土壤温度和含水量互作对抑制剂抑制氮素转化效果的影响
周 旋,吴良欢,戴 锋
土壤温度和含水量互作对抑制剂抑制氮素转化效果的影响
周 旋1,2,吴良欢1,2※,戴 锋3
(1. 教育部环境修复与生态健康重点实验室/浙江大学环境与资源学院,杭州 310058;2. 浙江省农业资源与环境重点实验室/浙江大学环境与资源学院,杭州 310058;3. 浙江奥复托化工有限公司,上虞 312300)
为比较生化抑制剂组合对土壤氮素转化的抑制效果,揭示不同土壤温度和含水量互作对尿素水解抑制效应的影响。该文采用室内模拟培养方法,研究土壤含水量(60%和80%田间最大持水量,water holding capacity, WHC)和土壤温度(15、25和35 ℃)互作对生化抑制组合[N-丁基硫代磷酰三胺(N-(n-butyl) thiophosphoric triamide, NBPT)、N-丙基硫代磷酰三胺(N-(n-propyl) thiophosphoric triamide, NPPT)和2-氯-6(三氯甲基)吡啶(2-chloro-6(trichloromethyl)pyridine, CP)在黄泥田土壤中抑制氮素转化效果的影响。结果表明:土壤温度和含水量对生化抑制组合在黄泥田土壤中抑制尿素水解效应显著,以土壤温度影响更大。随着土壤温度增加,尿素水解转化增强,有效作用时间降低,硝化作用增强,脲酶和硝化抑制效应减弱;随着土壤含水量降低,尿素水解转化缓慢,有效作用时间延长,硝化作用减弱,脲酶和硝化抑制效应增强。不同土壤温度和含水量条件下,NBPT/NPPT或配施CP处理有效抑制黄泥田土壤脲酶活性,延缓尿素水解;CP或配施NBPT/NPPT处理有效抑制NH4+-N向NO3--N转化,保持土壤中较高NH4+-N含量长时间存在。新型脲酶抑制剂NPPT单独施用及与CP配施的土壤尿素水解抑制效果与NBPT相似。黄泥田土壤中生化抑制组合应用最佳的土壤温度和含水量分别为25 ℃和60%WHC。总之,针对不同土壤温度和含水量条件,在黄泥田土壤中应采用脲酶抑制剂与硝化抑制剂相结合的施肥方式。
土壤;土壤含水量;氮;脲酶抑制剂;硝化抑制剂;N-丁基硫代磷酰三胺(NBPT);N-丙基硫代磷酰三胺(NPPT);2-氯-6(三氯甲基)吡啶(CP);土壤温度
0 引 言
大量研究指出,施入土壤中的尿素只有30%~60%的氮(N)被作物吸收利用,在中国平均只有35%[1],导致N肥大量浪费流失和生态环境污染加剧。通过在普通尿素或NH4+-N肥中添加脲酶抑制剂或硝化抑制剂,控制其在土壤中的转化,即尿素的水解和NH4+的转化过程,从而实现N肥长效和缓效目的,是未来新型肥料发展的重要方向[2-3]。脲酶抑制剂N-丁基硫代磷酰三胺(N-(n-butyl) thiophosphoric triamide,NBPT)主要通过与尿素竞争脲酶活性部位起作用;硝化抑制剂2-氯-6(三氯甲基)吡啶(2-chloro-6(trichloromethyl) pyridine,CP)主要通过竞争性抑制氨单加氧酶(ammonia monooxygenase,AMO)进而对硝化作用的第一步反应(NH4+氧化为NO2-过程)进行抑制。同时,抑制剂的施用效果除取决于其自身物理、化学性质及降解特性外,还受土壤类型、有机质含量、pH值、温度、湿度及土肥管理措施等环境因素的影响[4-5]。
相关研究表明,土壤脲酶对尿素的亲和系数和尿素水解反应的最大初速度受土壤类型、温度和培养时间的影响[6]。土壤中尿素转化的NH4+-N含量与土壤湿度和土壤温度呈正相关;其中,20~25 ℃为施用尿素后产生NH4+-N的最敏感温区,而土壤含水量80 g/kg为最敏感湿度[7]。可见,土壤温度和含水量均是土壤尿素态N转化过程中的重要影响因素。
黄泥田属于渗育型水稻土亚类,是中国南方稻区重要的中低产田之一[8],面积约有140万hm2。其土壤速效养分含量低,肥力贫瘠,保肥性能差,盲目施肥易导致养分的大量损耗[9]。冉炜等[6]比较发现,潮土和黄棕壤中同时存在高亲和性和低亲和性尿素水解反应,而灰钙土仅表现为低亲和性反应。杨剑波等[10]研究发现,在黑土和红壤中,尤其是红壤,硝化过程相对缓慢,施用抑制剂虽降低土壤表观硝化率,但降低程度低于水稻土和潮土。可见,不同类型土壤N素水解转化存在较大差异,且抑制剂施用效果也大不相同。作者前期工作发现,NBPT和CP在偏酸性的黄泥田土壤中具有良好的协同抑制效果[11]。浙江奥复托化工公司经多次筛选发现一款有良好应用前景的脲酶抑制剂—N-丙基硫代磷酰三胺(N-(n-propyl) thiophosphoric triamide,NPPT),具有一定的抑制作用[12]。
相关研究认为,不同抑制剂或者同一抑制剂在不同施用环境条件下对N素转化的调控效果存在较大变异[13]。因此,开展不同土壤温度和含水量对生化抑制组合在黄泥田土壤中抑制尿素态N水解转化效应的研究,旨在找出抑制剂施用达到最佳效果的外界环境条件,为研制适于黄泥田施用的添加生化抑制剂的缓释尿素肥料及推广应用提供科学理论依据。
1 材料与方法
1.1 试验材料
