沙格列汀对高糖环境下成骨细胞增殖、分化和凋亡的影响
郭丽艳,王金兰,谭音玲,陈福莲,董振华
(1 潍坊医学院附属益都中心医院,山东潍坊262500;2 山东大学附属济南市中心医院)
沙格列汀对高糖环境下成骨细胞增殖、分化和凋亡的影响
郭丽艳1,王金兰1,谭音玲1,陈福莲1,董振华2
(1 潍坊医学院附属益都中心医院,山东潍坊262500;2 山东大学附属济南市中心医院)
目的 观察沙格列汀对高糖环境下成骨细胞增殖、分化和凋亡的影响,为糖尿病性骨质疏松患者选择合理的降糖药物提供理论依据。方法 取成骨细胞MC3T3-E1置于a-MEM培养基中常规培养、传代。取传3代的对数生长期细胞,随机分为正常对照组、高糖组、高糖联合沙格列汀组,即刻更换a-MEM培养基,正常对照组更换为葡萄糖浓度5.5 mmol/L的培养液,高糖组更换为葡萄糖浓度30.0 mmol/L的培养液,高糖联合沙格列汀组更换为葡萄糖浓度30.0 mmol/L+沙格列汀1 μmol/L的培养液,继续培养48 h。收集各组细胞,分别采用CCK-8法和流式细胞术检测细胞增殖能力和细胞凋亡率,ELISA法检测碱性磷酸酶(ALP)活性,qRT-PCR法检测成骨细胞分化相关基因Ⅰ型胶原(COL-Ⅰ)、成骨特异性转录因子2(Runx2)、骨钙蛋白(OCN)、骨桥蛋白(OPN)mRNA表达。结果 与正常对照组比较,高糖组、高糖联合沙格列汀组细胞增殖能力、ALP活性及成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA相对表达量均明显降低,细胞凋亡率均明显升高(P均<0.05);与高糖组比较,高糖联合沙格列汀组细胞增殖能力、ALP活性和成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA相对表达量均明显升高,细胞凋亡率均明显降低(P均<0.05)。结论 沙格列汀能在一定程度上逆转高糖环境对小鼠成骨细胞增殖、分化和凋亡的影响,为糖尿病性骨质疏松患者的降糖治疗提供了理论依据。
糖尿病性骨质疏松症;沙格列汀;成骨细胞;细胞增殖;细胞分化;细胞凋亡
糖尿病性骨质疏松是糖尿病在骨骼系统的重要并发症之一,患者骨组织微结构改变,骨强度降低、脆性增加,骨折发生风险明显增加[1]。有研究显示,20%~60%糖尿病患者可并发糖尿病性骨质疏松[2,3]。沙格列汀属于二肽基肽酶Ⅳ(DPP-4)抑制剂类降糖药,通过选择性抑制DPP-4调节机体血糖。Monami等[4]研究发现,沙格列汀可增加糖尿病性骨质疏松患者的骨密度,可降低骨折的发生风险。但目前沙格列汀对糖尿病患者骨代谢影响的具体作用机制尚不完全清楚。2016年5~10月,我们观察了沙格列汀对高糖环境下小鼠成骨细胞MC3T3-E1增殖、分化及凋亡的影响,旨在为糖尿病性骨质疏松患者选择合理的降糖药提供依据。
1 材料与方法
1.1 材料 小鼠前体成骨细胞株MC3T3-E1,购自中国科学院上海生命科学研究院细胞资源中心。沙格列汀,英国阿斯利康公司。PCR引物由广州市锐博生物科技有限公司设计合成。CCK-8试剂盒,购自江苏碧云天生物技术研究所;Anexin V-FITC/PI双染试剂盒,购自南京凯基生物科技发展有限公司;TRIzol试剂,购自美国Invitrogen公司;RNA逆转录试剂盒、qRT-PCR试剂盒,购自大连宝生物工程有限公司;碱性磷酸酶(ALP)检测试剂盒,购自上海高创医疗科技有限公司。LightCycler®96实时荧光定量PCR仪,瑞士罗氏公司。SP-Max 2300A2型多功能酶标仪,上海闪谱生物科技有限公司;FACSCalibur型流式细胞仪,美国BD公司;HERAcell 150i CO2细胞培养箱,美国Thermo公司。
1.2 细胞培养及分组处理 将成骨细胞MC3T3-E1接种于96孔板(含10% FBS、1%青-链霉素的a-MEM基础培养基),37 ℃、5% CO2、饱和湿度的细胞培养箱中培养,每3天换液1次,按1∶2进行传代。取传3代对数生长期细胞,随机分为正常对照组、高糖组、高糖联合沙格列汀组,每组设3个复孔;即刻更换a-MEM基础培养基,正常对照组更换为葡萄糖浓度5.5 mmol/L的培养液,高糖组更换为葡萄糖浓度30.0 mmol/L的培养液,高糖联合沙格列汀组更换为葡萄糖浓度30.0 mmol/L+沙格列汀1 μmol/L的培养液,继续培养48 h。
1.3 相关指标观察
