磁共振管壁成像评价老年人胸主动脉粥样硬化斑块的特征
周长武
赵锡海2 ZHAO Xihai
李 澄3 LI Cheng
乔会昱2 QIAO Huiyu
何 乐2 HE Le
苑 纯2,4YUAN Chun
磁共振管壁成像评价老年人胸主动脉粥样硬化斑块的特征
周长武1ZHOU Changwu
赵锡海2ZHAO Xihai
李 澄3LI Cheng
乔会昱2QIAO Huiyu
何 乐2HE Le
苑 纯2,4YUAN Chun
目的胸主动脉粥样硬化斑块是老年人缺血性卒中栓子的重要来源。本研究拟应用三维磁共振管壁成像技术评价老年人胸主动脉粥样硬化易损斑块的特征,从而积极预防心脑血管并发症的发生。资料与方法前瞻性选取60岁以上且无任何严重心脑血管症状的老年人53例,分为60~74岁组(A组)和75~89岁组(B组)。全部受试者均接受磁共振胸主动脉管壁多对比度序列扫描。将胸主动脉分为升主动脉段、主动脉弓段和降主动脉段3个节段,评价其粥样硬化斑块的特征。定量测量老年人群胸主动脉粥样硬化斑块的负荷特征,并定性分析其成分特征。结果老年人胸主动脉粥样硬化斑块内出血的发生率为26.4%(14/53),脂质核的发生率为94.3%(50/53)。同时,B组升主动脉段、主动脉弓段和降主动脉段3个节段的最大管壁厚度均显著大于 A 组 [(3.1±0.6)mm 比(3.0±0.4)mm,P<0.05;(3.2±0.7)mm比(3.1±0.7)mm,P<0.05;(3.0±0.8)mm 比(2.9±0.7)mm,P<0.001];B 组 3 个节段的标准化管壁指数均显著大于A组[(26.9±3.5)%比(26.7±2.9)%,P<0.001;(31.9±5.1)% 比(31.0±5.1)%,P<0.001;(34.6±5.0)% 比(34.1±4.6)%,P<0.001]。结论老年人胸主动脉粥样硬化斑块内出血的发生率较高,且斑块负荷随年龄增长而逐渐增大。因此,应用磁共振管壁成像早期识别老年人胸主动脉的易损斑块,将有助于脑卒中的预防及治疗。
动脉粥样硬化;主动脉,胸;磁共振成像;磁共振血管造影术;主动脉造影术;老年人
动脉粥样硬化(atherosclerosis,AS)是随着年龄增长而出现的血管疾病,中老年时期加重、发病,近年来成为老年人死亡的主要原因之一[1]。随着我国进入老龄化社会,高龄已成为除高血压、糖尿病、高脂血症、吸烟等以外的另一项引起AS性疾病的重要危险因素[1-3]。60岁以上的老年人AS的发生率超过80%[4]。
AS易损斑块破裂、脱落是缺血性卒中栓子的主要来源,其中约1/5来源于胸主动脉[5-6]。既往研究显示,主动脉弓AS不稳定斑块好发于缺血性脑卒中患者[7]。另有研究发现,缺血性卒中患者中,胸主动脉AS易损斑块的发生率为34.4%[8]。
早期学者采用经食管超声心动图和二维磁共振管壁成像研究胸主动脉AS斑块的特征,取得了初步成果,但其自身的缺陷,限制了其发展和应用[9-10]。因此,本研究应用三维磁共振管壁成像技术评价老年人胸主动脉AS易损斑块的特征,从而积极预防心脑血管并发症的发生。
1 资料与方法
1.1 研究对象 选取2013年5月-2014年12月于清华大学生物医学影像研究中心进行胸主动脉磁共振管壁成像的受试者53例。纳入标准: 60岁以上无任何严重心脑血管症状的老年人。排除标准:①有MRI检查禁忌证者,如体内含金属磁性异物等;②不同意签署知情同意书者;③幽闭恐惧症患者。根据WHO对老年人的划分,将受试者分为A、B两组,即60~74岁组(29例)和75~89岁组(24例)。两组在性别、高密度脂蛋白、三酰甘油、踝-肱指数、吸烟、糖尿病、高血压病、高脂血症、冠心病史及脑卒中史等方面比较,差异均无统计学意义(P>0.05)。见表1。本研究通过伦理委员会审核,且所有受试者知情同意。
