SR 59230A对心衰大鼠心脏MicroRNAs表达的影响*
景佳妮, 李凡璐, 王 茜, 宛 欣, 赵倩倩, 崔香丽
(山西医科大学生理学系, 细胞生理学山西省重点实验室, 太原 030001)
SR59230A对心衰大鼠心脏MicroRNAs表达的影响*
景佳妮, 李凡璐, 王 茜, 宛 欣, 赵倩倩, 崔香丽△
(山西医科大学生理学系, 细胞生理学山西省重点实验室, 太原 030001)
目的观察β3肾上腺素受体(β3-AR)对心衰大鼠心脏MicroRNAs表达的影响及可能的作用机制。方法大鼠冠脉左前降支结扎造成心衰模型,假手术大鼠只穿线不结扎。造模成功大鼠再随机分为:心衰组(CHF control group)和心衰+SR 59230A组(CHF+SR group);假手术大鼠也随机分为假手术组(Sham group)和假手术+SR 59230A组(Sham+SR group)。Sham+SR组和CHF+SR组每天两次腹腔注射SR(85 mmol/L,1 ml),连续注射7周。结果①miScript miRNA PCR Arrays显示,在体阻断β3-AR后,假手术组与心衰组有18种MicroRNAs共同表达下调;经文献比对,与NF-κB相关的MicroRNAs有6种,分别为miR-125b-5p,miR-143-3p,miR-145-5p,miR-26a-5p,miR-30a-5p和miR-320-5p。②大鼠心脏组织切片观察到NF-κB在心衰大鼠心肌细胞核与细胞质中均有分布,而p53在心肌细胞质分布较多,NF-κB和p53表达明显高于假手术组(P<0.05)。阻断β3-AR后,心衰组心脏NF-κB和p53表达显著减少(P<0.05),而假手术组NF-κB和p53表达略增加(P<0.05)。③Western blot结果发现心衰大鼠NF-κB p65表达高于假手术组(P<0.05),给予β3-AR阻断剂后,心衰组心脏NF-κB p65和 p53-Phospho-Serine 15表达均下降(P<0.05),而假手术组心脏阻断β3-AR后,NF-κB、p53和 p53-Phospho-Serine 15 表达均增加(P<0.05)。结论阻断β3肾上腺素受体有利于缓解心衰大鼠心脏的损伤;β3-AR可引起MicroRNAs表达变化且与NF-κB信号通路有关。
大鼠;心脏;β3肾上腺素受体;SR 59230A;MicroRNAs; NF-κB
慢性心力衰竭(chronic heart failure, CHF)是各种因素引起的心血管疾病发展的终末阶段,是导致患者死亡最普遍的原因[1]。心血管疾病发展为慢性心力衰竭的原因有很多,交感神经系统过度激活是慢性心力衰竭发生发展过程中的显著特点[2],参与心肌纤维化,心室重构甚至心肌细胞凋亡等多种病理变化过程。β3肾上腺素受体(β3-adrenaline receptor, β3-AR)在心血管系统中发挥负性肌力作用[3],心衰时,β3-AR在心脏的表达水平可增加2~3倍,并在心力衰竭发生发展中起重要作用。在心衰早期,交感神经兴奋,β1-AR因过度激活而脱敏,此时心肌组织中的β3-AR激活并表达上调,降低心肌收缩力,可防止心肌细胞受损,改善心脏的舒张功能,在心衰末期,β1-AR和β2-AR衰竭下调,β3-AR在高浓度的儿茶酚胺类物质的刺激下不易脱敏,其发挥的负性肌力效应破坏与正性肌力的平衡,使心功能恶化[4]。我们前期的研究发现激动心衰大鼠的β3-AR,大鼠心功能受抑制,心肌细胞收缩幅度,钙瞬变和钙敏感性降低[5]。而采用β3-AR阻断剂SR 59230A可以缓解由心衰引起的血管功能受损,使大鼠胸主动脉MicroRNAs表达变化,其中多种MicroRNAs与NF-κB信号途径有关[6]。在心衰发展过程中,儿茶酚胺类物质的持续刺激,产生心肌炎症反应。在炎症反应过程中,β3-AR的激活包含多种信号通路,免疫炎性反应可能是心力衰竭发展的重要机制之一[7],但目前β3-AR在心脏的机制还不清楚,而且关于其机制的研究报道较少,本课题采用在体给予大鼠特异性β3-AR阻断剂SR 59230A来研究β3-AR对心脏MicroRNAs表达的影响以及可能的机制,为阐明β3-AR的作用提供实验依据。
1 材料与方法
1.1 实验动物
100只清洁级(SPF级)SD雄性大鼠180~230 g,均由中国人民解放军军事医学科学院实验动物中心提供,许可证号:SCXK-(军)2012-0004。实验动物在室温22℃~25℃,相对湿度50%~60%,12 h/12 h明暗交替的环境下分笼饲养,饮食与饮水自由且充足,受试动物在动物观察室内饲养一周后开始实验。