1.3.1 细胞增殖能力 采用CCK-8法。取各组处理48 h的成骨细胞MC3T3-E1,接种于96孔板,每孔100 μL(约含1×104个细胞),避光条件下每孔加入CCK-8试剂10 μL,37 ℃、5% CO2细胞培养箱中避光孵育2 h,酶标仪450 nm波长处检测每孔的吸光度(A)值。以A450值代表细胞增殖能力。
1.3.2 细胞凋亡率 采用流式细胞术。取各组处理48 h的成骨细胞MC3T3-E1,制成密度为1×106个/L的细胞悬液,分别取细胞悬液100 μL置于5 mL流式管中,依次加入5 μL Annexin V-FITC和5 μL PI,避光反应15 min。上机检测时加入400 μL结合缓冲液,流式细胞仪检测各组细胞凋亡情况,采用Flow-JO软件分析细胞凋亡率。细胞凋亡率=早期凋亡率+晚期凋亡率。
1.3.3 细胞ALP活性 采用ELISA法。取各组处理48 h的成骨细胞MC3T3-E1,PBS冲洗3次,用0.2%的Triton x-100裂解液处理,按ALP检测试剂盒说明加入新鲜配置的底物,37 ℃孵育30 min,0.1 mol/L的NaOH终止反应。酶标仪410 nm波长处检测每孔的A值。以A410值表示细胞ALP活性。
1.3.4 成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA表达 采用qRT-PCR法。取各组处理48 h的成骨细胞MC3T3-E1,TRIzol法提取细胞总RNA,紫外分光光度计检测总RNA的浓度和纯度合格后,根据RNA逆转录试剂盒说明将总RNA逆转录为cDNA,按qRT-PCR试剂盒说明进行PCR扩增。引物序列:COL-Ⅰ上游引物:5′-CAGGCTGGTGTGATGGGATT-3′,下游引物: 5′-AAACCTCTCTCGCCTCTTGC-3′;Runx2上游引物:5′-GTGGCCTTCAAGGTTGTAG-3′,下游引物:5′-GGGTAAG-ACTGGTCATAGG-3′;OCN上游引物: 5′-TCTGACAAAGCCTTCATGTCC-3′,下游引物:5′-AAATAGTGAACCGAGATGCG-3′;OPN上游引物: 5′-GTGGCG-GTYATGACTTCAGC-3′,下游引物: 5′-TCACGAACCACGTTAGCATC-3′;β-actin上游引物: 5′-TATGCT-CTCCCTCACGCCA-3′,下游引物: 5′-TTTACGGATGTCAACGTCACAC-3′。PCR反应体系共25 μL:SYBR® Premix Ex TaqTMⅡ(2×)12.5 μL,cDNA 2 μL,上下游引物各1 μL,无酶水8.5 μL;反应条件:95 ℃ 3 min,95 ℃ 10 s、59 ℃ 30 s共40个循环。每个样本设3次复孔。以β-actin为内参,2-ΔΔCt法计算目的基因mRNA相对表达量。
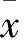
2 结果
2.1 各组细胞增殖能力、细胞凋亡率及ALP活性比较 见表1。
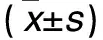
表1 各组细胞增殖能力、细胞凋亡率及ALP活性比较
注:与正常对照组比较,*P<0.05;与高糖组比较,#P<0.05。
2.2 各组成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA表达比较 见表2。
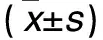
表2 各组成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA相对表达量比较
注:与正常对照组比较,*P<0.05;与高糖组比较,#P<0.05。
3 讨论