供试土壤为黄泥田水稻土,于2014年10月采自浙江省金华市婺城区琅琊镇金朱村(29°01¢19²N,119°27¢96²E)的0~20 cm耕层土壤。土样采回后剔除杂物及根系,过2 mm筛备用。供试土壤基本理化性质为pH5.31(土∶水=1∶1),有机质和全N质量分数分别为25.6、1.87 g/kg,碱解N、有效磷和速效钾质量分数分别为118.4、7.21和93.0 mg/kg。
供试尿素含N46%为分析纯,由国药集团化学试剂有限公司生产;2-氯-6-(三氯甲基)吡啶(CP)24%乳油剂型、N-丁基硫代磷酰三胺(NBPT)和N-丙基硫代磷酰三胺(NPPT)为分析纯,由浙江奥复托化工有限公司生产。
1.2 试验设计
试验前先将土壤含水量调节至田间最大持水量(water holding capacity,WHC)的60%左右,然后放置于培养箱(江南,HWS智能型)中25 ℃培养1周,以恢复土壤的生物学活性。预培养结束后,以尿素(U)为N源开展以下试验。
试验设置3种土壤温度(15、25和35 ℃,参照当地温度条件设置)、2种土壤含水量(60%WHC和80%WHC,参照当地水分条件设置)和6种生化抑制剂组合(U、U+NBPT、U+NPPT、U+CP、U+NBPT+CP、U+NBPT+CP)及不施N处理(CK),共42个处理。各抑制剂处理中NBPT/NPPT或CP用量分别为尿素态N量的0.5%或0.3%。
采用塑料瓶(500 mL)盛土,每瓶装干土300 g,尿素用量为N 300 mg/kg。试验时将尿素和生化抑制剂按上述比例加入培养瓶中与土壤混匀,并加蒸馏水使土壤含水量分别达到60%WHC和80%WHC,分别于15、25和35 ℃培养箱中黑暗条件恒温、恒湿培养,定期补充水分。于2016年5月16日开始进行,在培养第1、3、6、9、12、18、24、30天取样,重复3次。采用非破坏性取样[1],即取样时先在第一组塑料瓶中取,第一组塑料瓶中土取完后,再从第二组中取,以此类推。测定土壤中NH4+-N、NO3--N及尿素态N含量,若浸提液不能立即测,放在4 ℃冷冻箱里储存。
1.3 测定项目与方法
采用常规分析方法测定土壤基本理化性质[14];尿素态N采用KCl-乙酸苯汞浸提,用二乙酰一肟比色法测定;NO3--N和NH4+-N采用2mol/L KCl浸提,分别用双波长分光光度法和靛酚蓝比色法测定[14]。
抑制率计算公式[11]如下:
土壤脲酶活性抑制率(%)=(0−1)/0×100%
式中0为不加脲酶抑制剂处理的尿素态N水解量,mg/kg;1为添加脲酶抑制剂处理的尿素态N水解量,mg/kg;尿素态N水解量为尿素态N加入量减去测定尿素态N剩余量,mg/kg。
土壤硝化抑制率(%)=(0−1)/0×100%
式中0为尿素处理NO3--N含量;1为同期硝化抑制剂与尿素配合施用处理的NO3--N含量。
土壤表观硝化率(%)=NO3--N/(NH4+-N+NO3--N)×100%。
1.4 数据处理
试验数据采用Microsoft Excel 2003和SPSS 17.0进行统计分析,处理间差异显著性比较采用邓肯氏新复极差检验法。
2 结果与分析
2.1 土壤尿素态N含量
由图1可知,施N处理整个培养期间土壤尿素态N含量总体呈下降趋势。不同土壤温度处理土壤尿素水解时间长短表现为15 ℃(6~9 d)长于25 ℃(3 d)和35 ℃(3 d);不同土壤含水量处理时间长短表现为60%WHC(9 d,15 ℃)长于80%WHC(6 d,15 ℃)。添加NBPT/NPPT处理尿素态N含量分别于第6~9天(15 ℃)、第1~3天(25 ℃)、第1天(35 ℃)开始先快后慢逐渐下降,且水解速率表现为随着土壤温度和含水量的增加而增加。添加NBPT/NPPT处理培养期间土壤尿素态N含量显著高于U和U+CP处理。不同土壤温度和含水量条件下,NBPT/NPPT在黄泥田土壤中有效作用时间为6~30 d。
2.2 土壤脲酶抑制率
由表1可知,添加抑制剂处理整个培养期间土壤脲酶抑制率总体呈下降趋势。不同温度处理脲酶抑制率第1天均值大小表现为43.5%(35 ℃)>31.7%(15 ℃)>22.9%(25 ℃),之后3~9 d内尿素大量水解,土壤脲酶抑制率降低,分别于第24天(15 ℃)、第12天(25 ℃)、第9天(35 ℃)之后无脲酶抑制效果。不同土壤含水量处理脲酶抑制率第1天均值大小表现为45.9%(60%WHC)>19.5(80%WHC),之后培养期间处理间变化差异不大。
方差分析表明,土壤温度对培养期间(1~12 d)土壤脲酶抑制率效应极显著(<0.001),而土壤含水量效应不显著(第1天除外)(>0.05),两者交互效应不显著(第1天除外)(>0.05);抑制剂组合对培养期间(1~12 d)土壤脲酶抑制率效应极显著(<0.001),与土壤温度交互效应极显著(<0.001),与土壤含水量交互效应不显著(>0.05);三者交互效应不显著(第1天除外)(>0.05)。

注:WHC、CK、U、U+NBPT、U+NPPT、U+CP、U+NBPT+CP和U+NPPT+CP分别表示田间最大持水量、对照、尿素、尿素配施N-丁基硫代磷酰三胺、尿素配施N-丙基硫代磷酰三胺、尿素配施2-氯-6-(三氯甲基)吡啶、尿素配施N-丁基硫代磷酰三胺与2-氯-6-(三氯甲基)吡啶和尿素配施N-丙基硫代磷酰三胺与2-氯-6-(三氯甲基)吡啶,下同。
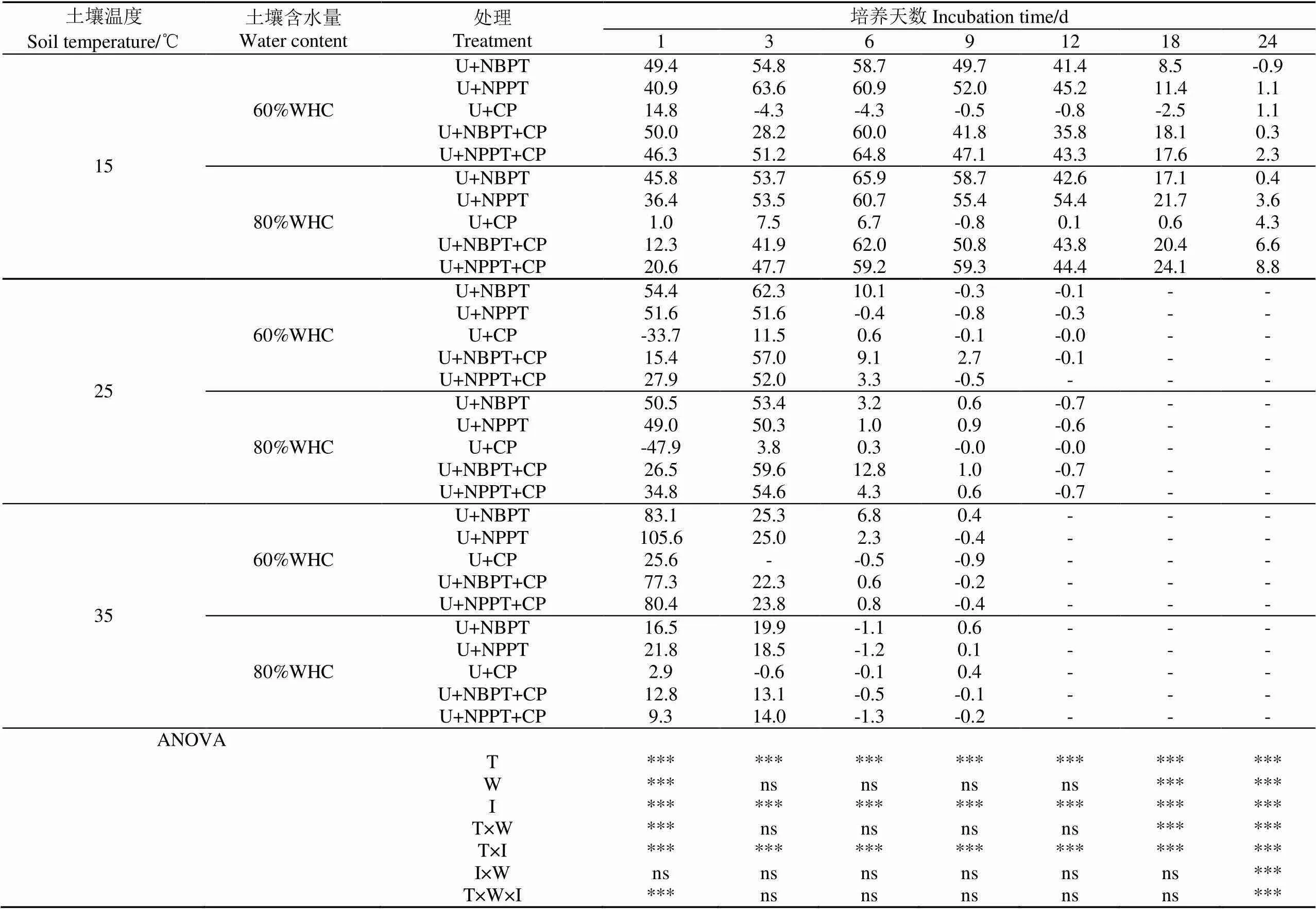
