体素内不相干运动扩散加权成像定量参数评估胰腺癌的分化程度
马婉玲 MA Wanling
魏梦绮 WEI Mengqi
任 静 REN Jing
潘 奇 PAN Qi
文娣娣 WEN Didi
宦 怡 HUAN Yi
体素内不相干运动扩散加权成像定量参数评估胰腺癌的分化程度
马婉玲 MA Wanling
魏梦绮 WEI Mengqi
任 静 REN Jing
潘 奇 PAN Qi
文娣娣 WEN Didi
宦 怡 HUAN Yi
作者单位
第四军医大学西京医院放射科 陕西西安710032
目的 胰腺癌早期诊断缺乏有效的筛查工具,本研究通过对比不同病理级别胰腺癌的体素内不相干运动扩散加权成像(IVIM-DWI)表现,探讨IVIM-DWI定量参数在胰腺癌病理分级评估中的应用价值。资料与方法 收集病理证实的胰腺癌患者16例(高-中分化10例,低分化6例),采用3.0T MR扫描仪行胰腺多b值DWI。应用IVIM双指数模型分析多b值DWI测量参数,测量并比较高-中分化胰腺癌和低分化胰腺癌的慢表观扩散系数(ADCslow)、快表观扩散系数(ADCfast)和灌注分数(f)。结果 高-中分化胰腺癌的ADCslow值低于低分化胰腺癌的ADCslow值[(0.546±0.041)×10-3mm2/s比(0.677±0.120)×10-3mm2/s,P<0.05]。高 -中分化胰腺癌的 f值高于低分化胰腺癌的f值[(59.3±8.8)%比(41.7±22.4)%,P<0.05]。鉴别高-中分化胰腺癌和低分化胰腺癌时,ADCslow的曲线下面积大于f的曲线下面积(0.850>0.750)。当ADCslow值≤0.599×10-3mm2/s,鉴别高-中分化胰腺癌和低分化胰腺癌的敏感度为100.00%,特异度为83.33%。当f值>44.7%时,鉴别高-中分化胰腺癌和低分化胰腺癌的敏感度为100.00%,特异度为66.67%。结论 IVIM-DWI定量参数ADCslow值和f值能够鉴别高-中分化胰腺癌和低分化胰腺癌,能够术前预测胰腺癌病理分级。鉴别高-中分化胰腺癌和低分化胰腺癌时,ADCslow值和f值具有较高的诊断效能。
胰腺肿瘤;磁共振成像;扩散加权成像;病理学,外科
胰腺癌预后很差,是全球癌症死亡的首要原因之一[1]。手术完全切除肿瘤是唯一可以治愈胰腺癌的方法,但胰腺癌确诊时能进行手术治疗的患者不足10%,而这些患者的5年生存率不足20%[2-3]。大部分胰腺癌诊断时已经侵犯周围结构或发生远处转移,错过了最佳的手术时机,不可切除胰腺癌平均生存时间仅4~6个月[3]。因此,早期诊断和鉴别胰腺癌对于评估肿瘤分期、判断能否手术切除、提高生存率和转归至关重要,对于无症状的早期胰腺癌,目前尚缺乏有效的筛查工具[4]。本研究应用体素内不相干运动(intravoxel incoherent motion,IVIM)扩散加权成像(DWI),双指数模型分析多b值DWI,探讨其定量参数慢表观扩散系数(ADCslow)、快表观扩散系数(ADCfast)和灌注分数(perfusion fraction,f)值在评估胰腺癌分化程度中的应用价值。
1 资料与方法
1.1 研究对象 收集第四军医大学西京医院2014年6月-2016年3月经病理证实并有明确分化程度的胰腺癌患者16例,其中男10例,女6例;年龄20~73岁,平均(51.9±15.3)岁。高-中分化胰腺癌10例,低分化胰腺癌6例。临床主要表现为腹胀、腹痛、消瘦、厌食、消化不良、体重减轻、黄疸等。