糖尿病性骨质疏松是糖尿病的慢性并发症之一,可导致骨折的发生风险明显增加,是糖尿病致残的重要原因之一[5,6]。有研究发现,应用噻唑烷二酮类降糖药者骨折的发生率最高,其次是胰岛素,磺脲类降糖药最低[7,8],提示各类降糖药均可不同程度影响糖尿病患者的骨代谢[9]。已有研究证实,胰岛素可通过提高成骨细胞数量及骨钙素水平,促进矿物质沉积,减轻高血糖对成骨细胞的抑制[10,11]。二甲双胍能够使成骨细胞ALP活性增加,可促进成骨细胞分化和骨矿化[12,13],而噻唑烷二酮类降糖药可激活PPAR-γ,继而促进骨髓间充质干细胞向脂肪细胞转化,抑制成骨细胞形成,导致机体骨量减少[14,15]。但至今有关DDP-4抑制剂类降糖药对2型糖尿病患者骨代谢影响的研究国内外均较少。沙格列汀是一种DDP-4抑制剂类新型降糖药,可通过选择性抑制DPP-4来调节糖代谢,单用或与磺酰脲类、二甲双胍、胰岛素等合用均能有效控制患者血糖水平[16~19]。近期有研究发现,沙格列汀可明显增加糖尿病性骨质疏松患者骨密度,继而有可能减少骨折的发生率[4]。陈珺等[20]研究发现,沙格列汀能使糖尿病性骨质疏松大鼠骨量增加,骨小梁数目增多。但目前其相关作用机制尚未阐明。
成骨细胞是骨形成的主要功能细胞,负责骨基质的合成、分泌,参与骨矿化,在骨重建过程中起关键作用[21,22]。当成骨细胞功能下降或数量减少时,就会出现骨吸收大于骨形成,导致机体骨量降低,从而引起骨质疏松[23,24]。成骨细胞分化是骨形成的重要阶段,成骨细胞分化主要包括细胞增殖、细胞外基质成熟和矿化[25]。ALP和Runx2是成骨细胞分化早期的重要指标,其中ALP活性是成骨细胞分化的功能性指标[26]。ALP在体内钙化过程中具有关键作用,其活性可反映成骨细胞的成熟程度[27]。ALP活性越高,表明成骨细胞的分化程度越高[28,29]。Runx2主要在骨原细胞和多向分化的间质细胞中表达,是成骨细胞分化和骨形成过程中必需的转录因子[30]。OPN是磷酸化、糖基化的细胞外基质蛋白编码基因,是骨形成和骨重建的标志物之一[31]。COL-Ⅰ和OCN是成骨细胞分化晚期的标志物之一,在骨矿化过程中具有重要作用[32,33]。MC3T3-E1细胞是成骨细胞的前体细胞,具有成骨细胞的表型和分化特征,是体外研究成骨细胞模型的常用细胞[34,35]。本研究观察了MC3T3-E1细胞的增殖能力,结果发现,高糖组和高糖联合沙格列汀组细胞增殖能力较正常对照组明显降低,提示高糖环境能抑制MC3T3-E1细胞增殖;高糖联合沙格列汀组细胞增殖能力较高糖组明显升高,提示沙格列汀可一定程度上反转高糖环境对MC3T3-E1细胞增殖的抑制作用。本研究还发现,高糖组和高糖联合沙格列汀组细胞凋亡率较正常对照组明显升高,提示高糖环境能够明显促进MC3T3-E1细胞凋亡;且高糖联合沙格列汀组细胞凋亡率较高糖组明显降低,提示沙格列汀可一定程度上反转高糖环境对MC3T3-E1细胞凋亡的促进作用。进一步研究发现,与正常对照组比较,高糖组和高糖联合沙格列汀组ALP活性下降,成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA相对表达量明显降低,提示高糖环境能抑制MC3T3-E1细胞分化;与高糖组比较,高糖联合沙格列汀组MC3T3-E1细胞ALP活性明显上升,成骨细胞分化相关基因COL-Ⅰ、Runx2、OCN、OPN mRNA相对表达量明显升高,提示沙格列汀可一定程度上反转高糖环境对MC3T3-E1细胞分化的抑制作用。
综上所述,沙格列汀能在一定程度上反转高糖环境对小鼠成骨细胞MC3T3-E1增殖、分化和凋亡的影响。本研究为糖尿病性骨质疏松患者选择合理的降糖药提供了理论依据。
[1] Hamann C, Kirschner S, Günther KP, et al. Bone,sweet bone-osteoporotic fractures in diabetes mellitus[J].Nat Rev Endocrinol,2012,8(5):297-305.
[2] Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants[J]. Lancet, 2011,378(9785):31-40.
[3] Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults[J]. J Bone Miner Res, 2005,20(4):596-603.
[4] Monami M, Dicembrini I, Antenore A, et al. Dipeptidyl peptidase-4 inhibitors and bone fractures: a meta-analysis of randomized clinical trials[J]. Diabetes Care, 2011,34(11):2474-2476.
