含蜡原油管输过程化学组成与应用研究进展①
甘亦凡 成庆林 孙 巍 苏文坤 刘 扬
(东北石油大学提高油气采收率教育部重点实验室)

甘亦凡 成庆林 孙 巍 苏文坤 刘 扬
(东北石油大学提高油气采收率教育部重点实验室)



1 化学基准物选取




2 含蜡原油管输过程化学反应
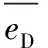
(1)
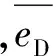

(2)




图1 各油田原油化学反应计算结果对比
3 含蜡原油管输过程化学扩散
(3)
(4)

在计算含蜡原油活度系数时,可采用较为精确的正规溶液理论模型,即:
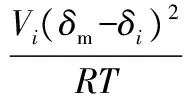
(5)
其中,Vi为组分摩尔体积,δi为组分溶解度参数,T为溶液温度,溶解度参数δm=∑δiφi,φi=xiVi(∑xiVi)-1。因此,只要计算出组分i的溶解度参数和摩尔体积就可以得到活度系数。对于溶解度参数,Riazi M R和Al-Sahhaf T A提出了一个正构烷烃液相溶解度参数的关系式[38],Leelavanichkul P等建立了一个异构环烷烃、芳香烃和组分i固相溶解度参数的表达式[39]。对于组分i的液相摩尔体积,可以由ViL=Mi/di25和Leelavanichkul P等建立的各碳氢化合物液相密度求得。对于组分i的固相摩尔体积,当体系中组分碳数不小于7时,可从相关化工手册查询,当碳数大于7时,可由经验公式计算。
4 含蜡原油管输过程化学传递



5 结束语
[1] 万宇飞,邓道明,刘霞,等.稠油掺稀管道输送工艺特性[J].化工进展,2014,33(9):2293~2297.
[2] 郑志,王树立,武玉宪,等.改善油气管道输送性能的相关技术[J].油气储运,2010,29(2):100~106.
[3] 侯磊,齐世明,李静.原油管道输送系统节能降耗工作的若干思考[J].油气田地面工程,2008,27(11):62~63.
[4] Mohammadi A,Mehrpooya M.Exergy Analysis and Optimization of an Integrated Micro Gas Turbine,Compressed Air Energy Storage and Solar Dish Collector Process[J].Journal of Cleaner Production,2016,139(15):372~383.
[5] Ameri M,Askari M.Enhancing the Efficiency of Crude Oil Transportation Pipeline:A Novel Approach[J].International Journal of Exergy,2013,13(4):523~542.
[6] Pal R.Exergy Destruction in Pipeline Flow of Surfactant Stabilized Oil-in-Water Emulsions[J].Energies,2014,7(11): 7602~7619.



[11] 日本能量变换恳话会编.能量有效利用技术[M].北京:化学工业出版社,1984:131~139.


[14] Szargut J.International Progress in Second Law Analysis[J].Energy,1980,5(8-9):709~718.
[16] Rickert L.The Efficiency of Energy-Utilization in Chemical Process[J].Chemical Engineering Science,1974,29(7): 1613~1620.
[17] 袁一,胡德生.化工过程热力学分析法[M].北京:化学工业出版社,1985:177~216.


[20] Szargut J,Styrylska T.Angenaherte Bestimmung der Exergie von Brennostoffen[J].Brennst Wärme Kraft,1964,16(12): 589~596.
[21] Shich J H,Fan L T.Estimation of Energy(Enthalpy) and Exergy(Availability) Contents in Structurally Complicated Materials[J].Energy Soures,1982,6(1-2):l~46.


[24] Song G,Xiao J,Zhao H,et al.A Unified Correlation for Estimating Specific Chemical Exergy of Solid and Liquid Fuels[J].Energy,2012,40(1):164~173.
[25] Kaushik S C,Singh O K.Estimation of Chemical Exergy of Solid,Liquid and Gaseous Fuels Used in Thermal Power Plants[J].Journal of Thermal Analysis &Calorimetry,2014,115(1):903~908.

[27] Nishida K,Takagi T,Kinoshita S.Analysis of Entropy Generation and Exergy Loss during Combustion[J].Proceedings of the Combustion Institute,2002,29(1):869~874.