表1 不同土壤温度和含水量处理下土壤脲酶抑制率变化
注:T:土壤温度;W:土壤含水量;I:抑制剂;T×W:温度和含水量互作;T×I:温度和抑制剂互作;I×W:抑制剂和含水量互作;T×W×I:温度、含水量和抑制剂互作;ns:不显著。*,**,***分别表示在0.05、0.01和0.001水平上差异显著。下同。
Note:T: Soil temperature; W: Soil water content; I: Inhibitor; T×W: Temperature - water interaction; T×I: Temperature - inhibitor interaction; I×W: Inhibitor - water interaction; T×W×I: Temperature- water- inhibitor interaction; ns: no significant. *, **, *** represent significant difference at 0.05, 0.01 and 0.001 levels, respectively. The same as below.
培养第3天,不同土壤含水量处理脲酶抑制率均值大小表现为60%WHC(35.0%)>80%WHC(32.7%);不同土壤温度处理均值大小表现为25 ℃(45.6%)>15 ℃(39.8%) >35 ℃(16.1%);不同抑制剂组合处理均值大小表现为U+NBPT(44.9%)>U+NPPT(43.7%)>U+NPPT+CP(40.5%) >U+NBPT+CP(37.0%)>U+CP(3.0%)。
2.3 土壤NH4+-N含量
由图2可知,施N处理整个培养期间土壤NH4+-N含量总体呈先升后降的趋势。第3~24天(15 ℃)、第3~9天(25 ℃)或3~6天(35 ℃),U和U+CP处理土壤中NH4+-N含量急剧增加,而添加NBPT/NPPT处理增长缓慢。施N处理分别于第24天(15 ℃)或第6天(25 ℃、35 ℃)后陆续达到峰值,上升速率表现为随着土壤温度和含水量的增加而增加。第24天(15 ℃)或第12天(25 ℃和35 ℃),施N处理土壤中NH4+-N含量开始逐渐下降,而添加CP处理土壤NH4+-N含量明显高于U和U+NBPT/NPPT处理,且可以维持较高NH4+-N含量超过30 d。
2.4 土壤NO3--N含量
由图3可知,施N处理整个培养期间土壤NO3--N含量总体呈上升趋势。第12~30天(15 ℃)或第6~30天(25 ℃、35 ℃),U处理和添加NBPT/NPPT处理土壤NO3--N含量大幅增加,上升速率表现为随着土壤温度和含水量的增加而增加。添加CP处理土壤NO3--N含量明显低于U和U+NBPT/NPPT处理,且可以维持较低NO3--N含量超过30 d。U和U+NBPT/NPPT处理(35 ℃)土壤NO3--N含量分别于第18和24天达到峰值后出现下降。

图2 不同土壤温度和含水量处理下土壤NH4+-N含量变化

图3 不同土壤温度和含水量处理下土壤NO3--N含量变化
2.5 土壤表观硝化率
由表2可知,不同处理土壤表观硝化率整个培养期间总体呈先降后升趋势,以CK处理最高。第6~30天,施N处理土壤表观硝化率急剧增加,且上升幅度表现为80%WHC高于60%WHC。添加CP处理土壤表观硝化率低于U和U+NBPT/NPPT处理。
方差分析表明,土壤温度和含水量分别对培养期间土壤表观硝化率效应显著或极显著(土壤含水量第1天除外)(<0.05),两者交互效应极显著(第1天除外)(<0.01);抑制剂组合对培养期间土壤表观硝化率效应极显著(<0.001),与土壤温度交互效应显著或极显著(<0.05),与土壤含水量交互效应不显著(第12天除外)(>0.05);三者交互效应不显著(>0.05)。培养第30天,不同土壤含水量处理表观硝化率均值大小表现为60%WHC(22.1%)< 80%WHC(28.0%);不同土壤温度处理均值大小表现为25 ℃(30.2%)>35 ℃(28.7%)>15 ℃(16.4%);不同抑制剂组合处理均值大小表现为CK(50.4%)>U(29.2%)>U+NPPT(29.0%)>U+NBPT(28.4%)>U+NPPT+CP(13.2%)>U+NBPT+CP(13.2%)>U+CP(12.3%)。

表2 不同土壤温度和含水量处理下土壤表观硝化率变化
2.6 土壤硝化抑制率
由表3可知,添加抑制剂处理土壤硝化抑制率整个培养期间总体呈先升后降趋势。土壤含水量处理均值分别于第18天(60%WHC:43.3%)和12天(80%WHC:48.7%)达到峰值;土壤温度处理均值分别于第24天(15 ℃:34.4%;25 ℃:52.9%)和12天(35 ℃:56.9%)达到峰值;抑制剂组合处理均值分别于第12天(U+NBPT:32.1%)、第9天(U+NPPT:23.9%)和第24天(U+CP:67.9%;U+NBPT+CP:57.1%;U+NPPT+CP:53.9%)达到峰值。不同土壤含水量处理培养期间硝化抑制率变化趋势差异不大;不同土壤温度和抑制剂处理培养前期变化趋势差异不大,后期差异逐渐增大。