1.2 试剂
SR 59230A(3-(2-Ethylphenoxy)-1-[[(1S)-1,2,3,4-tetrahydronaphth-1-yl]amino]-(2S)-2-propanol oxalate salt),DMSO 均购于美国sigma公司,浓缩型DAB试剂盒,SABC免疫组化染色试剂盒购于上海博士德生物有限公司,miRNeasyMini 试剂盒,miScriptⅡ RT 试剂盒与miScript SYBRGreen PCR 试剂盒购于德国凯杰生物工程有限公司,其他试剂均购自上海生工生物工程有限公司。
1.3 大鼠心衰模型的建立与分组
将100只SD大鼠(180~230 g)随机分为假手术组(40只)和手术组(60只),采用结扎心脏冠脉左前降支的方法[8],术前记录正常心电图,术中实时检测心电图变化,术后四周做超声心动检测,测定左心室舒张末期内径(left ventricular end-diastolic dimension, LVEDD),左心室舒张末期容积(left ventricle end-systolic volume, LVEDV),射血分数(ejection fraction, EF)和左心室短轴缩短率(%FS),建立心衰模型;然后将手术成功的心衰组大鼠随机分两组:CHF control组和CHF+SR 59230A组,假手术组也随机分两组:Sham control组和Sham+SR 59230A组。在相同的条件下,SR组大鼠腹腔注射含SR 59230A 85 mmol/L 的生理盐水,control组大鼠腹腔注射等量的生理盐水,每天2次,连续7周后[9],各组大鼠随机选取6只断头处死,迅速摘取心脏,留取心脏左室组织分别用于免疫组化,Western blot和荧光定量PCR实验。
1.4 HE染色
将固定于福尔马林溶液中的心肌组织进行脱水、透明、包埋,然后经过石蜡切片后,进行苏木素、伊红染色,其后常规脱水、透明、封片,待风干后进行组织形态学观察。
1.5 MicroRNAs array 分析
取各组大鼠心脏左室前壁组织于RNA Later中保存,其后每组分别取100 mg心肌组织充分匀浆,应用miScriptⅡRT 和miRNeasyMini试剂盒提取心肌总RNA,再进行反转录生成cDNA,然后应用miScript SYBR Green PCR试剂盒,将反转录产生的MicroRNAs的特异性cDNA移入96孔板miScript miRNA PCR Arrays,在CFX96 Real-time PCR仪中进行程序反应,条件为95℃预热15 min, 94℃变性15 s, 55℃退火30 s,然后70℃延伸30 s,如此进行40个循环。根据2(ΔΔCt)法计算每个基因各组MicroRNAs相对于Sham control组表达的变化倍数。
1.6 免疫组织化学法
分别取各组大鼠左室前壁穿线下或结扎线下心肌组织,浸入福尔马林溶液中固定,其后进行石蜡包埋,切片厚度5 μm,再脱蜡,抗原修复,封闭,孵育单克隆小鼠抗兔NF-κB p65一抗(购自美国Cell Signaling公司, 1∶800)和p53一抗(购自武汉三鹰生物公司,1∶1 000),SABC二抗试剂盒以及DAB显色剂显色后,镜下观察NF-κB p65和p53的分布以及表达。按人工定性和半定量评分方法判定,选取镜下所有视野心肌细胞着色范围和着色强度分别进行评分,着色范围,估算阳性细胞百分比:0(0%);1(0~10%);2(10%~50%);3(51%~80%);4(>80%)。着色强度,阳性细胞的着色程度:0(阴性);1(弱阳性);2(中等阳性);3(强阳性),将两者乘积作为评分分数(IRS)评分结果范围在0 到 12之间[10]。
1.7Westernblot检测NF-κB,p53和p53-Phospho-Serine15表达
取各组大鼠心肌组织约300 mg用Rap裂解液充分裂解,使用考马斯亮蓝试剂盒(Bio-Rad Protein assay)操作检测蛋白浓度,其后于100℃的金属浴煮沸使蛋白变性,进行SDS-PAGE(十二烷基硫酸钠-聚丙烯酰胺)凝胶电泳,电泳后将蛋白转到PVDF膜上,用5%脱脂牛奶封闭,再分别孵育一抗抗β-actin(购自武汉三鹰生物公司,1∶1 000),抗NF-κB p65,抗p53和抗p53-Phospho-Serine-15抗体,4℃过夜,次日用TBS-T液洗膜3次,室温孵育山羊抗兔二抗(全式金生物公司,1∶2 000)1 h后置于ChemiDocTM MP Imaging 系统显影成像。