1.2 仪器与方法 所有患者检查前一晚口服番泻叶清洁肠道,检查前禁食、禁水4 h。采用GE Discovery MR750 3.0T MR扫描仪,32通道腹部相控阵线圈,进行胰腺常规MRI扫描,包括轴位T1WI、轴位T2WI扫描。轴位T1WI采用LAVA-Flex序列,轴位T2WI采用FSE序列,应用呼吸门控和脂肪抑制技术,扫描范围包含整个胰腺。
多b值DWI序列采用呼吸触发技术,应用EPI序列进行轴位DWI扫描,b值取 0、10、20、40、60、80、100、150、200、400、800、1000、1200、1500、2000、3000、4000 s/mm2。扫描参数:TR 6600 ms,TE 57.5~67.5 ms,层厚4.0 mm,层间距1.0 mm,矩阵128×128,视野 380 mm×304 mm,激励次数 1~8,翻转角90°,扫描时间10~14 min,带宽250 Hz,范围包含整个胰腺。
1.3 图像后处理及感兴趣区(ROI)的选取 将所采集的多b值DWI原始数据导入工作站上(AW 4.6: GE Healthcare,Milwaukee,WI),利用工作站自带的MADC软件,对包含17个b值信息的DWI数据进行处理,得到ADCslow、ADCfast和f的参数图。
参照T2WI和T1WI图像,在显示胰腺癌灶和癌周胰腺组织对比最清晰的b值序列DWI图上,采用手绘法在显示胰腺癌灶最大的连续3个层面(小病灶至少选定显示病灶清晰的连续2个层面),沿距离病灶边缘1 mm处勾画出3个不同的不规则形ROI,相同部位的形状、大小一致的ROI会自动生成匹配在相同层面的ADCslow、ADCfast和f值参数图上(图1)。测量中尽量避开坏死、囊变、出血、血管和扩张的胰胆管区域。由2位腹部影像诊断副主任医师分别记录胰腺癌的测量面积及各参数值,取2位3次测量结果的平均值作为最终结果。
1.4 统计学方法 采用SPSS 17.0软件,高-中分化胰腺癌和低分化胰腺癌的ADCslow、ADCfast、f值比较采用成组资料t检验。应用MedCalc(version 12.3,MedCalc Software, Mariakerke, Belgium)软件对ROC曲线进行分析,评估IVIM-DWI各参数值鉴别高-中分化胰腺癌和低分化胰腺癌的诊断效能。P<0.05表示差异有统计学意义。
2 结果
2.1 不同分化程度胰腺癌的IVIM-DWI各参数值比较高-中分化胰腺癌的ADCslow值明显低于低分化胰腺癌的ADCslow值,而高-中分化胰腺癌的f值明显高于低分化胰腺癌的f值,差异有统计学意义(P<0.05)。高-中分化胰腺癌的ADCfast值较低分化胰腺癌的ADCfast值低,但两者差异无统计学意义(P>0.05)。见表1。
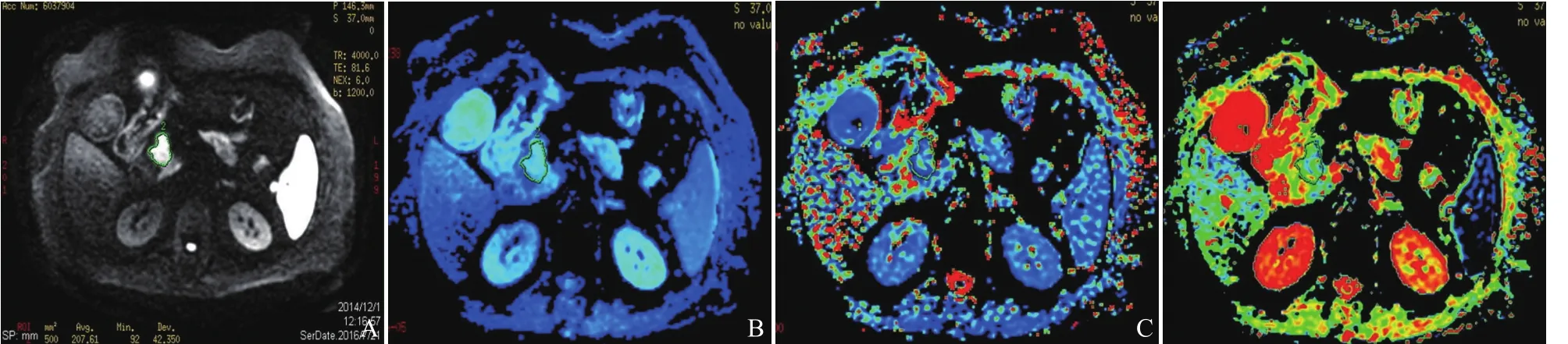
图1 女,71岁,胰头癌。在显示胰头癌灶最清晰的IVIM-DWI参数图上,沿病灶边缘1 mm勾画不规则形ROI,相同形状、大小的ROI自动匹配生成在IVIM-DWI的各参数图胰头癌灶区。DWI图示胰头癌呈不规则形不均匀高信号(b=1200 s/mm2,A);ADCslow参数图(B);ADCfast参数图(C);灌注分数f参数图(D)
