硫与Mn2O3空心球的复合结构及其在锂硫电池中的应用
王瑛 弭侃 熊胜林*,
(1山东玉皇新能源科技有限公司,菏泽274000)(2山东大学化学与化工学院,济南250100)
硫与Mn2O3空心球的复合结构及其在锂硫电池中的应用
王瑛1弭侃2熊胜林*,2
(1山东玉皇新能源科技有限公司,菏泽274000)
(2山东大学化学与化工学院,济南250100)
在水热条件下,以碳球为模板合成了Mn2O3空心球,并用作锂硫电池的载硫基底材料。测试结果表明载硫量为51%的Mn2O3-S复合材料显示了较高的比容量,良好的循环稳定性和倍率性能。循环100圈后,最终可逆容量仍保持657 mA·g-1,证明该Mn2O3空心球是一种有潜力的载硫基底材料。
电化学;空心球;三氧化二锰;锂硫电池
0 Introduction
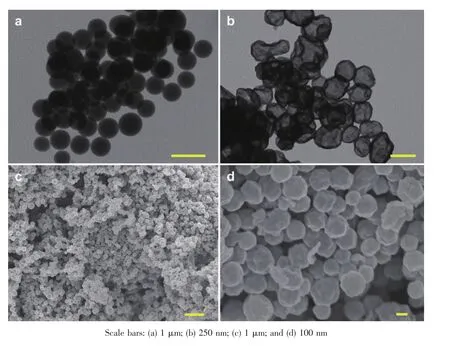
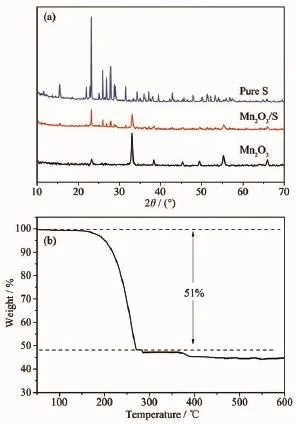
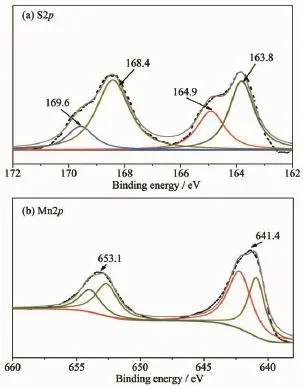
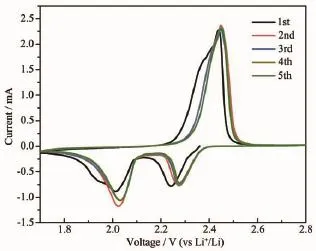
Inrecentyears,Lithium-sulfurbatteriesare receiving increasing attentions from all around the world due to its numerous advantages,including its low cost,nontoxic nature and remarkable theoretical specific capacity and energy density(1 675 mAh·g-1and 2 500 Wh·kg-1).However,the practical application of Li-S batteries is still hindered by many problems.For example,the insulation of sulfur can lower the electrochemical utilization of active material and lead to the poor rate capability.What is more,the dissolution of intermediate discharge products(Li2Sx,3 To overcome these issues of Li-S batteries,a lot of schemes have been carried out to improve the correspondingelectrochemicalperformance.First, carbon materials with different morphology(like hollow carbon spheres[8-10],mesoporous carbon spheres[11-13]or nanotubes[14],and graphene[15-17]and so on)have been designed for the holder of sulfur in consideration ofthe excellent conductivity and the advantageous pore structure which favors suppressing the loss of sulfur and the discharge produces.As a result,the related cycling performance and reversible capacity of composites with sulfur are obviously enhanced.However, the capacity of Li-S batteries is still decayed during the long-term electrochemical cycle,which may be attributed to the nonpolar nature of carbon.Recently, besides pure carbon matrix,the polar metal oxides (like TiO2[18-19],Al2O3[20],V2O5[21],MnO2[22-23]and so on) are reported as holder or absorber for Li-S batteries. Due to that the dissolution of polysufides is effectively buffered by the surficial chemical adsorption of metal oxides,theenhancedcyclingperformancesare obtained.It is no doubt that the cycling stability of cells is largely improved by the usage of those metal oxides. In this paper,Mn2O3hollow spheres were developed with purpose to incorporate sulfur,which were synthesized by using carbon spheres as hard template. When evaluated as cathode in Li-S batteries,Mn2O3-S composites exhibited a reversible capacity of 657 mAh·g-1after 100 cycles at the current density of 900 mA·g-1. 1.1 Synthesis of carbon spheres and Mn2O3hollow spheres Firstly,carbon spheres were synthesized by a modified method[24].Typically,3.83 g glucose and 0.27 g cetyl trimethyl ammonium bromide(CTAB)was dissolved totally in 40 mL of deionized water.Then, the solution was transferred into a 65 mL Teflon-lined autoclave and heated for 9 h at 180℃.The final product was collected by centrifugation with water and ethanol for several times.To produce the Mn2O3hollow spheres,0.15 mol·L-1manganese acetate solution was formed with 40 mL of absolute ethanol as the solvent. After the PH was adjusted to be 5 by hydrochloric acid,0.5 g of carbon spheres was introduced into the abovesolutionandthenfollowedbyultrasonic treatment for about 2 h.The final mixture was centrifuged by water and ethanol before it was aged for 8 h at 50℃in an oven.The powder was dried at 60℃for overnight and finally heated at 500℃for 2.5 h with a heating ratio of 2℃·min-1to attain Mn2O3hollow spheres. 