miRNA在结直肠癌中的研究现状
王小昆,张玉成,吴晓冬
(吉林大学中日联谊医院 科学研究中心,吉林 长春130033)
*通讯作者
miRNA在结直肠癌中的研究现状
王小昆,张玉成,吴晓冬*
(吉林大学中日联谊医院 科学研究中心,吉林 长春130033)
MicroRNA(微小RNA,miRNA)是一类大小约19-25nt的短链非编码RNA,人类已发现1000多种miRNA,参与调节了人60%以上的人类基因[1]。MiRNA由RNA polymeraseⅡ转录成较长的初级产物pri-miRNA,然后在Drosha和DGCR8酶的作用下,剪切为约70nt并具有独特发卡结构的pre-miRNA,后者在胞质中Dicer酶的作用下被剪切为双链互补的RNA,双链解开,释放成熟的单链miRNA。成熟miRNA整合到miRISC(miRNA诱导沉默复合体)中发挥生物学作用[2]。miRNA发挥作用需要与靶基因的3’端非编码区(3’UTR)结合,完全匹配的结合可使靶基因mRNA降解,而不完全匹配的结合则使靶基因mRNA的翻译受到抑制[3]。但Lytle J R等[4]研究表明,miRNA不仅可以与靶基因3’UTR 结合,也可与靶基因5’UTR甚至与编码区结合而发挥作用。研究发现,一个基因可以同时受到多种miRNA的调节,同时一个miRNA也可以参与调节多个基因,miRNA与靶基因的相互作用是复杂的网状结构[5]。miRNA在进化上高度保守,这使得通过生物信息学分析预测miRNA的靶基因成为可能,已有少部分靶基因被鉴定和实验确证。
结直肠癌是消化系统最常见的恶性肿瘤,2014年美国癌症协会最新数据表明,结直肠癌发病率和死亡率居所有癌症的第三位[6]。随着饮食习惯的改变和检测手段的发展,在过去认为发病率不高的我国,结直肠癌的发病率也呈升高态势。目前,根治结直肠癌最有效的方法仍是手术切除联合化疗。约有三分之一的患者会有淋巴结或远处转移,早期患者五年生存率可达到80%-90%,晚期和转移癌患者五年生存率仅为15%[7],所以早发现、早诊断、早治疗成为结直肠癌最重要的防治手段。结直肠癌的发病机制未明确,是一个多因素,多步骤的渐进过程[8]。运用高通量的筛查和定量PCR的确证的方法已发现多种miRNA在结直肠癌中存在异常表达(表1)。
1 miRNA在结直肠癌患者粪便中的表达与作用
最近几年研究发现,结直肠癌患者粪便中miRNA存在表达异常。异常的粪便miRNA可能来自癌细胞,随癌细胞脱落入粪便,或由癌细胞破碎降解后作为自由的核酸分子进入粪便[9]。相比正常个体,结直肠癌患者粪便中的miR-21、miR-106a和miR-92呈高表达[19],而miR-4478、-1295b-3p、-29a、-145、-223、-224呈低表达[11,12]。MiR-20a-5p、miR-21-3p和miR-141在外科术后患者粪便中的表达水平与术前相比明显降低[9],提示这组miRNA可以作为可信赖的结直肠癌二级预防指标。香港中文大学的一项研究表明,miR-221和miR-18a在结直肠癌患者粪便中的表达明显增高,miR-221和miR-18a的AUC(area under receiver operating characteristic curve,受试者特征曲线下面积)分别是0.73和0.67,证明它们可以作为无创性的结直肠癌诊断指标[10]。