1.8 统计学处理
2 结果
2.1 超声心动检测确定造模成功
超声心动测定假手术组(Sham)和心衰组(CHF)大鼠LVIDS及LVIDD、EF, 并计算FS值, 结果显示CHF组大鼠心功能较Sham组明显下降。而与Sham组比较, CHF组LVIDS及LVIDD明显增大(P<0.05),LVEF及FS则明显降低(P<0.01, 图1,表1)。
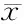

GroupLVEDD(mm)LVEDV(mm)EF(%)FS(%)Sham5.19±0.242.94±0.0580.00±3.2743.00±3.27CHF6.51±0.71*5.04±0.69*56.80±7.95**25.80±4.66**
Sham: Sham operation group; CHF: Chronic heart failure group; LVEDD: Left ventricular end-diastolic dimension; LVEDV: Left ventricle end-systolic volume; EF: Ejection fraction; FS: Fractional shortening
*P<0.05,**P<0.01vssham group
2.2 SR 59230A对大鼠心肌形态学的影响
HE染色观察各组心肌组织病理学改变,在假手术组,心肌组织完好,未见损伤,心肌细胞排列整齐,核染色清晰;而在心衰组,心肌组织大面积皱缩,心肌纤维肿胀,断裂,间质水肿,此外,心肌细胞排列紊乱,松散,经SR 59230A在体处理后,心衰组大鼠心肌细胞排列紊乱减轻,心肌纤维断裂减轻,炎性细胞减少(图2,见彩图页Ⅱ)。

Fig.1Echocardiograms of rat hearts after 4 weeks of surgery
CHF: Chronic heart failure
2.3SR59230A对大鼠心脏MicroRNAs表达的影响
本实验结果显示大鼠在体给予SR 59230A后,Sham control组与CHF control组有18种共同表达下调的MicroRNAs(表2),即这18种MicroRNAs与β3受体的功能有关。其中miR-125b-5p(P<0.01,Sham+SR υs Sham),miR-143-3p(P<0.01,Sham+SR υs Sham;P<0.05, CHF+SR υs CHF),miR-145-5p(P<0.01,Sham+SR υs Sham;P<0.05,CHF+SR υs CHF),miR-26a-5p(P<0.05,Sham+SR υs Sham),miR-30a-5p(P<0.01,Sham+SR υs Sham)和miR-320-5p(P<0.05,CHF+SR υs CHF)与NF-κB信号通路有关。

Tab. 2 miRNAs related to β3-AR
β3-AR: β3 adrenaline receptor; NF-κB: Nuclear factor-kappa B; CHF: Chronic heart failure
*P<0.05,**P<0.01vssham group;#P<0.05vsCHF group
2.4SR59230A对大鼠心脏NF-κBp65和p53表达的影响
2.4.1 免疫组化 镜下可见心衰组大鼠心脏NF-κB p65在胞核与胞质均有表达,在假手术组未见明显表达;大鼠心脏p53在胞质表达多于胞核。免疫组化评分结果显示,CHF control组NF-κB表达较Sham control组增加(P<0.05),在体给予SR 59230A后,Sham control组NF-κB p65,p53表达增加(P<0.05),而CHF control组NF-κB p65,p53表达下降(P<0.05,P<0.01,图3、图4均见彩图页Ⅲ)。
2.4.2 Western blot 手术组NF-κB p65表达明显高于假手术组(P<0.05),p53 和 p53-Phospho-Serine 15 蛋白表达在手术组高于假手术组,但结果没有统计学差异。在体给予SR 59230A后,手术组大鼠心脏NF-κB p65和p53-Phospho-Serine 15蛋白表达下降(P<0.05,P<0.01),但仍高于假手术组(P<0.05),而p53蛋白在两组比较中没有显著性差异,假手术组大鼠在体给予SR 59230A后,NF-κB p65,p53和p53-Phospho-Serine 15蛋白表达均显著增加(P<0.05,图5)。