1.2 仪器与方法 采用Philips 3.0T MR扫描仪(Achieva TX,The Netherlands)和32通道心脏线圈,行三维冠状位扫描,仰卧位,头先进,以胸主动脉为中心。扫描参数:①3D SNAP序列:快速场回波(FFE),TR 7.5 ms,TE 3.7 ms,空间分辨率1.5 mm×1.5 mm×1.5 mm,视野(FOV) 220 mm×280 mm×37 mm;②3D T2-VISTA序列:快速自旋回波,FOV 250 mm×160 mm×64 mm,TR 800 ms,TE 64 ms,空间分辨率1.25 mm×1.25 mm×1.25 mm。
1.3 图像分析 由2名具有5年以上心脑血管MR图像判读经验的放射诊断医师(1名主治医师,1名主任医师)采用盲法阅片。当判读结果有争议时,协商解决。采用清华大学自主研发的3D CASCADE图像后处理软件进行图像分析。

表1 两组受试者基本资料比较
本研究分析胸主动脉AS斑块的成分主要包括斑块内出血(intraplaque hemorrhage,IPH)和富含脂质的坏死核(lipid rich necrotic core,LRNC),IPH/MT表现为SNAP序列上的高信号,LRNC表现为T2-VISTA序列上的低信号,而钙化表现为更低信号。将胸主动脉分为3个节段,即升主动脉段(ascending aorta,AAO):自左心室出口至主动脉弓近端;主动脉弓段(aortic arch,AOA)以及降主动脉段(descending aorta,DAO):自主动脉弓远端至膈肌水平。胸主动脉各段AS斑块负荷的形态学参数主要包括管腔面积(lumen area,LA)、血管总面积(total vessel area,TVA)、管壁面积(wall area,WA)、最大管壁厚度(maximum wall thickness,Max WT)以及标准化管壁指数(normalized wall index,NWI)等,其中 NWI=WA/[LA+WA]×100%。
1.4 一致性检验 由2名图像判读医师对所有受试者胸主动脉AS斑块的负荷和成分特征进行测量和分析,并比较2次结果的一致性。
1.5 统计学方法 采用SPSS 16.0软件。胸主动脉各段AS斑块负荷参数比较采用独立样本t检验。不同年龄组胸主动脉AS斑块成分及负荷的差异比较采用χ2检验和Fisher确切概率法。根据胸主动脉斑块负荷的组内相关系数(intraclass correlation coef fi cient,ICC)和相应的95% CI判断2次斑块负荷测量结果的可重复性。同时,2次评价胸主动脉AS斑块成分结果的一致性评价采用Kappa检验。P<0.05表示差异有统计学意义。
2 结果
2.1 胸主动脉AS斑块的成分特征 本研究中胸主动脉AS斑块IPH的发生率为26.4%(14/53),LRNC的发生率为94.3%(50/53)。但两组间IPH(27.6%比25.0%,P>0.05)和 LRNC(80.0%比 82.9%,P>0.05)差异均无统计学意义(P>0.05)。
2.2 胸主动脉AS斑块的负荷特征 本研究中,随着年龄的增长,老年人群胸主动脉各段的LA、TVA以及WA均逐渐增大,且差异均有统计学意义(P<0.05)。两组胸主动脉各段WA比较见图1。
B组胸主动脉3个节段的Max WT均显著大于A组,差异有统计学意义(P<0.05)。见图2。
B组胸主动脉3个节段的NWI[(26.9±3.5)%比(26.7±2.9)%,P<0.001;(31.9±5.1)% 比(31.0±5.1)%,P<0.001;(34.6±5.0)% 比(34.1±4.6)%,P<0.001]均显著大于A组。同时,两组DAO段的NWI均最大,其次为AOA段和AAO段(P<0.05)。见图3。

图3 两组胸主动脉各段NWI的比较
2.3 一致性评价 采用磁共振管壁图像测量胸主动脉AS斑块负荷的一致性良好,LA的ICC值分别为AAO段 0.958(95% CI:0.940~0.971)、AOA 段 0.977(95%CI:0.972~0.981)和 DAO 段 0.984(95% CI:0.982~0.987)(P<0.05)。同时,胸主动脉AS斑块成分的一致性亦良好,AAO段(Kappa=0.848)、AOA段(Kappa=0.921)和DAO段(Kappa=0.96)(P<0.05);尤其是IPH,胸主动脉各段的结果均为Kappa=1.000(P<0.05)。老年人胸主动脉AS易损斑块的MR图像,见图4、5。