2 miRNA在结直肠癌患者循环系统中的表达与作用
2.1 循环miRNA的发现
Lawrie等的研究表明,弥漫大细胞淋巴瘤患者血清中miR-155、miR-21和miR-210表达明显增高,且miR-21与患者的无瘤生存期密切相关[20]。此后,很多研究报道了循环miRNA与多种肿瘤的早期诊断、治疗效果和预后相关。循环miRNA以其稳定性高,重复性好和易于取材等优点,显示了巨大的临床应用价值[21]。
2.2 循环miRNA与结直肠癌
研究表明,循环miR-15b、-17-3p、-17-5p、-18a、-19a、-19b、-29a、-335在结直肠癌中表达明显上调,循环miR-9、miR-30-3p和miR-101在结直肠癌表达明显下调[13,14]。同时,miR-18a仅在高级别的腺瘤中呈高表达,提示循环miRNA的表达也有相对特异的变化。结直肠癌患者血清中的miR-17-3p的相对表达量明显高于正常对照,其表达与淋巴结转移、分化程度、T N M分期均相关,ROC曲线(receiver operating characteristic curve)分析表明miR-17-3p对结肠癌的诊断敏感性为87%,特异性为62%,AUG为0.910,明显优于CEA等传统肿瘤标志物[22]。Huang Z等[23]研究表明,结直肠癌患者循环miR-29a能区分正常人和高级别腺瘤患者(敏感度为62.2%,特异性为84.7%)以及正常人和结肠癌患者(敏感度为69.0%,特异性为89.1%),同时结直肠癌患者循环miR-92a也能区分正常人和高级别腺瘤患者(敏感度为64.9%,特异性为81.4%)以及正常人和结肠癌患者(敏感度为84%,特异性为71.2%),将miR-29a和miR-92a联合考虑诊断结肠癌则更价值,其敏感度为83.0%,特异性为84.7%。也有研究表明,高水平的或者术后重新升高的循环miR-155是结直肠癌化疗耐药的标志,高水平的或者术后重新升高的循环miR-155、miR-200c、miR-210则预示结直肠癌的复发、转移和预后不良[24]。对循环miRNA越来越多的研究表明,循环miRNA对于结直肠癌早期诊断、疗效评估和预后判断有着广阔的应用前景。
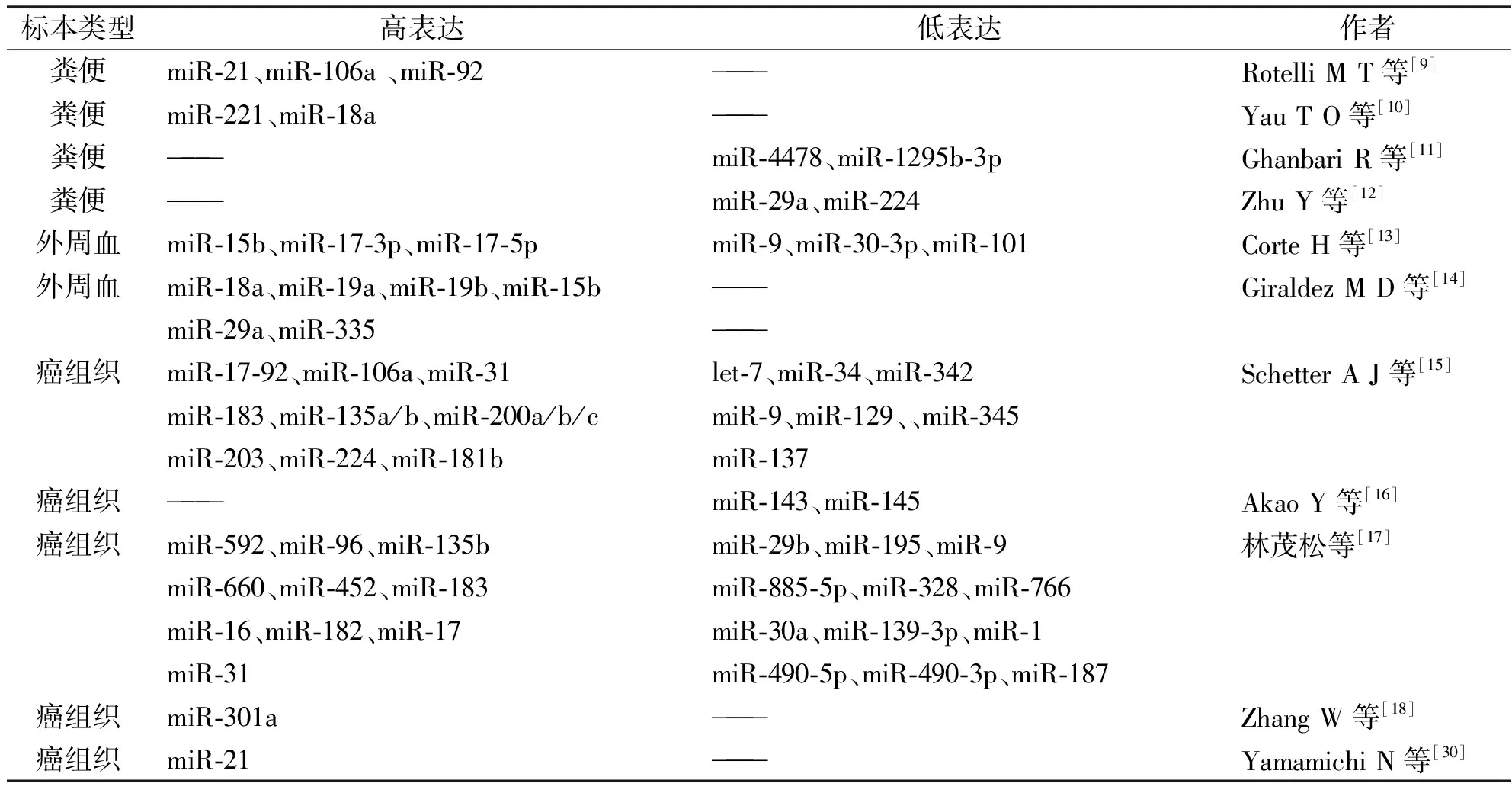
表1 结直肠癌中异常表达的microRNA
3 miRNA在结直肠癌组织中的表达与作用
3.1 miRNA在结直肠癌组织中的表达
目前大量的研究集中在miRNA在结直肠癌组织中的异常表达,结直肠癌组织是研究miRNA与结直肠癌关系最常用的材料。通过基因测序、微阵列分析、定量PCR和原位杂交等方法,确证了数百种在结直肠癌中异常表达的miRNA。研究表明,miR-17-92家族、-106a、-31、-181b、-183、-135a/b、-200a/b/c家族、-203、-224、-376a、-592、-96、-135b、-660、-452、-16、-182、-21、-19a等在结直肠癌组织表达显著增高,而let-7、miR-34、-342、-345、-9、-129、-137、-143、-145、-29b、-195、-885-5p、-328、-766、-30a、
