, Todd J. The neurobiology of MMN and implications for schizophrenia.Biol Psychol. 2016; 116: 90-97. doi: http://dx.doi.org/10.1016/j.biopsycho.2016.01.011
12. Zhang Y, Fan M, Wang Q, He G, Fu Y, Li H, Yu S.Polymorphisms in MicroRNA Genes And Genes Involving in NMDAR Signaling and Schizophrenia: A Case-Control Study in Chinese Han Population. Sci Rep. 2015; 5: 12984. doi:http://dx.doi.org/10.1038/srep12984
13. Cohen J. Statistical Power Analysis for the Behavioral Sciences.2nd ed. Mahwah, NJ: Lawrence Erlbaum; 1988
14. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analyzing data and undertaking Meta-analyses. In: Higgins JPT, Green S.Cochrane Handbook for Systematic Reviews of Interventions.Version 5.1.0. The Cochrane Collaboration; 2011
15. He Q, Huang YX, Kang WJ. [A bibliometric analysis on injury prevention research in China, 2001-2010]. Zhong Hua Ji Bing Kong Zhi Za Zhi. 2014; 18(10): 913-916. Chinese
16. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ. 2011; 343: d4002
17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ.1997; 315(7109): 629-634
18. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56(2): 455-463
19. Lin YT, Liu CM, Chiu MJ, Liu CC, Chien YL, Hwang TJ, et al. Differentiation of schizophrenia patients from healthy subjects by mismatch negativity and neuropsychological tests. Plos One. 2012; 7(4):e34454. doi: http://dx.doi.org/10.1371/journal.pone.0034454
20. Yuan GZ, Zhou ZH, Yao JJ. Effect of quetiapine on cognitive function in schizophrenia: a mismatch negativity potentials study. Acta Neuropsychiatrica. 2009; 21(1): 26-33. doi: http://dx.doi.org/10.1111/j.1601-5215.2008.00337.x
21. Zhao J, Yang LQ, Dai J, Wu XQ, Zhang Y, Li YP. [Eventrelated potential study of abnormal cognition processing in treatment-refractory schizophrenia]. Zhong Hua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2012; 21(10): 910-912. doi: http://dx.chinadoi.cn/10.3760/cma.j.issn.1674-6554.2012.10.014
22. Zhou Z, Zhu H, Chen L. Effect of aripiprazole on mismatch negativity (MMN) in schizophrenia. Plos ONE. 2013;8(1):e52186. doi: http://dx.doi.org/10.1371/journal.pone.0052186
23. Zha Y, Tang YJ, Liu PX. [Comparative Study on the Mismatch Negativity MMN between First-episode Schizophrenia and Recurrence Schizophrenia]. Zhongguo Jian Kang Xin Li Xue Za Zhi. 2015; 23(4): 498-501. Chinese. doi: http://dx.chinadoi.cn/10.13342/j.cnki.cjhp.2015.04.006
24. Zhang LN. [Event-related potential mismatch negativity study in patients with schizophrenia]. Shanxi Yi Ke Da Xue Xue Bao. 2012; 43(10): 780-782. Chinese. doi: http://dx.chinadoi.cn/10.3969/J.ISSN.1007-6611.2012.10.016
25. Yang LQ, Zhao J. [Event-related potential MMN study of abnormal cognition in patients with schizophrenia].Shen Jing Ji Bing Yu Jing Shen Wei Sheng. 2012; 12(6):561-563. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1009-6574.2012.06.006
26. Haigh SM, Coffman BA, Salisbury DF. Mismatch Negativity in First-Episode Schizophrenia: A Meta-Analysis. Clinical Eeg & Neuroscience. 2017; 48(1): 3. doi: http://dx.doi.org/10.1177/1550059416645980
27. Hammer TB, Oranje B, Skimminge A, Aggernæs B, Ebdrup BH, Glenthøj B, et al. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with firstepisode schizophrenia. J Psychiatry Neurosci. 2013; 38(1): 34-42. doi: http://dx.doi.org/10.1503/jpn.110129
28. Lv WQ, Mao Y, Chen JM, Chen C, Tang YX. [Application of eletroencephalography mismatch negativity in patient with schizophrenia]. Shanghai Arch Psychiatry. 2007;19(2): 92-94. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1002-0829.2007.02.008
29. Li Z, Deng W, Wang Q. [A comparative study about mismatch negativity between patients with schizophrenia and bipolar I disorder]. Jing Shen Yi Xue Za Zhi. 2015;28(1): 1-5. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1009-7201.2015.01.001
30. Sun FL, Wu RQ, Huang JB, Chen XS. [Impacts of repetitive transcranial magnetic stimulation upon the measurements of event related potential in patients with schizophrenia].Lin Chuang Yi Xue Za Zhi. 2014; 5: 312-314. Chinese
31. Yao JJ, Zhang ZJ, Zhou ZH. [Effect of treatment with Aripiprazole on mismatch negativity potentials in schizophrenia]. Shen Jing Ji Bing Yu Jing Shen Yi Xue Wei Sheng. 2012; 12(1): 24-26. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1009-6574.2012.01.008
中国精神分裂症患者失匹配负波特征的Meta分析
熊燕兵,李先宾,赵 蕾,王传跃
精神分裂症、中国汉族、失匹配负波、Meta分析
Background:Previous meta-analysis revealed that mismatch negativity(MMN) amplitude decreased in patients with schizophrenia compared with healthy controls (Cohen's d, d about 1), leading to the possibility of mismatch negativity being used as a biomarker for schizophrenia. However, it is unknown whether MMN is reliably changed in Chinese patients. It is necessary to carry out a meta-analysis on MMN of Han Chinese patients with schizophrenia.