Fig.5Expressions of NF-κB p65,p53 and p53-Phospho-Serine 15 by Western blot in left ventricle(n=6)
A: Western blot of NF-κB p65,p53 and p53-Phospho-Serine 15; B: Expression level of NF-κB p65; C: Expression level of p53; D: Expression level of p53-Phospho-Serine
*P<0.05vssham group;#P<0.05,##P<0.01vsCHF group
3 讨论
本实验通过对大鼠心脏MicroRNAs表达的观察以及炎症相关蛋白的检测,证明在体阻断β3-AR,有助于缓解心衰大鼠的心肌损伤,其机制与NF-κB信号通路有关。β3的基因最早发现于人类脂肪组织,其后发现β3-AR在机体其它组织中均有分布,且β3-AR激活在心脏中发挥负性肌力作用[3]。心衰时,β3-AR在心脏表达上调,这种上调对心脏到底是代偿性保护作用还是损伤作用还有争议,而阻断β3-AR有利于缓解心衰大鼠的心功能下降[9]。因此本实验通过在体给予β3-AR阻断剂研究其在心脏的作用及其可能机制。心衰时交感神经系统持续激活产生的儿茶酚胺类物质以及炎性分子的作用会促进心脏在疾病状态下氧化应激产物的增加[11],而炎症是心血管疾病发病的主要因素[12]。NF-κB是一种介导细胞凋亡的多效型的转录因子,在心脏缺血再灌注损伤与心衰等病理过程中可调控促炎性细胞因子的释放,引起心肌肥大,诱导心肌凋亡、纤维化、引起心室重构,阻碍心脏行使正常的收缩功能,促进心力衰竭的发生发展。有报道显示NF-κB在p53介导的细胞死亡中有重要作用,抑制NF-κB的活性将减少p53介导的细胞凋亡[13]。但β3-AR在衰竭心脏中的作用与NF-κB和p53的关系并不清楚。
MicroRNAs作为生物进程中重要的调节因子,参与心血管疾病的过程[14]。研究发现评估与心衰有关的MicroRNAs表达,检测胎儿期基因重组可以为心衰治疗提供线索[15]。在应答β肾上腺素系统调节中,伴随有miR-133b 和miR-92下调以及miR-100和miR-195上调[16]。心肌损伤后多种MicroRNAs的表达发生改变,包括miR-1-3p、miR-133b-3p、miR-208a-3p、miR-499-5p、miR-21-5p、miR-423-5p和miR-320-3p等。本实验在阻断β3-AR的前提下对大鼠心脏与炎症有关的84种MicroRNAs进行筛查,结果发现在体阻断β3-AR后,在假手术组与心衰组有18种MicroRNAs共同表达下调,其中miR-125b-5p[17],miR-143-3p[18],miR-145a-5p[19],miR-26a-5p[20],miR-30a-5p[21]and miR-320-5p都与NF-κB信号通路有关,提示β3-AR对心脏MicroRNA的调节作用与NF-κB信号通路有相关性。
通过免疫组织化学法检测NF-κB p65和p53在各组心肌组织的分布,发现心衰时NF-κB p65在心肌细胞核与胞质均有表达,而p53在胞质表达多于胞核;在体阻断β3-AR后,心衰组NF-κB p65和p53表达下降,而假手术组两种蛋白表达增加(图3,4)。Western blot结果发现心衰组大鼠NF-κB p65,p53和p53-Phospho-Serine 15蛋白表达均增加,而在体给予SR 59230A后,心衰组大鼠3种蛋白均有下调的趋势,假手术组3种蛋白表达上调(图5),提示在体阻断β3-AR缓解心衰大鼠心肌损伤与NF-κB p65和p53表达变化有关,可能是通过NF-κB途径实现的。
综上所述,本研究证明在体阻断β3-AR,可影响心脏MicroRNAs的表达,有利于缓解心衰的发展和心肌损伤,其作用可能与NF-κB信号通路有关。本研究为β3-AR在心衰中的作用机制提供实验依据。
[1] Lange P. Chronic heart failure [J].AustFamPhysician, 2011, 40(6): 362.