-139-3p、-1、-490-5p、-490-3p、-187等在结肠癌组织中表达显著降低[15-17,25-27]。其中,miR-21在至少七篇报道中被证明在结肠癌中高表达[26]。总之,有大量的miRNA已被确证和结直肠癌相关,并且深入研究发现了miRNA在直肠癌中有广泛的调节作用。
3.2 miRNA在结直肠癌中的作用
3.2.1 miRNA与结直肠癌的肿瘤形成相关 结直肠癌可由结直肠腺瘤演化进展而来,研究表明,miRNA在早期的结直肠腺瘤中存在异常表达,提示miRNA有引发肿瘤形成的作用,miRNA的表达差异可以用来区分正常结直肠、结直肠腺瘤和结直肠癌[28]。MiR-21在结直肠腺瘤和结直肠癌中均存在高表达,通过原位杂交法证实,从结直肠腺瘤到结直肠癌的转变过程中,miR-21的表达量显著增加,且在裸鼠体内高表达的miR-21可以促进肿瘤形成,更证明了miR-21有促进肿瘤形成的作用[29-31]。进一步研究表明,miRNA可以通过影响Wnt/β-catenin信号通路的强度进而影响肿瘤形成,且有超过90%的结直肠癌存在信号通路的变化[32]。Nagel R等[33]研究表明,结直肠腺瘤中高表达的miR-135b作用于靶基因APC,并且APC基因也是Wnt信号通路的重要组成部分,在结直肠腺瘤中存在活跃的APC基因突变。APC基因突变检测已成为结直肠癌的重要筛查指标应用于临床。
此外,靶基因mRNA上的miRNA结合位点出现的SNP(single nucleotide polymorphism,单核苷酸多态性)与肿瘤发病风险相关,也可作为部分肿瘤的遗传易感性标记[34]。
3.2.2 miRNA与结直肠癌的TNM分期相关 TNM分期是结直肠癌患者选择治疗方式的重要参考因素,研究表明,高表达的miR-31与结直肠肿瘤的TNM分期和原位侵袭密切相关[35]。MiR-29a在结直肠癌中高表达,且与较晚的TNM分期相关[36]。对这些miRNA的深入研究有望为结直肠癌治疗方法的选择提供参考。
3.2.3 miRNA与结直肠癌的增殖、转移和侵袭相关 研究表明,高表达的miR-21通过调节多个靶基因发挥了促进结直肠癌细胞增殖、侵袭和转移的作用。MiR-21的众多靶基因中,已经被实验证实的有PDCD4(programmed cell death 4)[37]、RHOB(ras homolog gene family member B)[38]、PTEN(phosphatase and tensin homolog)[39]、Cdc25A(Cell division cycle 25 homolog A)[40]、TPM1(tropomyosin 1)[41]等。
此外,miR-610通过抑制HDGF(hepatoma-derived growth factor)而抑制结肠癌细胞增殖、转移和侵袭[42]。MiR-31通过抑制RhoBTB1(Rho-related BTB domain containing 1 )而抑制结肠癌细胞系的增殖和转移[43]。Wu J等[44]研究表明,miR-34a属于P53相关基因,且通过调节Fra-1(Fos-related antigen-1)抑制结肠癌细胞的增殖和侵袭。