1.2 Preparation of Mn2O3-S composites In a typical synthesis,sulfur and Mn2O3hollow spheres were mixed with the mass ratio of 55∶45.Then, a moderated amount of CS2was added and stirred untilitwasevaporatedcompletely.Finally,the mixture was heated at 155℃for 10 h to get the Mn2O3-S composites. 1.3 Sample characterization TEM and SEM images were obtained from a transmission electron microscope(TEM,JEM-1011, Japan)and a scanning electron microscope(SEM,JSM -7600F,Japan),respectively.X-ray powder diffraction (XRD)pattern was examined on a Bruker D8 advance X-ray diffractometer with Cu Kα as a radiation(λ= 0.154 18 nm,U=40 kV,I=40 mA,2θ=8°~80°).The sulfur content of Mn2O3/S composites was collected from a Mettler Toledo TGA/SDTA851 thermal analyzer with a heating rate of 5℃·min-1under a nitrogen atmosphere.The chemical analysis of the samples was performedusingX-rayphotoelectronspectroscopy (XPS,ESCALAB 250 spectrometer,Perkin-Elmer). 1.4 Electrochemical measurements Typically,75%of Mn2O3-S composites,15%of carbon black,and 10%of polyvinylidene fluoride (PVDF)(mass ratio=75∶15∶10)were mixed in N-methylpyrrolidinone(NMP)to form a slurry and coated on aluminum foil to prepare the cathode.The foil was cut into discs with the diameter of 12 mm and the areal mass of sulfur for each disc is about 1.0~1.2 mg·cm-2.The discs were assembled with a Li metal foil as the counter electrode and the Celgard 2400 as the separator.All cells were assembled in an argonfilled glovebox.The electrolyte used was 1 mol·L-1bis (trifluoromethane)sulfonamide lithium salt(LiTFSI)in a mixed solvent of 1,3-dioxolane(DOL)and 1,2-dimethoxyethane(DME)(the volumetric ratio of 1∶1) with 2%LiNO3to prohibit the shuttle effect.The charge-discharge behaviors were tested in a potential window of 1.7~2.8 V at 30℃by using a LANDCT2001A instrument.The Cyclic voltammograms(CV) were obtained from an LK2005A electrochemical workstation at a scan rate of 0.1 mV·s-1. 2.1 Structure characterization Fig.1a indicates TEM image of carbon spheres, which were synthesized by the hydrothermal method using glucose as precursor.It can clearly seen that the sizes of those carbon spheres were ranging from 500 to 700 nm.The hydrophilic surface of carbon spheresrenderslotsoffunctionalgroups(like hydroxyl)which could adsorb Mn2+from the ethanol solution.After calcination and removal of carbon spheres,Mn2O3hollow spheres were obtained(the corresponding XRD was displayed in Fig.2).TEM image in Fig.1b demonstrates the hollow structure of the as-prepared Mn2O3spheres with the thickness of the shell about 30 nm.As shown in Fig.1c,the panoramic SEM image reveals the large area of uniform spheres with diameters of 300~500 nm.Fig. 1d shows SEM image of Mn2O3-S composites.It is clear that the sphere structure of Mn2O3was still remained even after the sulfur was infiltrated into Mn2O3hollow spheres.More importantly,the massive sulfur aggregates were hardly observed on the surface ofcomposites,whichrevealedthattheuniform dispersion of sulfur in the oxide matrix. The XRD patterns of Mn2O3hollow spheres and Mn2O3-S composites are indicated in Fig.2a.The characteristic diffraction peaks of the product after calcination process in laboratory air matched perfectly with cubic Mn2O3(JCPDS Card No.65-7467).As for theMn2O3-Scomposites,theintensityofsulfur diffraction peaks becomes obviously lower than that of pure elemental sulfur,which suggests the sulfur has been distributated well into Mn2O3hollow spheres and concides with the above FESEM observation.The TGA calculations reveal that the actual mass ratio of sulfur in the Mn2O3-S composites is approximately 51%(Fig.2b). Fig.1(a)TEM image of carbon sphere;(b,c)TEM and SEM images of Mn2O3hollow spheres;(d)SEM image of Mn2O3-S composites Fig.3 showed the XPS spectra of S2p and Mn2p of