Aim:To investigate whether MMN could be used as a biomarker for Han Chinese patients with schizophrenia
Methods:A literature search was conducted to identify clinical trials on MMN in Han Chinese schizophrenia patients published before May 8, 2017, by searching the Chinese language databases CNKI, WanFang Data,VIP Data and PubMed. The effects of MMN deficits were evaluated for MMN amplitude by calculating standard mean difference (SMDs) between schizophrenia patient groups and healthy control groups.
Results:A total of 11 studies were included in the analysis. The total quality of all the studies were more than 6 as evaluated by Newcastle-Ottawa Scale (NOS). Meta-analysis of data from these studies had a pooled sample of 432 patients with schizophrenia and 392 healthy controls. There exists significant MMN deficit in schizophrenia patients compared to healthy controls (Cohen's d=1.004). When studies were excluded due to heterogeneity, the pooled effect size of the MMN differences between the patient group and healthy controls dropped to 0.79 (Cohen's d=0.79). Subgroup analysis showed that MMN amplitude deficits of schizophrenia over three years had the pooled effect size of 0.95, and less than three years had the pooled effect size of 0.77. Publication bias conducted via Egger regression test (t = 1.83; p = 0.101),suggested that there was no publication bias.
Conclusion:The effect size of MMN amplitude between Chinese patients with schizophrenia and healthy controls is consistent with other meta-analyses published on this topic, suggesting that Han Chinese patients with schizophrenia also exhibited MMN deficits.
PROSPERO registration number:CRD42016048326
[Shanghai Arch Psychiatry. 2017;29(5): 259-267.
http://dx.doi.org/10.11919/j.issn.1002-0829.217103]
Beijing Key Laboratory of Mental Disorders, Department of Psychiatry, Beijing Anding Hospital, Capital Medical University, Beijing 100088, China; Center of Schizophrenia, Beijing Institute for Brain Disorders, Laboratory of Brain Disorders (Capital Medical University), Ministry of Science and Technology,Beijing 100088, China.
# equal contribution
*correspondence: Chuanyue Wang; Mailing address: No 5 Alley, Deshengmen, Xicheng District, Beijing, China. Postcode: 100088; E-Mail: wang.cy@163.net
背景:国外对精神分裂症患者失匹配负波(mismatch negativity, MMN)波幅进行Meta分析发现其明显低于健康对照组,区分效应值(Cohen's d,)d在1.00左右,使之可能成为精神分裂症早期诊断的生物标志物.但检索文献未发现纳入中国精神分裂症人群MMN研究的Meta分析报道,因此有必要对中国精神分裂症患者的MMN进行Meta分析.
目的:通过Meta分析计算中国精神分裂症患者MMN的平均效应值,探讨其是否可作为中国精神分裂症患者的生物标志物.
方法:计算机检索中国知网(China National Knowledge Infrastructure, CKNI)、万方数据库(WanFang Data)、维普数据库(Vip Citation Databases,VIP)、PubMed数据库,收集2017年5月8日前公开发表的关于中国汉族精神分裂症患者MMN研究的相关文献.MMN受损的影响通过计算精神分裂症患者组和健康对照组之间的标准平均差(SMDs)来评估MMN波幅.
结果:共有11篇文献纳入分析.Newcastle-Ottawa Scale (NOS)评估所有研究的总体质量超过6.这些研究的meta分析数据包括精神分裂症患者432例,健康对照人群392例.Meta分析结果显示,精神分裂症组与健康对照组相比MMN受损显著(Cohen's d=1.004).剔除异质性大的文献2个,患者组和健康对照组之间MMN差异的效应值降为0.79(Cohen's d=0.79).亚组分析显示精神分裂症病程大于3年的患者MMN波幅缺失的效应值为0.95,而病程小于3年的效应值为0.77.通过Egger回归分析得出的发表偏移(t = 1.83; p= 0.101)提示没有发表偏移.
结论:中国精神分裂症患者和健康对照者之间的MMN波幅效应值与其他关于该研究的meta分析结果是一致的,提示中国汉族精神分裂症患者也表现有MMN受损.

Xiong Yanbing, obtained a bachelor's degree in medicine in 2013 and a master's degree in psychiatry and mental health in 2016 from Shanxi Medical University. He is now working in the PhD program in psychiatry and mental health at Capital Medical University. His main research interest is electrophysiology for the treatment of schizophrenia.
- 上海精神医学的其它文章
- Pleasure Experience and Emotion Expression in Patients with Schizophrenia
- Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study
- Pretreatment Serum MCP-1 Level Predicts Response to Risperidone in Schizophrenia
- A Cross-Sectional Study on the Characteristics of Tardive Dyskinesia in Patients with Chronic Schizophrenia
- Multidimensional Approaches for A Case of Severe Adult Obsessive - Compulsive Disorder
- Psychiatry and Cinema: What Can We Learn from the Magical Screen?