2.2 不同分化程度胰腺癌的ROC曲线分析 鉴别高-中分化胰腺癌和低分化胰腺癌时,ADCslow值的曲线下面积(AUC)大于f值(0.850>0.750),具有更高的诊断效能,但两者差异无统计学意义(P>0.05)。当ADCslow值≤0.599×10-3mm2/s、f值>44.7%时,两者鉴别高-中分化胰腺癌和低分化胰腺癌的敏感度均为100.00%,准确度均达85%以上,ADCslow值的特异度为83.33%,而f值的特异度比较低,为66.67%。见图2、3及表2。

图2 ADCslow值鉴别不同分化程度胰腺癌的ROC曲线
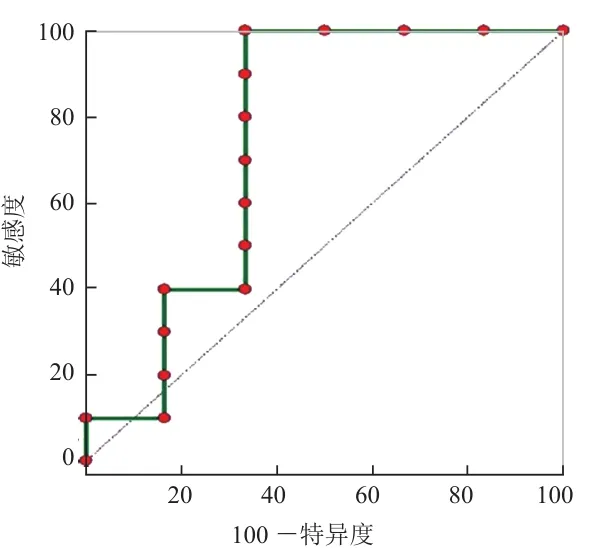
图3 f值鉴别不同分化程度胰腺癌的ROC曲线
3 讨论
胰腺位于腹膜后,位置较深,早期胰腺癌缺乏特异性临床表现,对于无症状的早期胰腺癌,目前尚无有效的筛查工具[4]。增强MRI与多层螺旋CT增强扫描是临床评估胰腺癌最常用的检查方法,已有研究显示,两者在胰腺癌检出和分期方面价值相当[5]。DWI可在分子和细胞水平研究活体组织内水分子扩散的特征,间接反映活体组织的微观细胞结构,所得量化参数ADC值可用来量化水分子的扩散程度[6]。以往采用高斯单指数扩散模型计算的ADC值,同时包含了水分子扩散和微循环灌注两方面的信息,因此ADC值在量化活体组织扩散特征时同时受到微循环灌注的影响。
活体组织内水分子的扩散一般呈非高斯分布,既往研究发现了IVIM现象,应用非高斯IVIM双指数扩散模型分析多b值DWI,可以分离活体组织内水分子的真实扩散和毛细血管微循环灌注,同时提供活体组织扩散和灌注两方面的信息,更准确地反映活体组织的生理病理特征[7-10]。应用更多的大b值进行IVIMDWI,所得ADCslow值能更好地去除微循环灌注效应[11],从而更准确地反映活体组织的真实扩散特征[12-13]。本研究IVIM-DWI采用的17个b值中有9个b值≥200 s/mm2,最高b值达4000 s/mm2,所得ADCslow值基本接近活体组织内水分子的真实扩散情况。
胰腺癌分化程度最重要的形态特征是肿瘤性腺体的形成[14]。高-中分化胰腺癌由丰富的大导管样结构和肿瘤性腺体构成,位于大量纤维结缔组织增生形成的间质中[14-16]。高-中分化胰腺癌中丰富的大导管样结构内含大量黏液,黏液中富含的大分子蛋白限制水分子的扩散,引起ADCslow值减低。高-中分化胰腺癌中大量的纤维结缔组织进一步限制了水分子的扩散[17]。而低分化胰腺癌含有少量小腺体,黏液减少或无黏液产生[14],其内纤维化含量多少不一,对水分子扩散的限制不及高-中分化胰腺癌明显[17]。本研究显示,高-中分化胰腺癌的ADCslow值明显低于低分化胰腺癌的ADCslow值,这可能与不同病理级别胰腺癌的组织学特征不同有关。

表1 不同分化程度胰腺癌IVIM-DWI各参数值比较
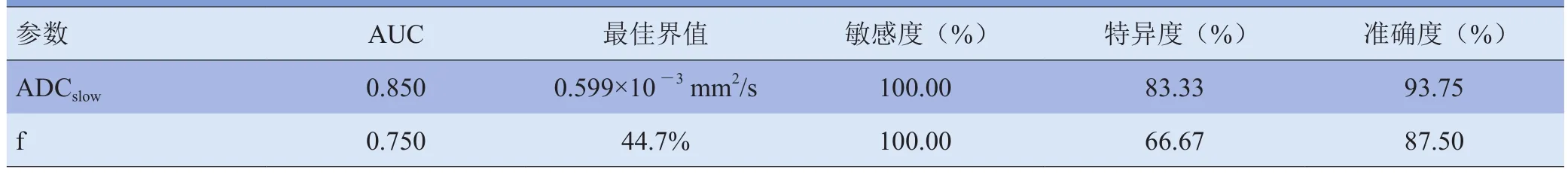
表2 IVIM-DWI各参数值评估不同分化程度胰腺癌的ROC曲线分析
IVIM-DWI采用的低b值(<200 s/mm2)越多,所得灌注参数能越好地反映活体组织的灌注特征[7-11,18]。IVIM-DWI无需注射对比剂即可得到活体组织微循环灌注的信息,尤其适用于肾功能较差或对比剂过敏的患者[9,19]。IVIM-DWI所得f值代表微循环中扩散的部分占组织总体扩散成分的比例,反映了活体组织的血管密集程度[7-10,16]。研究证实IVIM-DWI所得f值与动态增强扫描的相对血容量相关[20]。本研究IVIM-DWI采用了8个低b值(<200 s/mm2),所得f值能更好地反映胰腺癌的灌注特征,结果显示高-中分化胰腺癌的f值明显高于低分化胰腺癌的f值。IVIM-DWI无需注射对比剂,所得f值能够反映胰腺癌的肿瘤血管特征。