3 讨论
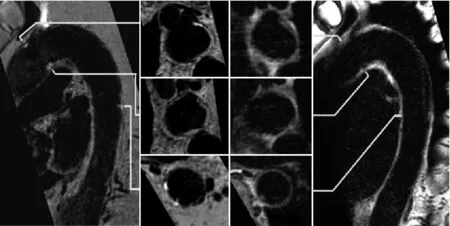
图4 男,69岁,主动脉弓段AS易损斑块的MR图像。SNAP序列图像(A矢状位;B横轴位),T2-VISTA序列图像(C横轴位;D矢状位);图像显示主动脉弓段多发AS斑块(箭),其中SNAP序列图像中箭示高信号代表IPH/MT,提示该斑块为易损斑块

图5 男,79岁,降主动脉AS斑块的MR图像。SNAP序列图像(A矢状位;B横轴位),T2-VISTA序列图像(C横轴位;D矢状位);图像显示降主动脉多发AS斑块(箭),其中SNAP序列图像中箭示高信号为IPH/MT,提示该斑块为易损斑块
本研究应用三维磁共振管壁成像技术评价老年人胸主动脉AS斑块的成分及负荷等特征。结果发现,老年人胸主AS斑块IPH的发生率较高,并且随着年龄的增长,胸主动脉AS斑块的负荷亦逐渐增大,与既往研究一致,也符合AS斑块形成的病理生理机制[11-12]。IPH是AS易损斑块的一项重要指标[13]。本研究中,约1/4的老年人存在胸主动脉IPH。Bitar等[14]应用MRI研究发现,13%的脑血管病患者胸主动脉存在IPH,其中10.9%发生于上部胸主动脉。既往研究表明,颈动脉的IPH能够加速AS病变纤维帽的破裂,并且能够抵消他汀类药物治疗AS斑块的效果[15]。发生于颅内动脉和颈动脉的IPH与脑卒中事件有显著相关性[16-17]。另外,Wehrum等[18]研究发现,在降主动脉近端反流性血流机制普遍存在,尤其多见于缺血性卒中患者[19-20]。因此,胸主动脉AS斑块的IPH也可以预测缺血性卒中的风险。
早期部分学者应用经食管超声心动图评价胸主动脉AS斑块的特征,结果发现仅能定性分析斑块的分布(好发于主动脉弓和降主动脉),不能定量测量斑块的负荷以及鉴别斑块的成分,尤其是IPH[21]。本研究中,3D T2-VISTA和3D SNAP 序列不仅能定量测量斑块的负荷,还能准确判断IPH等成分。既往研究表明,3D VISTA序列在测量胸主动脉斑块负荷方面具有良好的可重复性和可操作性[11]。同时,3D T2-VISTA序列能获得充分的血流抑制[22]。因此,对于斑块内LRNC的识别,T2-VISTA序列具有较高的敏感性,呈稍低信号(相对于钙化的更低信号和纤维帽的稍高信号)。Wang等[23]报道3D SNAP序列可同时获得黑血管壁图像和MRA图像,因此能够重点突出斑块内出血的正信号,对IPH的显示非常敏感。同时,颈部斑块病理已经证实3D SNAP 图像上的高信号代表IPH[21],并且提示为易损斑块[23]。因此,三维磁共振管壁成像技术在鉴别斑块的成分方面(尤其是IPH、LRNC)具有无限的潜力和广阔的临床应用前景。
本研究的局限性:①研究样本量较小,而且为60岁以上的老年人,不足以代表所有人群;②有5例受试者(A组3例,B组2例)因呼吸运动伪影较重无法测量斑块负荷,其升主动脉段的图像未纳入统计分析;③由于不能准确地鉴别纤维帽,本研究未对纤维帽的完整性进行评价。针对上述不足,在未来的研究中应增大样本量、运用技术手段提高图像质量、增加T1加权[24-25]、T2-mapping[26]等特异性成像序列,更加全面地分析易损斑块的特征。
本研究发现,老年人胸主动脉易损斑块的发生率较高,且斑块的负荷随着年龄的增长而逐渐增大。因此,在后续研究中,将同时对颅内动脉、颈动脉及胸主动脉进行磁共振管壁成像,以期能够更加直观地发现脑卒中栓子的来源,从而积极预防脑卒中的发生。
[1] Booth GL, Kapral MK, Fung K, et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet, 2006, 368(9529): 29-36.