Mn2O3-S composites.From Fig.3a,the spectrum of S2p was divided into two sets of peaks.The peaks located at 163.8 and 164.9 eV are the typical signals of pure S,while the other two peaks loacted at 168.4and 169.6 eV were characteristic of SOxspecies, which may be caused by Mn2O3covalently bonding with S[23].In addition,the binding energies of Mn2p3/2and Mn2p1/2in Fig.3b are 641.4 and 653.1 eV,which can be attributed to the spin-orbit splitting.Like some literatures reported,the binding energy difference between Mn2p1/2and Mn2p3/2is 11.7 eV,indicating the existence of Mn3+in Mn2O3-S composites[25-26].It is worth noting that the signals of Mn2p peaks displayed an apparent shift to the lower binding energy,which is related with an increased electron cloud density from the adjacent sulfur element[23]. Fig.2(a)XRD patterns of pure sulfur,Mn2O3hollow spheres and Mn2O3-S composites;(b)TGA curve of the Mn2O3-S composites Fig.3XPS spectra of S2p(a)and Mn2p(b)of Mn2O3-S composites 2.2 Electrochemical performance The electrochemical property of the as-fabricated product was first investigated by cyclic voltammetry. Fig.4 indicates the first five consecutive cyclic voltammograms(CVs)of the electrode made from Mn2O3-S composites.Two reduction peaks can be found in the cathodic polarization process of the first cycle.The first reductive peak located at 2.3 V can be assigned to the conversion of element sulfur(S8)to long-chain lithium polysulfides(Li2Sx,5 Fig.4First five consecutive CVs of the electrode made from Mn2O3-S composites at a scan rate of 0.1 mV·s-1 Fig.5aindicatesthetypicaldischarge-charge voltage profiles of the Mn2O3-S composites at a current density of 0.9 A·g-1.There are two typical plateaus located at 2.3 and 2.1 V in the curves,which are in agreement with the above CV observation.Fig.5b shows the cycling performance of Mn2O3-S electrode was measured at the current density of 0.9 A·g-1. When the sulfur mass in the composites is 51%,the Mn2O3-S electrode delivers high first-cycle discharge and charge capacities of 1 003.3 and 989.5 mAh·g-1respectively,corresponding to a Coulombic efficiency (CE)of 101.4%.A discharge capacity of 917.1 mAh· g-1is delivered in the second cycle,followed by a charge capacity of 937.2 mAh·g-1,resulting in a high CE of about 97.9%.Furthermore,the final discharge capacity can remained at 657 mAh·g-1after 100 cycles.Although the reversible capacity is still decaying during the cycling process,which may attributed to some soluble polysulfides escaped from the Mn2O3matrixes and dissolved into the electrolyte,The final retention of reversible capacity can still remain at 65.5%.In addition,the CE can keep approximately 100%during the discharge-charge process,which suggestedthegoodreversibilityoftheMn2O3-S materials.The rate performance was also tested at various rates(Fig.5c).As can be seen,the reversible capacities are 1 061,735,586,464 mAh·g-1at the current density of 0.3,0.9,1.8,3.6 A·g-1,respectively, which reveals that the Mn2O3-S electrode has the good electrochemical performance even at the high rates. When the rate went back to 0.9 and 0.3 A·g-1after 40 cycles,the corresponding capacities can remain at 592 and 659 mAh·g-1,receptively.Furthermore,both the discharge and charge capacities could be stable at different current densities and the CE was almost 100%,indicating a good inhibition of the shuttle effect,which is considered as a primary shortcoming of Li-S batteries. Fig.5Electrochemical performance of Mn2O3-S composites: (a)cycling performance at a current density of 0.9 A·g-1and the corresponding Coulombic efficiency; (b)discharge-charge voltage profiles for the 1st,2nd, 50th,and 100th cycles at a