3.2.4 miRNA与结直肠癌化疗敏感性相关 将化学合成的miR-34a类似物转染到DLD-1细胞系后,在观察到明显的细胞增殖能力降低的同时发现DLD-1细胞系对化疗药物5-氟尿嘧啶敏感性增强[45]。在一项包括中美两国人群的研究中发现,miR-21的表达与5-氟尿嘧啶化疗效果直接相关,即高表达的miR-21导致结直肠癌组织对5-氟尿嘧啶产生耐药抵抗,进一步研究发现,miR-21诱发结肠癌细胞系对5-氟尿嘧啶的抵抗作用是通过下调DNA修复蛋白MSH2(MutS homolog 2)而发挥作用[46,47]。
也有研究表明,BER(base excision repair)基因3′UTR上与miRNA结合区域的单核苷酸多态性(single nucleotide polymorphism,SNP)影响结直肠癌5-氟尿嘧啶的化疗效果[48]。种种研究表明,miRNA在结直肠癌化疗中有巨大的潜在应用价值。
3.2.5 miRNA与结直肠癌患者预后相关 多个研究表明,高表达的miR-21与结直肠癌患者生存期和治疗效果呈负相关,miR-21甚至可以作为一个独立的结肠癌预后标志物[15,46]。类似研究表明,miR-320、-498、-185、-133b、-376a等多种miRNA均与结直肠癌患者治疗效果和生存期相关[25,49,50]。
3.2.6 miRNA在不同人种的结直肠癌中作用存在明显差异 值得注意的是,一项对5种miRNA(包括miR-20a、-21、-106a、-181b、-203)的研究,标本取自106名非裔美国人患者(African American patients)和239名白人患者(non-Hispanic Caucasian patients),发现这些miRNA对于不同人种和分期的结直肠癌的预后价值有明显差异,结果显示,高表达的miR-203与白人IV期结直肠癌不良预后相关,而高表达的miR-203则与黑人I 期和II期结直肠癌不良预后相关,高表达miR-181b则仅与黑人III期结直肠癌不良预后相关[51]。另一项在非裔美国人和白人患者之间的研究发现,非裔美国人中miR-1207-5p表达量相比对照组增高八倍以上,而白人中miR-1207-5p表达量仅为对照组1.2倍左右,这种高表达与非裔美国人中miR-1207-5p的靶基因PVT1(plasmacytoma variant translocation 1)的表达增高相关[52]。
4 展望
粪便miRNA和循环miRNA由于其存在环境的复杂,仍需更多的研究对其是否来自于结肠癌细胞进行确证,并且同一病人的粪便miRNA、循环miRNA和癌组织miRNA表达水平的相关性仍少有研究。
为将miRNA应用于结直肠癌的诊治,仍有多个难题需要攻克:其一,能够用来发现结直肠癌的miRNA或其组合仍不能确定。其二,由于miRNA和靶基因之间的复杂网状关系,以miRNA为基础精准的打击靶基因成为又一难题。其三,miRNA表达存在明显的种族差异甚至个体差异,也大大增加了miRNA应用于临床诊治的难度。
对miRNA在结直肠癌中的作用和机制仍需大量深入的研究,将来miRNA定能应用于结直肠癌的预防、诊断、和治疗。
[1]Xuan Y,Yang H,Zhao L,et al.MicroRNAs in colorectal cancer:Small molecules with big functions[J].Cancer Letters,2015,360(2):89.