[5] Sta Romana M, Li-Yu JT. Investigation of the relationship between type 2 diabetes and osteoporosis using Bayesian inference[J]. J Clin Densitom, 2007,10(4):386-390.
[6] Abdulameer SA, Sulaiman SA, Hassali MA, et al. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do[J]. Patient Prefer Adherence, 2012(6):435-448.
[7] Hayakawa N, Suzuki A. Diabetes mellitus and osteoporosis. effect of antidiabetic medicine on osteoporotic fracture[J]. Clin Calcium, 2012,22(9):1383-1390.
[8] Zinman B, Haffner SM, Herman WH,et al. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes[J]. J Clin Endocrinol Metab, 2010,95(1):134-142.
[9] Hayakawa N, Suzuk A. Diabetes mellitus and osteoporosis. Effect of anti-diabetic medicine on osteoporotic fracture[J]. Clin Calcium, 2012,22(9):1383-1390.
[10] Angela M,Inzerillo E,Solomon E. Osteoporosis and diabetes mellitus[J]. Endocr Metab Dis,2004,5(2):261-268.
[11] Gopalakrishnan V, Vignesh RC, Arunakaran J, et al. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages[J]. Biochem Cell Biol, 2006,84(1):93-101.
[12] Wu W, Ye Z, Zhou Y, et al. AICAR, a small chemicalmolecule,primes osteogenic differentiation of adult mesenchymal stem cells[J]. Int J Artif Organs, 2011,34(12):1128-1136.
[13] Zhen D, Chen Y, Tang X. Metformin reverses the deleterious effects of high glucose on osteoblast function[J]. J Diabetes Complicat, 2010,24(5):334-344.
[14] Beck GR Jr, Khazai NB, Bouloux GF, et al. The effects of thiazolidinediones on human bone marrow stomal cell differentiation in vitro an in thiazolidinedione-treated patients with type 2 diabetes[J]. Transl Res, 2013,161(3):145-155.
[15] Grey A. Thiazolidinedione-induced skeletal fragility-mechanisms and implications[J]. Diabetes Obes Metab, 2009,11(4):275-284.
[16] Jain R. Utility of saxagliptin in the treatment of type 2 diabetes: review of efficacy and safety[J]. Adv Ther, 2015,32(11):1065-1084.
[17] Derosa G, Maffioli P. Patient considerations and clinical utility of a fixed dose combination of saxagliptin/metformin in the treatment of type 2 diabetes[J]. Diabetes Metab Syndr Obes, 2011(4):263-271.
[18] Li FF, Jiang LL, Yan RN, et al. Effects of saxagliptin add-on therapy to insulin on blood glycemic fluctuations in patients with type 2 diabetes: a randomized, control, open-labeled trial[J]. Medicine (Baltimore), 2016,95(43):e5229.
[19] Seong JM, Choi NK, Shin JY, et al. Differential cardiovascular outcomes after dipeptidyl peptidase-4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population-based cohort study[J]. PLoS One, 2015,10(5):e0124287.
[20] 陈珺,杨力,麦静娥,等.沙格列汀和艾塞那肽对糖尿病性骨质疏松大鼠松质骨的影响[J].中国药学杂志,2016,51(12):976-980.
[21] Cicuéndez M, Silva VS, Hortigüela MJ, et al. MC3T3-E1 pre-osteoblast response and differentiation after graphene oxide nanosheet uptake[J]. Colloids Surf B Biointerfaces, 2017(158):33-40.
[22] Tang X, Lin J, Wang G, et al. MicroRNA-433-3p promotes osteoblast differentiation through targeting DKK1 expression[J]. PLoS One, 2017,12(6):e0179860.
[23] Sanghani-Kerai A, Coathup M, Samazideh S, et al. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4[J]. Bone Joint Res, 2017,6(6):358-365.
[24] Xu D, Gao Y, Hu N, et al. miR-365 ameliorates dexamethasone-induced suppression of osteogenesis in MC3T3-E1 cells by targeting HDAC4[J]. Int J Mol Sci, 2017,18(5).pii:E977.
[25] Jin X, Sun J, Yu B, et al. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like MG-63 cells via estrogen receptor-dependent MEK/ERK and PI3K/Akt activation[J]. Nutr Res, 2017(42):20-30.
[26] Umeda H, Mano T, Harada K, et al. Appearance of cell-adhesion factor in osteoblast proliferation and differentiation of apatite coating titanium by blast coating method[J]. J Mater Sci Mater Med, 2017,28(8):112.
[27] Ling M, Huang P, Islam S, et al. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase[J]. Cell Biosci, 2017(7):27.