current density of 0.9 A·g-1;(c)rate performance at various current rates In summary,Mn2O3hollow spheres were synthesized by using colloidal carbon spheres as the hard template and aimed at the holder for Li-S battery. When the sulfur loading was as high as 51%,the corresponding reversible capacity can remain at 657 mA·g-1after 100 cycle at the current density of 0.9 A·g-1.The rate performance of these Mn2O3-S composites were also attractive even at high rate.The good electrochemical performance was mainly contributed by the hollow structure of Mn2O3spheres,which can suppress the volume expansion of sulfur and the sulfides during the charge-discharge process.Importantly,the diffusion of polysulfides could be suppressed effectively by the surface chemical bonding ofMn2O3.The present study confirms the Mn2O3hollow spheres are a promising matrix for Li-S batteries. Further work is in progress. [1]Chen L,Shaw L L.J.Power Sources.,2014,267:770-783 [2]ZHOU Lan(周兰),YU Ai-Shui(余爱水).J.Electrochem.(电化学),2015,21(3):211-220 [3]Lin Z,Liang C.J.Mater.Chem.A,2015,3:936-958 [4]Manthiram A,Fu Y,Chung,et al.Chem.Rev.,2014,114: 11751-11787 [5]Pope M A,Aksay I A.Adv.Energy Mater.,2015,5:1500124 [6]DIAO Yan(刁岩),XIE Kai(谢凯),HONG Xiao-Bin(洪晓斌), et al.Acta Chim.Sinica(化学学报),2013,7(4):508-548 [7]WAN Wen-Bao(万文博),PU Wei-Hua(蒲薇华).Prog.Chem. (化学进展),2013,25(11):1830-1841 [8]He G,Evers S,Liang,et al.ACS Nano,2013,7:10920-10930 [9]Jayaprakash N,Shen J,Moganty S S,et al.Angew Chem. Int.Ed.,2011,50:5904-5908 [10]QuY,Zhang Z,Wang,etal.J.Mater.Chem.A,2013,1:14306 [11]Jung D S,Hwang T H,Lee,et al.Nano Lett.,2014,14:4418 -4425 [12]Wang M,Zhang H,Wang,et al.ACS Appl.Mater.Interfaces, 2015,7:3590-3599 [13]XU Jing-Jing(徐晶晶),LI Bin(李斌),LI Song-Hai(李松梅), et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(10): 2030-2036 [14]Mi K,Jiang Y,Feng J,et al.Adv.Funct.Mater.,2016,26: 1571-1579 [15]Ahn W,Lee D U,Song,et al.RSC Adv.,2015,5:29370-29374 [16]Duan X,Han Y,Huang L W,et al.J.Mater.Chem.A,2015, 3:8015-8021 [17]MAO Yan(毛艳),ZHANG Chuan-Hui(张传辉),ZHANG Yang(张杨),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(5):889-895 [18]Li J,Ding B,Xu G,et al.Nanoscale,2013,5:5743-5746 [19]Wei S Z,Li W Y,Cha J J,et al.Nat.Commun.,2013,4:1331 [20]Choi Y J,Jung B S,Lee,et al.B.Phys.Scr.,2007,T129: 62-65 [21]Li W,Hicks-Garner J,Wang J,et al.Chem.Mater.,2014, 26:3403-3410 [22]Liang X,Hart C,Pang Q,et al.Nat.Commun.,2015,6:5682 [23]Wang X,Li G,Li J,et al.Energy Environ.Sci.,2016,9: 2533-2538 [24]Sun X,Li Y.Angew.Chem.Int.Ed.,2004,43:597-601 [25]Bongu C S,Karuppiah S,Nallathamby K,et al.J.Mater. Chem.A,2015,3:23981-23989 [26]Kolathodi M S,Rao S N H,Natarajan T S,et al.J.Mater. Chem.A,2016,4:7883-7891 Immobilizing Sulfur in Mn2O3Hollow Spheres for Lithium-Sulfur Batteries WANG Ying1MI Kan2XIONG Sheng-Lin*,2 Uniform Mn2O3hollow spheres were synthesized by using carbon spheres as the hard template and were used as holder for Li-S batteries.The Mn2O3-S composites showed a high specific capacity,good cycling stability and rate performance.The final reversible capacity can remain at 657 mA·g-1after 100 cycles at the current density of 0.9 A·g-1with the 51%sulfur loading,which demonstrates that Mn2O3hollow spheres could be a promising matrix for Li-S batteries. electrochemistry;hollow spheres;manganic oxide;Li-S batteries O614.71+1;O613.51 A 1001-4861(2017)02-0243-06 10.11862/CJIC.2017.048 2016-09-01。收修改稿日期:2016-11-17。 山东省自然科学杰出青年基金(No.JQ201304)资助项目。* 。E-mail:chexsl@sdu.edu.cn1 Experimental
2 Results and discussion

3 Conclusions
(1Shandong Yuhuang New Energy Technology Co.,LTD,Heze,Shandong 274000,China)
(2School of Chemistry and Chemical Engineering,Shandong University,Jinan 250100,China)