[2] Rengo G, Pagano G, Vitale DF,etal. Impact of aging on cardiac sympathetic innervation measured by 123I-mIBG imaging in patients with systolic heart failure[J].EurJNuclMedMolImaging, 2016, 43(13): 2392-2400.
[3] Balligand JL. Beta3-adrenoreceptors in cardiovasular diseases: new roles for an “old" receptor[J].CurrDrugDeliv, 2013, 10(1): 64-66.
[4] Moniotte S, Kobzik L, Feron O,etal. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium[J].Circulation, 2001, 103(12): 1649-1655.
[5] 杨 岚, 李海清, 李晓鹏, 等. β3肾上腺素能受体通过降低钙敏感性介导正常和心衰大鼠心脏的负性肌力作用[J]. 中国心血管病研究, 2015, 13(7): 671-672.
[6] 赵倩倩, 景佳妮, 李海清, 等. β3-AR阻断剂 SR 59230A对大鼠胸主动脉张力及microRNA表达的影响[J]. 中国应用生理学杂志, 2017, 33(1): 6-10.
[7] Mamamtavrishvili ND, Kvirkveliia AA, Abashidze RI,etal. Role of immune inflammatory activity in chronic heart failure progress[J].GeorgianMedNews, 2008(160-161): 30-34.
[8] Gao E, Lei YH, Shang X,etal. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse [J].CircRes, 2010, 107(12): 1445-1453.
[9] Gan RT, Li WM, Xiu CH,etal. Chronic blocking of beta 3-adrenoceptor ameliorates cardiac function in rat model of heart failure [J].ChinMedJ(Engl), 2007, 120(24): 2250-2255.
[10]Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue[J].Pathologe, 1987, 8(3): 138-140.
[11]Rubattu S, Mennuni S, Testa M,etal. Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress[J].IntJMolSci, 2013, 14(11): 23011-23032.
[12]Maier HJ, Schips TG, Wietelmann A,etal. Cardiomyocyte-specific IkappaB kinase (IKK)/NF-kappaB activation induces reversible inflammatory cardiomyopathy and heart failure [J].ProcNatlAcadSciUSA, 2012, 109(29): 11794-11799.
[13]Ryan KM, Ernst MK, Rice NR,etal. Role of NF-kappaB in p53-mediated programmed cell death [J].Nature, 2000, 404(6780): 892-897.
[14]De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases [J].CircJ, 2014, 78(3): 567-575.
[15]Dirkx E, Gladka MM, Philippen LE,etal. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure[J].NatCellBiol, 2013, 15(11): 1282-1293.
[16]Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates [J].JMolCellCardiol, 2008, 45(2): 185-192.
[17]Eigsti RL, Sudan B, Wilson ME,etal. Regulation of activation-associated microRNA accumulation rates during monocyte-to-macrophage differentiation [J].JBiolChem, 2014, 289(41): 28433-28447.