方差分析表明,土壤温度对培养期间土壤硝化抑制率效应极显著(<0.001),土壤含水量效应显著或极显著(第6、18、30天除外)(<0.05),两者交互效应显著或极显著(第24天除外)(<0.05);抑制剂组合对培养期间土壤硝化抑制率效应极显著(<0.001),与土壤温度交互效应极显著(<0.001),与土壤含水量交互效应极显著(第6、18~30天除外)(<0.01);三者交互效应极显著(第18、30天除外)(<0.001)。
培养第30天,不同土壤含水量处理硝化抑制率均值大小表现为60%WHC(31.3%)>80%WHC(27.2%);不同土壤温度处理硝化抑制率均值大小表现为25 ℃(51.8%)>15 ℃(30.9%)>35 ℃(5.1%);不同抑制剂组合处理硝化抑制率均值大小表现为U+CP(60.8%)> U+NBPT+CP(47.9%)>U+NPPT+CP(40.4%)>U+NPPT(0.3%)>U+NBPT(−3.1%)。

表3 不同土壤温度和含水量处理下土壤硝化抑制率的变化
3 讨 论
3.1 抑制剂组合对尿素水解转化的影响
相关研究表明,脲酶抑制剂和硝化抑制剂分别对土壤中尿素态N水解转化的某一特定过程起到抑制作用,其单独作用不能对全过程进行有效控制,而两者配合施用更能有效延缓尿素水解及水解产物NH4+的进一步氧化,使其在土壤中存在相对较长的时间和较大的量[15-16]。郑福丽等[17]研究发现,NBPT与双氰胺(DCD)配施明显抑制NH4+-N向NO3--N的转化,延缓NO3--N释放高峰达40 d以上。邢卫等[18]研究发现,不同浓度NBPT与二甲基吡唑磷酸盐(DMPP)配施均延缓尿素水解,使更多NH4+-N保持在土壤中,增加土壤有效态N含量。李莉等[1,19]研究发现,NBPT+DMPP组合有效抑制土壤脲酶活性,缓释尿素施入4~7d;与仅添加DMPP对硝化作用抑制效果相同,两者能保持土壤中较高NH4+-N含量超过80 d。与上述研究结果一致,本研究中与仅添加NBPT/NPPT和CP处理相比,在黄泥田土壤中两者配施表现出对N素转化的协同抑制效果;不同作用条件下,既有效缓释尿素6~30 d,又能保持土壤中较高NH4+-N含量30 d以上。
3.2 土壤温度对抑制剂作用效果的影响
低温或pH值较低土壤将减少尿素的氨气挥发,提高N素利用率[20],而升高温度会利于解离酶—尿素复合体,提高酶促反应的初始速率[21]。程冬冬等[22]研究发现,土壤含水量相同,35 ℃土壤脲酶活性显著高于25 ℃。与本研究结果相符,土壤温度升高(15~35 ℃)将加剧尿素的水解,且缩短水解时间(图1)。
相关研究表明,土壤温度升高将降低NBPT有效性[23]。Bremner等[24]研究六种土壤添加NBPT于10、20、30和40 ℃培养14 d发现,随着温度的增加NBPT抑制效果显著下降50%。李涛等[25]研究发现,随着环境温度增高(20~30 ℃),NBPT降解速度略有提升(半衰期:7.6~6.5 d)。本研究中,在试验温度范围(15~35 ℃)内,黄泥田土壤中添加NBPT/NPPT对土壤尿素水解抑制效果显著,但作用时间及效果受温度的影响。Watson等[26]研究4种土壤、3种土壤温度(5、15和25 ℃)和不同NBPT添加量下尿素的NH3排放发现,NH3排放减少率为15 ℃(61%)小于5 ℃(83%)或25 ℃(74%)。隽英华等[21]研究认为,高温下尿素水解速度可能超过NBPT转换为NBPTO的速度,且抑制剂降解速度也可能加快。本研究结果表明,随着土壤温度升高,尿素水解速率增加,有效作用时间降低,脲酶抑制效应降低。土壤温度处理尿素水解时间大小表现为15 ℃(6~9 d)>25(3 d)和35 ℃(3 d);培养第3天脲酶抑制率均值大小表现为25 ℃(45.6%)>15 ℃(39.8%)>35 ℃(16.1%)。
相关研究表明,温度是影响硝化抑制剂效果及有效时间长短的重要因素:较高温度下抑制剂降解速率明显加快,半衰期缩短,挥发损失增多[27-28]。Menéndez等[29]研究认为,一定范围内硝化抑制剂在低温时抑制效果更好。Azam等[30]研究认为,温度升高加速DMPP在土壤中移动,有效抑制时间逐渐缩短。此外,高温可能增加微生物活性,加速DMPP在土壤中降解[31]。而低温环境有利于DMPP对硝化进程的抑制[32]。本研究中,在试验温度范围(15~35 ℃)内,黄泥田土壤中添加CP可以保持良好的硝化抑制效果,培养期间受温度影响较小。殷建祯等[32]采用好气培养发现,15 ℃有机无机N肥配施DMPP的NH4+-N含量(60 d)比25 ℃高56倍,NO3--N含量低18倍。Chaves等[33]研究发现,DMPP在粉质壤土中(2~14 ℃)有效控制蔬菜残体硝化作用的时间为95 d。在5~15 ℃和40%~60%含水量时,DMPP在黏壤土中硝化抑制作用时间达42 d[34-35]。本研究结果表明,培养第30天土壤温度处理表观硝化率均值大小表现为25 ℃(30.2%)>35 ℃(28.7%)>15 ℃(16.4%);硝化抑制率均值大小表现为25 ℃(51.8%)>15 ℃(30.9%)>35 ℃(5.1%)。史云峰等[36]研究发现,培养温度由10 ℃增高到30 ℃,砖红壤中DCD持续作用时间由90 d下降为30 d。本研究中,不同土壤温度条件下,CP在黄泥田土壤中有效作用时间超过30 d,具体作用周期还有待进一步研究确定。