[2] O'donnell MJ, Chin SL, Rangarajan SA, et al. Global and regional effects of potentially modi fi able risk factors associated with acute stroke in 32 countries (INTERSTROKE): a casecontrol study. Lancet, 2016, 388(146): 761-775.
[3] Wang D, Wang C, Zhou Y, et al. Different blood pressure indexes on intracranial arterial stenosis in asymptomatic polyvascular abnormalities in community study in China. J Hypertens, 2015, 33(7): 1452-1457.
[4] Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol, 2015, 12(5):267-277.
[5] Mendel T, Popow J, Hier DB, et al. Advanced atherosclerosis of the aortic arch is uncommon in ischemic stroke: an autopsy study. Neurol Res, 2002, 24(5): 491-494.
[6] Russo C, Jin Z, Rundek T, et al. Atherosclerotic disease of the proximal aorta and the risk of vascular events in a population-based cohort: the aortic plaques and risk of ischemic stroke(APRIS) study. Stroke, 2009, 40(7): 2313-2318.
[7] Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med, 1992, 326(4): 221-225.
[8] Cui X, Wu S, Zeng Q, et al. Detecting atheromatous plaques in the aortic arch or supra-aortic arteries for more accurate stroke subtype classi fi cation. Int J Neurosci, 2015, 125(2): 123-129.
[9] Fayad ZA, Nahar T, Fallon JT, et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography.Circulation, 2000, 101(21): 2503-2509.
[10] Ito A, Sugioka K, Matsumura Y, et al. Rapid and accurate assessment of aortic arch atherosclerosis using simultaneous multi-plane imaging by transesophageal echocardiography.Ultrasound Med Biol, 2013, 39(8): 1337-1342.
[11] Eikendal AL, Blomberg BA, Haaring C, et al. 3D black blood VISTA vessel wall cardiovascular magnetic resonance of the thoracic aorta wall in young, healthy adults: reproducibility and implications for efficacy trial sample sizes: a crosssectional study. J Cardiovasc Magn Reson, 2016, 18(1): 20.
[12] Papapanagiotou A, Siasos G, Kassi E, et al. Novel in fl ammatory markers in hyperlipidemia: clinical implications. Curr Med Chem, 2015, 22(23): 2727-2743.
[13] Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation, 2001, 104(17):2051-2056.
[14] Bitar R, Moody AR, Leung G, et al. In vivo identi fi cation of complicated upper thoracic aorta and arch vessel plaque by MR direct thrombus imaging in patients investigated for cerebrovascular disease. Am J Roentgenol, 2006, 187(1): 228-234.
[15] Underhill HR, Yuan C, Yarnykh VL, et al. Arterial remodeling in [corrected] subclinical carotid artery disease. JACC Cardiovasc Imaging, 2009, 2(12): 1381-1389.
[16] Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results.Stroke, 2006, 37(3): 818-823.
[17] Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance.Ann Neurol, 2012, 71(2): 195-198.
[18] Wehrum T, Kams M, Strecker C, et al. Prevalence of potential retrograde embolization pathways in the proximal descending aorta in stroke patients and controls. Cerebrovasc Dis, 2014,38(6): 410-417.
[19] Harloff A, Strecker C, Frydrychowicz AP, et al. Plaques in the descending aorta: a new risk factor for stroke? Visualization of potential embolization pathways by 4D MRI. J Magn Reson Imaging, 2007, 26(6): 1651-1655.
[20] Harloff A, Strecker C, Dudler P, et al. Retrograde embolism from the descending aorta: visualization by multidirectional 3D velocity mapping in cryptogenic stroke. Stroke, 2009, 40(4):1505-1508.