[28] 李军,靳爽,张娟,等.利拉鲁肽在高糖环境下对体外培养的小鼠前成骨细胞MC3T3-E1增殖、分化的影响[J].中华内分泌代谢杂志,2016,32(3):232-235.
[29] 刘斌,张琼.6-姜酚对MC3T3-E1成骨细胞增殖及分化的影响[J].中国生化药物杂志,2016,36(7):25-27.
[30] Yang J, Wang S, Wang F, et al. Downregulation of miR-10b promotes osteoblast differentiation through targeting Bcl6[J]. Int J Mol Med, 2017,39(6):1605-1612.
[31] Yin J, Zhuang G, Zhu Y, et al. MiR-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1[J]. Cell Biol Int, 2017,41(7):779-786.
[32] Li W, Ling W, Teng X, et al. Effect of advanced glycation end products, extracellular matrix metalloproteinase inducer and matrix metalloproteinases on type-I collagen metabolism[J]. Biomed Rep, 2016,4(6):691-693.
[33] Liu C, Jiang D. High glucose-induced LIF suppresses osteoblast differentiation via regulating STAT3/SOCS3 signaling[J]. Cytokine, 2017(91):132-139.
[34] Li JY, Liu SG, Xiao GN, et al. Fibroblast growth factor receptor 1 propagates estrogen and fluid shear stress driven proliferation and differentiation response in MC3T3-E1 cells[J]. Mol Biol (Mosk), 2017,51(2):342-355.
[35] Gapski R, Martinez EF. Behavior of MC3T3-E1 osteoblastic cells cultured on titanium and zirconia surfaces: an in vitro study[J]. Implant Dent, 2017,26(3):373-377.
Effects of saxagliptin on proliferation, differentiation, and apoptosis of osteoblasts in high glucose environment
GUOLiyan1,WANGJinlan,TANYinling,CHENFulian,DONGZhenhua
(1YiduCentralHospitalAffiliatedtoWeifangMedicalCollege,Weifang262500,China)
Objective To investigate the effects of saxagliptin on the proliferation, differentiation, and apoptosis of osteoblasts in high glucose environment, so as to provide the theoretical basis for the selection of rational hypoglycemic agents for patients with diabetic osteoporosis. Methods Osteoblasts MC3T3-E1 were cultured and passaged in a-MEM medium. The cells of 3 generations in the logarithmic phase were randomly divided into the normal control group, high glucose group, and high glucose + saxagliptin group. The a-MEM medium was immediately replaced with 5.5 mmol/L glucose medium in the normal control group, the a-MEM medium was immediately replaced with 30.0 mmol/L glucose medium in the high glucose group, and the a-MEM medium was immediately replaced with 30.0 mmol/L glucose +1 μmol/L saxagliptin medium in the high glucose + saxagliptin group. MC3T3-E1 osteoblasts were then cultured in different culture mediums for 48 h. CCK-8 method and flow cytometry were used to detect the proliferation ability and apoptosis rate, ELISA assay was used to detect alkaline phosphatase (ALP) activity, and qRT-PCR was used to detect the mRNA expression of COL-Ⅰ, Runx2, OCN and OPN. Results Compared with the normal control group, the proliferation, ALP activity and expression of COL-ⅠmRNA, Runx2 mRNA, OCN mRNA, and OPN mRNA were significantly decreased, and the apoptosis rate was significantly increased in the high glucose group and high glucose + saxagliptin group (allP<0.05). Compared with the high glucose group, the proliferation, ALP activity and expression of COL-ⅠmRNA, Runx2 mRNA, OCN mRNA, and OPN mRNA were significantly increased, and the apoptosis rate was significantly decreased in the high glucose + saxagliptin group (allP<0.05). Conclusion Saxagliptin can reverse the effects of high glucose environment on proliferation, differentiation, and apoptosis of MC3T3-E1 osteoblasts to a certain extent, which can be used for hypoglycemic therapy for patients with diabetic osteoporosis.
diabetic osteoporosis; saxagliptin; osteoblasts; cell proliferation; cell differentiation; apoptosis
国家自然科学基金资助项目(81400788)。
郭丽艳(1980-),女,大学本科,主要研究方向为内分泌系统疾病的诊治。E-mail: guoliyan2000@yeah.net
董振华(1984-),女,博士,主要研究方向为2型糖尿病骨骼肌微循环胰岛素抵抗的作用及其机制。E-mail: dongzhenhua_jn@yeah.net
10.3969/j.issn.1002- 266X.2017.28.005
R681
A
1002- 266X(2017)28- 0016- 04
2017- 02-12)