3.3 土壤含水量对抑制剂作用效果的影响
尿素水解需要水的存在。Cantarella等[37]研究发现,降雨过多导致施肥后NBPT效果降低。而周静等[38]研究发现,等量尿素施入红壤后,土壤含水量与氨挥发通量之间无显著相关性。本研究中,土壤含水量对培养期间土壤脲酶抑制率效应不显著(第1天除外),可能是由于尿素水解时间短,抑制剂作用时间短,受土壤含水量影响时效也相应缩短。Faria等[39]研究发现,NBPT的抑制效果随着时间的变化而降低,与土壤水分含量增加有关。本研究中,在试验含水量范围(60% WHC~80% WHC)内,随着土壤含水量降低,黄泥田土壤中尿素水解转化趋于缓慢,有效作用时间延长,脲酶抑制效应增强。不同土壤含水量处理尿素水解时间大小表现为60%WHC(9 d,15 ℃)>80%WHC(6 d,15 ℃);培养第1天,脲酶抑制率均值大小表现为60%WHC(45.9%)>80%WHC(19.5%)。
在一定土壤含水量范围,土壤硝化作用强度与土壤含水量呈正相关[40],且增加氨氧化细菌的种群数量和活性[41]。与本研究结果相符,培养第30天土壤含水量处理表观硝化率均值大小表现为60%WHC(22.1%)<80%WHC(28.0%)。相关研究指出,因抑制剂本身特性的不同,土壤含水量对硝化抑制剂抑制效果的影响存在差异[5]。Barth等[42]研究认为,在较低土壤含水量条件下,DMPP更易被土壤吸附,使得存留量增加。薛妍等[5]研究发现,随土壤含水量降低,DMPP降解趋于缓慢,硝化抑制效应增强。本研究中,在试验含水量范围(60% WHC~80% WHC)内,随着土壤含水量降低,硝化作用减弱,硝化抑制效应增强。培养第30天硝化抑制率均值大小表现为60%WHC(31.3%)>80%WHC(27.2%),可能是由于较低含水量条件下,通过底物限制作用或细胞脱水使硝化细菌的活性降低[43]。王改玲等[44]研究表明,低水分时施用CP可抑制硝化反应;高水分时施用砂子或砂子与CP配合,可有效抑制N2O排放。史云峰等[36]研究发现,土壤含水量12%和24%时,硝化抑制作用分别持续90和60 d,进而高含水量会降低DCD抑制效果。本研究结果表明,不同土壤含水量条件下,CP在黄泥田土壤中有效作用时间超过30 d,具体作用周期还有待进一步研究确定。
4 结 论
1)土壤含水量和土壤温度对生化抑制组合在黄泥田土壤中尿素水解转化作用效果显著,以土壤温度影响更大。不同土壤温度和含水量条件下,NBPT/NPPT在黄泥田土壤中有效作用时间为6~30 d,CP有效作用时间超过30 d。
2)本试验范围内,随着土壤温度增加,尿素水解转化增强,有效作用时间降低,硝化作用增强,脲酶和硝化抑制效应减弱;随着土壤含水量降低,尿素水解转化趋于缓慢,有效作用时间延长,硝化作用减弱,脲酶和硝化抑制效应增强。
3)不同土壤温度和含水量条件下,NBPT/NPPT或配施CP处理可以有效抑制土壤脲酶活性,延缓尿素水解;CP或配施NBPT/NPPT处理可以有效抑制NH4+-N向NO3--N转化,保持土壤中较高NH4+-N含量长时间存在,增加土壤有效态N含量。
[1] 李莉,李东坡,武志杰,等. 脲酶/硝化抑制剂对尿素氮在白浆土中转化的影响[J]. 植物营养与肥料学,2011,17(3):646-650.
Li Li, Li Dongpo, Wu Zhijie, et al. Effect of urease/nitrification inhibitors on transformation of urea-N in albic soil[J]. Plant Nutrition and Fertilizer Science, 2011, 17(3): 646-650. (in Chinese with English abstract)
[2] 史云峰,武志杰,张丽莉,等. 新型高效肥料创制的意义、现状及发展趋势[J]. 磷肥与复肥,2011,26(6):1-5.
Shi Yunfeng, Wu ZhiJie, Zhang Lili, et al. Significance, status and development trend of new high efficiency fertilizers in China[J]. Phosphate and Compound Fertilizer, 2011, 26(6): 1-5. (in Chinese with English abstract)
[3] 武志杰,石元亮,李东坡,等. 新型高效肥料研究展望[J]. 土壤与作物,2012,1(1):2-9.
Wu Zhijie, Shi Yuanliang, Li Dongpo, et al. Prospects for new-type fertilizers with high efficiency[J]. Soil and Crop, 2012, 1(1): 2-9. (in Chinese with English abstract)
[4] 孙志梅,武志杰,陈利军,等. 硝化抑制剂的施用效果、影响因素及其评价[J]. 应用生态学报,2008,19(7):1611-1618.