[21] Gupta V, Nanda NC, Yesilbursa D, et al. Racial differences in thoracic aorta atherosclerosis among ischemic stroke patients.Stroke, 2003, 34(2): 408-412.
[22] Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0Tesla. J Magn Reson Imaging, 2011,34(1): 22-30.
[23] Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med,2013, 69(2): 337-345.
[24] Cuvinciuc V, Viallon M, Momjian-Mayor I, et al. 3D fatsaturated T1 SPACE sequence for the diagnosis of cervical artery dissection. Neuroradiology, 2013, 55(5): 595-602.
[25] 吴婷婷, 陈振森, 赵锡海, 等. 在体测量血管壁T1值的双反转恢复多翻转角MRI序列研究. 中国医学影像学杂志,2014, 22(6): 465-469.
[26] Baranovicova E, Mlynarik V, Kantorova E, et al. Quantitative evaluation of cerebral white matter in patients with multiple sclerosis using multicomponent T2 mapping. Neurol Res,2016, 38(5): 389-396.
(本文编辑 张晓舟)
Characteristics of Atherosclerotic Plaque in Thoracic Aorta in the Elderly Evaluated by Magnetic Resonance Vessel Wall Imaging
PurposeThe thoracic aortic atherosclerotic plaque is an important source of ischemic stroke embolism in the elderly. This study aims to explore the characteristics of vulnerable plaque of atherosclerosis in the thoracic aorta in the elderly by using threedimensional multi-contrast magnetic resonance wall imaging technique, so as to actively prevent the occurrence of cardiovascular and cerebrovascular complications.Materials and MethodsFifty-three cases of elderly subjects (≥60 years old) without serious cerebrovascular diseases were recruited in this prospective study. All subjects were divided into A and B groups (60-74 and 75-90 years old). All subjects underwent MRI of multiple contrast sequences of the aortic wall. The thoracic aorta was divided into three segments of ascending aorta, aortic arch and descending aorta, and the characteristics of the atherosclerotic plaque were evaluated. The load characteristics of thoracic aortic atherosclerotic plaques in the elderly were calculated quantitatively, and the compositional characteristics were evaluated qualitatively.ResultsThe incidence of intraplaque hemorrhage in the thoracic aortic atherosclerotic plaque in the elderly was 26.4% (14/53),and the incidence of lipid nucleus was 94.3% (50/53). Meanwhile, the maximum wall thickness of three segments of ascending aorta, aortic arch and descending aorta in group B was signi fi cantly higher than that in group A [(3.1±0.6) mmvs(3.0±0.4) mm,P<0.05;(3.2±0.7) mmvs(3.1±0.7) mm,P<0.05; (3.0±0.8) mmvs(2.9±0.7) mm,P<0.001]; the normalized wall index of the three segments in group B was signi fi cantly higher than that in group A [(26.9±3.5)%vs(26.7±2.9)%,P<0.001; (31.9±5.1)%vs(31.0±5.1)%,P<0.001; (34.6±5.0)%vs(34.1±4.6)%,P<0.001)].ConclusionThe incidence of hemorrhage in the atherosclerotic plaque in the thoracic aorta in the elderly is higher, and the plaque load increases with age. Therefore, early screening of vulnerable plaque in the thoracic aorta in the elderly using magnetic resonance wall imaging will be helpful for prevention and treatment of stroke.
Atherosclerosis; Aorta, thoracic; Magnetic resonance imaging; Magnetic resonance angiography; Aortography; Aged
1. 扬州大学第二临床医学院放射科 江苏扬州225000
2. 清华大学生物医学工程系生物医学影像研究中心 北京 100084
3. 东南大学附属中大医院放射科 江苏南京210009
4. 美国华盛顿大学放射科 美国西雅图WA 98195-5502
赵锡海
Center for Biomedical Imaging Research,Tsinghua University, Beijing 100084, China
Address Correspondence to:ZHAO Xihai
E-mail: xihaizhao@tsinghua.edu.cn
国家自然科学基金(81271536)。
R445.2;R543.1
2016-12-28
修回日期:2017-03-02
中国医学影像学杂志2017年 第25卷 第8期:588-592
Chinese Journal of Medical Imaging 2017 Volume 25 (8): 588-592
10.3969/j.issn.1005-5185.2017.08.008