总之,IVIM-DWI的ADCslow值和f值能够鉴别高-中分化胰腺癌和低分化胰腺癌,具有较高的诊断效能。IVIM-DWI是无创性早期诊断和鉴别不同分化程度胰腺癌的理想方法之一。
[1] Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin, 2009, 59(4): 225-249.
[2] De Robertis R, Tinazzi Martini P, Demozzi E, et al. Diffusionweighted imaging of pancreatic cancer. World J Radiol, 2015,7(10): 319-328.
[3] Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study.Lancet Oncol, 2007, 8(9): 773-783.
[4] Gemmel C, Eickhoff A, Helmstädter L, et al. Pancreatic cancer screening: state of the art. Expert Rev Gastroenterol Hepatol,2009, 3(1): 89-96.
[5] Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging, 2009, 30(3): 586-595.
[6] Bammer R. Basic principles of diffusion-weighted imaging.Eur J Radiol, 2003, 45(3): 169-184.
[7] Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 1988, 168(2): 497-505.
[8] LeBihan D. IVIM method measures diffusion and perfusion.Diagn Imaging (San Franc), 1990, 12(6): 133-136.
[9] Iima M, Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology,2016, 278(1): 13-32.
[10] Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. Am J Roentgenol, 2011, 196(6): 1351-1361.
[11] Lemke A, Stieltjes B, Schad LR, et al. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging, 2011, 29(6): 766-776.
[12] Muhi A, Ichikawa T, Motosugi U, et al. High-b-value diffusionweighted MR imaging of hepatocellular lesions: estimation of grade of malignancy of hepatocellular carcinoma. J Magn Reson Imaging, 2009, 30(5): 1005-1011.