Sun Zhimei, Wu Zhijie, Chen Lijun, et al. Application effect, affecting factors, and evaluation of nitrification inhibitor: A review[J]. Chinese Journal of Applied Ecology, 2008, 19(7): 1611-1618. (in Chinese with English abstract)
[5] 薛妍,武志杰,张丽莉,等. 土壤含水量、pH及有机质对
DMPP硝化抑制效果的影响[J]. 应用生态学报,2012,23(10):2663-2669.
Xue Yan, Wu Zhijie, Zhang Lili, et al. Inhibitory effect of DMPP on soil nitrification as affected by soil moisture content, pH and organic matter[J]. Chinese Journal of Applied Ecology, 2012, 23(10): 2663-2669. (in Chinese with English abstract)
[6] 冉炜,沈其荣,郑金伟. 尿素浓度、培养时间和温度对3种土壤尿素水解过程的影响[J]. 南京农业大学学报,2000,23(2):43-46.
Ran Wei, Shen Qirong, Zheng Jinwei. Effect of urea concentration, incubation time and temperature on urea hydrolysis in 3 different soils[J]. Journal of Nanjing Agricultural University, 2000, 23(2): 43-46. (in Chinese with English abstract)
[7] 高鹏程,张一平,张海,等. 水热耦合作用下尿素转化为铵态氮的动力学模型[J]. 植物营养与肥料学报,2005,11(1):21-26.
Gao Pengcheng, Zhang Yiping, Zhang Hai, et al. The dynamic model of urea transforming into ammonium affected by interaction of temperature and water[J]. Plant Nutrition and Fertilizing Science, 2005, 11(1): 21-26. (in Chinese with English abstract)
[8] 李清华,王飞,林诚,等. 长期施肥对黄泥田土壤微生物群落结构及团聚体组分特征的影响[J]. 植物营养与肥料学报,2015,21(6):1599-1606.
Li Qinghua, Wang Fei, Lin Cheng, et al. Effects of long-term fertilization on soil microbial community structure and aggregate composition in yellow clayey paddy field[J]. Plant Nutrition and Fertilizing Science, 2015, 21(6): 1599-1606. (in Chinese with English abstract)
[9] 刘彦伶. 南方中低产黄泥田改良和产量提升技术研究[D]. 浙江大学,2013.
Liu Yanling. Study on the Technology of Soil and Grain Yield Improvement for Low-medium Yielding Yellow- clayed Paddy Field in south China[D]. Hangzhou: Zhejiang University, 2013. (in Chinese with English abstract)
[10] 杨剑波,李学超,徐晶晶,等. 两种硝化抑制剂在不同土壤中的效果比较[J]. 土壤,2014,46(2):319-324.
Yang Jianbo, Li Xuechao, Xu Jingjing, et al. Effects of nitrification inhibitors on nitrogen transformation in different Soils[J]. Soils, 2014, 46(2): 319-324. (in Chinese with English abstract)
[11] 周旋,吴良欢,戴锋. 生化抑制剂组合对黄泥田土壤尿素态氮转化的影响[J]. 水土保持学报,2015,29(5):95-100.
Zhou Xuan, Wu Lianghuan, Dai Feng. Effects of combined biochemical inhibitors on transformation of urea-N in yellow clayey soil[J]. Journal of Soil and Water Conservation, 2015, 29(5): 95-100. (in Chinese with English abstract)
[12] 周旋,吴良欢,戴锋. 新型磷酰胺类脲酶抑制剂对不同质地土壤尿素转化的影响[J]. 应用生态学报,2016,27(12):4003-4012.
Zhou Xuan, Wu Lianghuan, Dai Feng. Influence of a new phosphoramide urease inhibitor on urea-N transformation in different texture soil[J]. Chinese Journal of Applied Ecology, 2016, 27(12): 4003-4012. (in Chinese with English abstract)
[13] 刘建涛,许靖,孙志梅,等. 氮素调控剂对不同类型土壤氮素转化的影响[J]. 应用生态学报,2014,25(10):2901-2906.
Liu Jiantao, Xu Jing, Sun Zhimei, et al. Effects of different nitrogen regulators on nitrogen transformation in different soil types[J]. Chinese Journal of Applied Ecology, 2014, 25(10): 2901-2906. (in Chinese with English abstract)
[14] 鲁如坤. 土壤农业化学分析方法[M]. 北京:中国农业科技出版社,1999.
[15] Gioacchini P, Nastri A, Marzadori C, et al. Influence of urease and nitrification inhibitors on N losses from soils fertilized with urea[J]. Biology and Fertility of Soils, 2002, 36(2): 129-135.
[16] Xu X, Zhou L, Cleemput O V, et al. Fate of urea-15N in a soil-wheat system as influenced by urease inhibitor hydroquinone and nitrification inhibitor dicyandiamide[J]. Plant and Soil, 2000, 220(1/2): 261-270.
[17] 郑福丽,石元亮. 抑制剂对尿素转化及土壤中氮的影响研究[J]. 土壤通报,2007,38(2):412-414.
Zheng Fuli, Shi Yuanliang. Effects researches of inhibitors on urea transformation and N concentration and formation in soil[J]. Chinese Journal of Soil Science, 2007, 38(2): 412-414. (in Chinese with English abstract)
[18] 邢卫,陈利军,陈振华,等. NBPT与DMPP不同剂量组合对尿素氮转化的影响[J]. 土壤通报,2008,39(4):896-899.
Xing Wei, Chen Lijun, Chen Zhenhua, et al. Synergistic effect of different doses of NBPT and DMPP on urea-N transformation[J]. Chinese Journal of Soil Science, 2008, 39(4): 896-899. (in Chinese with English abstract)
[19] 李莉,李东坡. 白浆土中抑制剂不同浓度组合对尿素态氮的转化作用效果[J]. 水土保持学报,2010,24(6):99-104.
Li Li, Li Dongpo. Synergistic effect of different concentration of urease/nitrification inhibitor on the transformation of urea-N in albic soil[J]. Journal of Soil and Water Conservation, 2010, 24(6): 99-104. (in Chinese with English abstract)
[20] 蒋朝晖,曾清如,方至,等. 不同温度下施入尿素后土壤短期内pH的变化和氨气释放特性[J]. 土壤通报,2004,35(3):299-302.