[2]Carthew RW,Sontheimer EJ.Origins and Mechanisms of miRNAs and siRNAs[J].Cell,2009,136(4):642.
[3]Filipowicz W,Jaskiewicz L,Kolb FA,et al.Post-transcriptional gene silencing by siRNAs and miRNAs[J].Curr Opin Struct Biol,2005,15(3):331.
[4]Lytle JR,Yario TA,Steitz JA.Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5' UTR as in the 3' UTR[J].Proc Natl Acad Sci USA,2007,104(23):9667.
[5]Bimonte S,Barbieri A,Leongito M,et al.The Role of miRNAs in the Regulation of Pancreatic Cancer Stem Cells[J].Stem Cells Int,2016,2016:8352684.
[6]Ren A,Dong Y,Tsoi H,et al.Detection of miRNA as Non-Invasive Biomarkers of Colorectal Cancer[J].International Journal of Molecular Sciences,2015,16(2):2810.
[7]Wang J,Du Y,Liu X,et al.MicroRNAs as Regulator of Signaling Networks in Metastatic Colon Cancer[J].BioMed Research International,2015,2015:1.
[8]Kelley RK,Wang G,Venook AP.Biomarker use in colorectal cancer therapy[J].J Natl Compr Canc Netw,2011,9(11):1293.
[9]Rotelli MT,Di Lena M,Cavallini A,et al.Fecal microRNA profile in patients with colorectal carcinoma before and after curative surgery[J].Int J Colorectal Dis,2015,30(7):891.
[10]Yau TO,Wu CW,Dong Y,et al.MicroRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma[J].Br J Cancer,2014,111(9):1765.
[11]Ghanbari R,Mosakhani N,Asadi J,et al.Decreased expression of fecal miR-4478 and miR-1295b-3p in early-stage colorectal cancer[J].Cancer Biomark,2015,15(2):189.
[12]Zhu Y,Xu A,Li J,et al.Fecal miR-29a and miR-224 as the noninvasive biomarkers for colorectal cancer[J].Cancer Biomark,2016,16(2):259.
[13]Corte H,Manceau G,Blons H,et al.MicroRNA and colorectal cancer[J].Dig Liver Dis,2012,44(3):195.
[14]Giraldez MD,Lozano JJ,Ramirez G,et al.Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study[J].Clin Gastroenterol Hepatol,2013,11(6):681.
[15]Schetter AJ,Okayama H,Harris CC.The Role of MicroRNAs in Colorectal Cancer[J].The Cancer Journal,2012,18(3):244.
[16]Akao Y,Nakagawa Y,Hirata I,et al.Role of anti-oncomirs miR-143 and -145 in human colorectal tumors[J].Cancer Gene Ther,2010,17(6):398.
[17]林茂松.人结直肠癌microRNA表达谱及其临床意义的研究[D].苏州大学,2010.
[18]Zhang W,Zhang T,Jin R,et al.MicroRNA-301a promotes migration and invasion by targeting TGFBR2 in human colorectal cancer[J].Journal of Experimental & Clinical Cancer Research,2014,33(1):28.
[19]Ahmed FE,Jeffries CD,Vos PW,et al.Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue[J].Cancer Genomics Proteomics,2009,6(5):281.
[20]Lawrie CH,Gal S,Dunlop HM,et al.Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma[J].Br J Haematol,2008,141(5):672.
[21]Chen X,Ba Y,Ma L,et al.Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases[J].Cell Res,2008,18(10):997.
[22]郭秀丽,姚士伟,朱 东,等.外周血miRNA-17-3p的检测在结肠癌诊断中的临床意义[J].世界华人消化杂志,2014(30):4701.