[13] Anderson SW, Barry B, Soto J, et al. Characterizing nongaussian, high b-value diffusion in liver fibrosis: stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging, 2014, 39(4): 827-834.
[14] Klöppel G, Lingenthal G, Von Bülow M, et al. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology, 1985, 9(8): 841-856.
[15] Jaster R, Emmrich J. Crucial role of fibrogenesis in pancreatic diseases. Best Pract Res Clin Gastroenterol, 2008, 22(1): 17-29.
[16] Erkan M, Hausmann S, Michalski CW, et al. How fibrosis influences imaging and surgical decisions in pancreatic cancer.Front Physiol, 2012, 3: 389.
[17] Wang Y, Chen ZE, Nikolaidis P, et al. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas:association with histopathology and tumor grade. J Magn Reson Imaging, 2011, 33(1): 136-142.
[18] Klauss M, Lemke A, Grünberg K, et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol,2011, 46(1): 57-63.
[19] Lee HJ, Rha SY, Chung YE, et al. Tumor perfusion-related parameter of diffusion-weighted magnetic resonance imaging:correlation with histological microvessel density. Magn Reson Med, 2014, 71(4): 1554-1558.
[20] Wirestam R, Borg M, Brockstedt S, et al. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol, 2001,42(2): 123-128.
(本文编辑 周立波)
Intra voxel Incoherent Motion Diffusion-weighted Imaging Quantitative Parameters in Evaluating Differentiated Degrees of Pancreatic Cancer
Purpose To explore the application of intra voxel incoherent motion diffusionweighted imaging (IVIM-DWI) quantitative parameters in evaluating the pathological stage of pancreatic cancer by comparing the manifestations of IVIM-DWI in patients with pancreatic cancer in different differentiaed degrees as there lacked effective screening instrument for the early diagnosis of pancreatic cancer. Materials and Methods Sixteen patients with pathologically proved pancreatic cancer (10 with high-moderation differentiation while 6 with low differentiation) were enrolled, and 3.0T MRI was used to conduct pancreatic DWI with multiple b values. IVIM double-exponential model was used to analyze the measurement parameters of DWI with multiple b values, so as to measure the slow apparent diffusion coefficient (ADCslow), fast apparent diffusion coefficient (ADCfast)and filling fraction (f). Results The ADCslowvalue was evidently lower in patients with highmoderate differentiated pancreatic cancer than those with low differentiated pancreatic cancer[(0.546±0.041)×10-3mm2/s vs. (0.677±0.120)×10-3mm2/s, P<0.05], and f value was notably higher in patients with high-moderate differentiated pancreatic cancer than those with low differentiated pancreatic cancer [(59.3±8.8)% vs. (41.7±22.4)%, P<0.05]. The area under the curve of ADCslowwas higher than that of f when distinguishing highmoderate differentiated and low differentiated pancreatic cancer (0.850>0.750). The sensitivity and specificity were 100.00% and 83.33% when ADCslow≤ 0.599×10-3mm2/s,and were 100.00% and 66.67% when f>44.7% in distinguishing high-moderate differentiated and low differentiated pancreatic cancer, respectively. Conclusion ADCslowand f, as the quantitative parameters for IVIM-DWI, can distinguish high-moderate differentiated and low differentiated pancreatic cancer, and predict the pathological stage of pancreatic cancer before operation. Moreover, they also have high diagnostic efficacy in distinguishing highmoderate differentiated and low differentiated pancreatic cancer.
Pancreatic neoplasms; Magnetic resonance imaging; Diffusion-weighted imaging; Pathology, surgical
10.3969/j.issn.1005-5185.2017.09.006
马婉玲
Department of Radiology, Xijing Hospital,Fourth Military Medical University, Xi'an 710032, China
Address Correspondence to: MA Wanling E-mail: marynee@163.com
国家自然科学基金重大国际(地区)合作与交流项目(81220108011);国家自然科学基金青年面上连续项目(81370039)。
R445.2;R735.9
2017-04-20
修回日期: 2017-05-08
中国医学影像学杂志
2017年 第25卷 第9期:658-661
Chinese Journal of Medical Imaging 2017 Volume 25 (9): 658-661