Jiang Zhaohui, Zeng Qingru, Fang Zhi, et al. Short-term changes of pH and volatilization of ammonia from urea fertilizer in soil under different temperatures[J]. Chinese Journal of Soil Science, 2004, 35(3):299-302. (in Chinese with English abstract)
[21] 隽英华,陈利军,武志杰. 温度和脲酶抑制剂影响下的土壤脲酶动力学行为[J]. 辽宁工程技术大学学报,2009,28(6):1021-1025.
Juan Yinghua, Chen Lijun, Wu Zhijie. Kinetic behaviors of soil urease under influence of temperature and urease inhibitors[J]. Journal of Liaoning Technical University, 2009, 28(6): 1021-1025. (in Chinese with English abstract)
[22] 程冬冬,赵贵哲,刘亚青,等. 土壤温度、土壤含水量对高分子缓释肥养分释放及土壤酶活性的影响[J]. 水土保持学报,2013,27(6):216-220.
Cheng Dongdong, Zhao Guizhe, Liu Yaqing, et al. Influences of soil temperature and moisture on nutrients release of polymeric slow release fertilizer and soil enzyme activity[J]. Journal of Soil and Water Conservation, 2013, 27(6): 216-220. (in Chinese with English abstract)
[23] Carmona G, Christianson C B, Byrnes B H. Temperature and low concentration effects of the urease inhibitor N-(n-Butyl) thiophosphoric triamide (nBTPT) on ammonia volatilization from urea[J]. Soil Biology and Biochemistry, 1990, 22(7): 933-937.
[24] Bremner J M, Chai H S. Evaluation of N-butyl phosphorothioic triamide for retardation of urea hydrolysis in soil[J]. Communications in Soil Science and Plant Analysis, 1986, 17(3):337-351.
[25] 李涛,石元亮,李学文,等. NBPT在土壤中的降解及其影响因子[J]. 生态学杂志,2006,25(9):1082-1086.
Li Tao, Shi Yuanliang, Li Xuewen, et al. Degradation and its affecting factors of NBPT in soil[J]. Chinese Journal of Ecology, 2006, 25(9): 1082-1086. (in Chinese with English abstract)
[26] Watson C J, Akhonzada N A, Hamilton J T G, et al. Rate and mode of application of the urease inhibitor N-(n-butyl) thiophosphoric triamide on ammonia volatilization from surface-applied urea[J]. Soil Use and Management, 2008, 24(3): 246-253.
[27] 沈真实,许超,汤海涛,等. DMPP施用的环境效应、影响因素及其机理研究[J]. 湖南农业科学,2011(8):71-76.
Shen Zhenshi, Xu Chao, Tang Haitao, et al. Environmental effects, influencing factors and mechanism of DMPP application[J]. Hunan Agricultural Sciences, 2011(8): 71-76. (in Chinese with English abstract)
[28] 俞巧钢,殷建祯,马军伟,等. 硝化抑制剂DMPP应用研究进展及其影响因素[J]. 农业环境科学学报,2014,33(6):1057-1066.
Yu Qiaogang, Yin Jianzhen, Ma Junwei, et al. Effects of nitrification inhibitor DMPP application in agricultural ecosystems and their influencing factors: A review[J]. Journal of Agro-Environment Science, 2014, 33(6): 1057-1066. (in Chinese with English abstract)
[29] Menéndez S, Barrena I, Setien I, et al. Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions[J]. Soil Biology and Biochemistry, 2012, 53: 82-89.
[30] Azam F, Benckiser G, Müller C, et al. Release, movement and recovery of 3,4-dimethylpyrazole phosphate (DMPP), ammonium, and nitrate from stabilized nitrogen fertilizer granules in a silty clay soil under laboratory conditions[J]. Biology and Fertility of Soils, 2001, 34(2): 118-125.
[31] Zerulla W, Barth T, Dressel J, et al. 3, 4-Dimethypyrazole phosphate (DMPP): A new nitrification inhibitor for agriculture and horticulture[J]. Biology and Fertility of Soils, 2001, 34: 79-84.
[32] 殷建祯,俞巧钢,符建荣,等. 不同作用因子下有机无机配施添加DMPP对氮素转化的影响[J]. 土壤学报,2013,50(3):574-583.
Yin Jianzhen, Yu Qiaogang, Fu Jianrong, et al. Effects of combined application of organic and inorganic fertilizers plus DMPP on nitrogen transformation in soils as affected by different factors[J]. Acta Pedologica Sinica, 2013, 50(3): 574-583. (in Chinese with English abstract)
[33] Chaves B, Opoku A, Neve S D. Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues[J]. Biology and Fertility of Soils, 2006, 43(1): 62-68.
[34] Chen D, Suter H C, Islam A, et al. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea[J]. Soil Biology and Biochemistry, 2010, 42(4): 660-664.
[35] Yang J B, Li X C, Xu L, et al. Influence of the nitrification inhibitor DMPP on the community composition of ammonia-oxidizing bacteria atmicrosites with increasing distance from the fertilizer zone[J]. Biology and Fertility of Soils, 2013, 49: 23-30.
[36] 史云峰,赵牧秋,张丽莉. 双氰胺(DCD)在砖红壤中硝化抑制效果的影响因素研究[J]. 安徽农业科学,2011,12(33):1505-1508.
Shi Yunfeng, Zhao Muqiu, Zhang Lili. Research on the factors affecting nitrification inhibition of dicyandiamide (DCD) in Latosol[J].Journal of Anhui Agricultural Sciences, 2011, 12(33): 1505-1508. (in Chinese with English abstract)
[37] Cantarella H, Trivelin P C O, Contin T L M, et al. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets[J]. Scientia Agricola, 2008, 65(4): 397-401.
[38] 周静,崔键,王霞. 红壤不同含水量对尿素氨挥发的影响[J]. 土壤,2008,40(6):930-933.
Zhou Jing, Cui Jian, Wang Xia. Effects of red soil moisture on ammonia volatilization of urea[J]. Soils, 2008, 40(6): 930-933. (in Chinese with English abstract)
[39] Faria L D A, Bardella G R, Moura T A D, et al. NH3volatilization from urea-NBPT in Eucalyptus[J]. Communications in Soil Science and Plant Analysis, 2016, 47(6): 769-774.