[30] 张晋.以煤气化为核心的多联产系统的能量分析[D].北京:清华大学,2003.
[31] Lior N,Sarmiento W.The Exergy Fields in Transport Processes:Their Calculation and Use[J].Energy,2006,31(5): 553~578.
[32] Ji H Y,Tohidi B,Danesh A,et al.Wax Phase Equilibria:Developing a Thermodynamic Model Using a Systematic Approach[J].Fluid Phase Equilibria,2004,216(2):201~207.
[33] 陈五花.原油中石蜡沉积的热力学研究[D].大连:大连理工大学,2006.
[34] Broadhurst M G.An Analysis of the Solid Phase Behavior of the Normal Paraffins[J].Journal of Research of the National Bureau of Standards A,1962,66(3):241~249.
[35] Lira-Galeana C,Firoozabadi A,Prausnitz J M.Thermodynamics of Wax Precipitation in Petroleum Mixtures[J].Aiche Journal,1996,42(1):239~248.
[36] Pan H,Firoozabadi A,Fotland P.Pressure and Composition Effect on Wax Precipitation:Experimental Data and Model Results[J].SPE Production &Facilities,1997,12(4):579~592.
[37] Won K W.Thermodynamics for Solid Solution-Liquid-Vapor Equilibria:Wax Phase Formation from Heavy Hydrocarbon Mixtures[J].Fluid Phase Equilibria,1986,30(15):265~279.
[38] Riazi M R,Al-Sahhaf T A.Physical Properties of Heavy Petroleum Fractions and Crude Oils[J].Fluid Phase Equilibria,1996,117(1-2):217~224.
[39] Leelavanichkul P,Deo M D,Hanson F V.Crude Oil Characterization and Regular Solution Approach to Thermodynamic Modeling of Solid Precipitation at Low Pressure[J].Petroleum Science and Technology,2004,22(7-8):973~990.
[40] Gaggioli R A.The Concept of Available Energy[J].Chemical Engineering Science,1961,16(1-2):87~96.
[41] Gaggioli R A.The Concepts of Thermodynamic Friction,Thermal Available Energy,Chemical Available Energy and Thermal Energy[J].Chemical Engineering Science,1962,17(7):523~530.
[42] Soma J.The New Energy Hyperequation and Its Implications[J].Energy Engineering,1985,82(2):139~145.
[43] Soma J.Exergy Transfer:A New Field of Energy Endeavor[J].Energy Engineering,1985,82(4):219~225.
[44] 第六届全国热力学学术会议委员会.热力学分析与节能:论文集[M].北京:科学出版社,1993:75~81.






[52] Wang S P,Chen Q L,Yin Q H,et al.A Phenomenological Equation of Exergy Transfer and Its Application[J].Energy,2005,30(1):85~95.
[53] Acevedo L,Usón S,Uche J.Exergy Transfer Analysis of Microwave Heating Systems[J].Energy,2014,68(3):349~363.



ResearchProgressintheChemicalExergyCompositionandApplicationinWaxyCrudeOilPipelineTransportationProcess
GAN Yi-fan,CHENG Qing-lin,SUN Wei,SU Wen-kun,LIU Yang
(MOEKeyLaboraryforEnhancingOilandGasRecoveryRatio,NortheastPetroleumUniversity)
The theoretical achievements and development status of chemical exergy reference environment’s selection,chemical reaction exergy,chemical diffusion exergy and chemical exergy transfer at home and abroad were reviewed;through considering the actual application of chemical exergy analysis and chemical exergy transfer in engineering,the main problems existed in this field were indicated.
waxy crude oil pipeline transportation process,reaction exergy,diffusion exergy,chemical exergy transfer
国家自然科学基金项目(51534004);黑龙江省普通高校科技创新团队基金项目(2009td08)
甘亦凡(1992-),博士研究生,从事热力学分析与油气储运系统综合节能的研究,2905818298@qq.com。
TQ051.21
A
0254-6094(2017)04-0375-07
2017-01-19)