[18]Liu Q, Du GQ, Zhu ZT,etal. Identification of apoptosis-related microRNAs and their target genes in myocardial infarction post-transplantation with skeletal myoblasts [J].JTranslMed, 2015, 13: 270.
[19]Dong G, Fan H, Yang Y,etal. 17beta-Estradiol enhances the activation of IFN-alpha signaling in B cells by down-regulating the expression of let-7e-5p, miR-98-5p and miR-145a-5p that target IKKepsilon [J].BiochimBiophysActa, 2015, 1852(8): 1585-1598.
[20]Rasheed Z, Al-Shobaili HA, Rasheed N,etal. MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthaseviaactivation of NF-kappaB pathway in human osteoarthritis chondrocytes [J].ArchBiochemBiophys, 2016, 594: 61-67.
[21]Marques FZ, Vizi D, Khammy O,etal. The transcardiac gradient of cardio-microRNAs in the failing heart [J].EurJHeartFail, 2016, 18(8): 1000-1008.
SR59230AontheexpressionofMicroRNAsinmyocardiumofheartfailurerats
JING Jia-ni, LI Fan-lu, WANG Xi, WAN Xin, ZHAO Qian-qian, CUI Xiang-li△
(Department of Physiology, Cell Physiology Key laboratory of Shanxi Province, Shanxi Medical University,Taiyuan, Shanxi 030001, China)
Objective: To investigate the effects of β3-adrenoceptors(β3-AR) inhibitor SR 59230A on MicroRNAs expression in rat myocardium with chronic heart failure and the related mechanisms.MethodsOne hundred male SD rats were randomly divided into sham operated group(40)and chronic heart failure(CHF)group(60). Coronary artery ligation was used to induce CHF. Then the rats in CHF group were further randomly divided into CHF control group and CHF+SR 59230A group (CHF+SR). Rats in the sham group were divided into sham control group and sham+SR 59230A group (Sham+SR). The rats in Sham+SR group and CHF+SR group were treated with 1 ml SR 59230A(85 mmoL/L in 0.9% saline)twice a day for seven weeks by intraperitoneal injection, while the rats in control groups were injected with the same amount of saline for seven weeks separately. miScript miRNA PCR Arrays were used to determine the expression profile of MicroRNAs. Immunohistochemistry was used to evaluate the distribution of the related proteins in the heart tissue sections. Western blot was used to detect the expressions of nuclear factor-kappaB(NF-κB),p53 and p53-Phospho-Serine 15 in the heart.Results①Afterinvivoblockade of β3-AR by SR 59230A, there were 18MicroRNAs down-regulated in sham control group and CHF control group. Within them, 6 MicroRNAs were related to NF-κB signaling pathway, they were miR-125b-5p,miR-143-3p,miR-145-5p,miR-26a-5p,miR-30a-5p and miR-320-5p. ②Slides from the heart tissue showed that NF-κB was distributed both in nucleus and cytoplasm, while p53 in cytoplasm was more than that in nucleus in heart tissue sections. The expressions of NF-κB and p53 were higher in the CHF control group than those in the sham control group(P<0.05), but were lower in CHF+SR group than those in CHF control group(P<0.05),while they were elevated in Sham+SR group compared to the sham control group(P<0.05). ③ Compared with the sham control group, the protein expression of NF-κB p65 was increased significantly in the CHF control group (P<0.05). After treated with SR59230Ainvivo,the protein expressions of NF-κB and p53-Phospho-Serine 15 were decreased significantly in CHF rats(P<0.05),while the protein expressions of NF-κB, p53 and p53-Phospho-Serine 15 proteins were increased in the sham rats (P<0.05).ConclusionBlocking of β3-AR improved the damaged heart in CHF rats; β3-AR caused the change of MicroRNAs expression, and it related to NF-κB signal pathway.
rats; hearts; β3-adrenoceptors; SR 59230A; MicroRNAs; NF-κB
R3
A
1000-6834(2017)05-456-05
10.12047/j.cjap.5561.2017.109
△
Tel: 0351-4135329; E-mail: cuixlcxl@sina.com