[40] Klemedtsson L, Svensson B H, Rosswall T. Relationship between soil moisture content and nitrous oxide production during nitrification and denitrification.[J]. Biology and Fertility of Soils, 1988, 6(2): 106-111.
[41] Deirdreb G, Ankem H, Stephenj L, et al. Influence of water potential on nitrification and structure of nitrifying bacterial communities in semiarid soils[J]. Applied Soil Ecology, 2008, 40(1): 189-194.
[42] Barth G, von Tucher S, Schmidhalter U. Effectiveness of 3,4-Dimethylpyrazole Phosphate as nitrification inhibitor in soil as influenced by inhibitor concentration, application form, and soil matric potential[J]. Pedosphere, 2008, 18(3): 378-385.
[43] Stark J M, Firestone M K. Mechanisms for soil moisture effects on activity of nitrifying bacteria[J]. Applied and Environmental Microbiology, 1995, 61(1): 218-221.
[44] 王改玲,郝明德,陈德立. 硝化抑制剂和通气调节对土壤N2O排放的影响[J]. 植物营养与肥料学报,2006,12(1):32-36.
Wang Gailing, Hao Mingde, Chen Deli. Effect of nitrification inhibitor and aeration regulation on soil N2O emission[J]. Plant Nutrition and Fertilizer Science, 2006, 12(1): 32-36. (in Chinese with English abstract)
Inhibition effect of inhibitors on nitrogen transformation affected by interaction of soil temperature and water content
Zhou Xuan1,2, Wu Lianghuan1,2※, Dai Feng3
(1.;,310058,; 2.,310058,; 3.,312300,)
Agricultural intensification has led to high inputs of nitrogen fertilizers into cultivated land. Addition of urease and nitrification inhibitor to fertilizers can increase N uptake and reduce N losses, but field studies have shown that their efficiency varies greatly on the environmental conditions. Soil temperature and water content are key factors controlling urea hydrolysis in soils and are also key parameters for the variation in inhibition effect of urease and nitrification inhibitors. The objective of this study was to compare the inhibition effects of biochemical inhibitor combinations on soil nitrogen transformation, and to determine the effects of different soil temperature and water content on the inhibition of urea hydrolysis. We investigated the inhibition effect of combined biochemical inhibitors (N-(n-butyl) thiophosphoric triamide, NBPT/N-(n-propyl) thiophosphoric triamide, NPPT + 2-chloro-6(trichloromethyl) pyridine, CP) on urea-nitrogen transformation as affected by soil water content (60% and 80% water holding capacity, WHC) and soil temperature (15, 25 and 35 ℃) interaction in yellow clayey soil using an 30-day aerobic incubation test. Results showed that soil temperature and water content had significant effects on the inhibition effects of inhibitor combinations in yellow clayey soil (< 0.05), especially soil temperature. With the increase of soil temperature, urea hydrolysis increased by the effective time of the combinations reduced, and the inhibition effect of urease and nitrification weakened accompanied by increase in nitrification. With the decrease of soil water content, urea hydrolysis tended to slow by the effective time of the combinations prolonged, and the inhibition effect of urease and nitrification increased accompanied by nitrification weakened. The urease inhibition rate (Day 1) and nitrification inhibition rate (Day 30) under different soil water content treatments ranked as 60%WHC> 80%WHC, and apparent nitrification rate (Day 30) ranked as 60%WHC< 80%WHC. The urease inhibition rate (Day 3) and nitrification inhibition rate (Day 30) under different soil temperature treatments ranked as 25 ℃>15 ℃>35 ℃, and apparent nitrification rate (Day 30) ranked as 25℃> 35 ℃>15 ℃. The urease inhibition rate (Day 3) under different biochemical inhibitor combinations ranked as U+NBPT> U+NPPT>U+NPPT+CP>U+NBPT+CP>U+CP, and nitrification inhibition rate (Day 30) ranked as U+CP>U+NBPT+CP> U+NPPT+CP>U+NPPT>U+NBPT, and apparent nitrification rate (Day 30) ranked as CK>U>U+NPPT>U+NBPT> U+NPPT+CP>U+NBPT+CP>U+CP. NBPT/NPPT or combined with CP treatments can effectively inhibit soil urease activity, and slow urea hydrolysis. CP or combined with NBPT/NPPT treatments can effectively inhibit the transformation of NH4+-N to NO3--N, and keep high NH4+-N content in soil for longer time under different soil temperature and water content conditions. Application of a new urease inhibitor NPPT alone or with CP had the similar inhibition effect of soil urea hydrolysis with that of NBPT. The optimum condition of soil temperature and water content by the application of biochemical inhibitor combinations in yellow clayey soil were 25℃ and 60%WHC, respectively. In conclusion, urea application combined with both urease inhibitor and nitrification inhibitor should be adopted in yellow clayey soil according to different soil temperature and water content conditions.
soils; soil water content; nitrogen; urease inhibitor; nitrification inhibitor; N-(n-butyl) thiophosphoric triamide (NBPT); N-(n-propyl) thiophosphoric triamide (NPPT); 2-chloro-6(trichloromethyl)pyridine (CP); soil temperature
10.11975/j.issn.1002-6819.2017.20.014
S143.1
A
1002-6819(2017)-20-0106-10
2017-05-14
2017-08-14
苕溪流域农村污染治理技术集成与规模化工程示范(2014ZX07101-012);国家重点基础研究发展计划项目(2015CB150502);浙江省“三农六方”科研协作计划项目;浙江大学—浙江奥复托化工有限公司合作项目
周旋,博士生,主要从事肥料与养分资源管理研究。 Email:zhouxuan_123@126.com
※通信作者:吴良欢,博士,教授,主要从事有机营养与养分综合管理研究。Email:finm@zju.edu.cn
周 旋,吴良欢,戴 锋. 土壤温度和含水量互作对抑制剂抑制氮素转化效果的影响[J]. 农业工程学报,2017,33(20):106-115. doi:10.11975/j.issn.1002-6819.2017.20.014 http://www.tcsae.org
Zhou Xuan, Wu Lianghuan, Dai Feng. Inhibition effect of inhibitors on nitrogen transformation affected by interaction of soil temperature and water content[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(20): 106-115. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2017.20.014 http://www.tcsae.org