[23]Huang Z,Huang D,Ni S,et al.Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer[J].Int J Cancer,2010,127(1):118.
[24]陈建梅.血浆miRNAs预测结肠癌远处转移和化疗耐药性研究[D].中南大学,2014.
[25]Mo Z,Wu X,Li S,et al.Expression and Clinical Significance of MicroRNA-376a in Colorectal Cancer[J].Asian Pacific Journal of Cancer Prevention,2014,15(21):9523.
[26]Luo X,Burwinkel B,Tao S,et al.MicroRNA signatures: novel biomarker for colorectal cancer[J].Cancer Epidemiol Biomarkers Prev,2011,20(7):1272.
[27]Huang L,Wang X,Wen C,et al.Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-α induced epithelial-to-mesenchymal transition in colorectal cancer[J].Scientific Reports,2015,5:13350.
[28]Oberg AL,French AJ,Sarver AL,et al.miRNA expression in colon polyps provides evidence for a multihit model of colon cancer[J].PLoS One,2011,6(6):e20465.
[29]Schetter AJ,Leung SY,Sohn JJ,et al.MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma[J].JAMA,2008,299(4):425.
[30]Yamamichi N,Shimomura R,Inada K,et al.Locked nucleic acid in situ hybridization analysis of miR-21 expression during colorectal cancer development[J].Clin Cancer Res,2009,15(12):4009.
[31]Medina PP,Nolde M,Slack FJ.OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma[J].Nature,2010,467(7311):86.
[32]Thorstensen L,Lind GE,Lovig T,et al.Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability[J].Neoplasia,2005,7(2):99.
[33]Nagel R,le Sage C,Diosdado B,et al.Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer[J].Cancer Res,2008,68(14):5795.
[34]Lu J,Getz G,Miska E A,et al.MicroRNA expression profiles classify human cancers[J].Nature,2005,435(7043):834.
[35]Wang CJ,Zhou ZG,Wang L,et al.Clinicopathological significance of microRNA-31,-143 and -145 expression in colorectal cancer[J].Dis Markers,2009,26(1):27.
[36]Xing J,Wan S,Zhou F,et al.Genetic polymorphisms in pre-microRNA genes as prognostic markers of colorectal cancer[J].Cancer Epidemiol Biomarkers Prev,2012,21(1):217.
[37]Lu Z,Liu M,Stribinskis V,et al.MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene[J].Oncogene,2008,27(31):4373.
[38]Liu M,Tang Q,Qiu M,et al.miR-21 targets the tumor suppressor RhoB and regulates proliferation,invasion and apoptosis in colorectal cancer cells[J].FEBS Lett,2011,585(19):2998.
[39]Meng F,Henson R,Wehbe-Janek H,et al.MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer[J].Gastroenterology,2007,133(2):647.
[40]Wang P,Zou F,Zhang X,et al.microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells[J].Cancer Res,2009,69(20):8157.
[41]Zhu S,Si M L,Wu H,et al.MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1)[J].J Biol Chem,2007,282(19):14328.
[42]Sun B,Gu X,Chen Z,et al.MiR-610 inhibits cell proliferation and invasion in colorectal cancer by repressing hepatoma-derived growth factor[J].Am J Cancer Res,2015,5(12):3635.
[43]Xu RS,Wu XD,Zhang SQ,et al.The tumor suppressor gene RhoBTB1 is a novel target of miR-31 in human colon cancer[J].Int J Oncol,2013,42(2):676.
[44]Wu J,Wu G,Lv L,et al.MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1[J].Carcinogenesis,2012,33(3):519.
[45]Akao Y,Noguchi S,Iio A,et al.Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells[J].Cancer Lett,2011,300(2):197.
[46]Schetter AJ,Leung SY,Sohn JJ,et al.MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma[J].JAMA,2008,299(4):425.
[47]Valeri N,Gasparini P,Braconi C,et al.MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2)[J].Proc Natl Acad Sci U S A,2010,107(49):21098.
[48]Pardini B,Rosa F,Barone E,et al.Variation within 3'-UTRs of base excision repair genes and response to therapy in colorectal cancer patients: A potential modulation of microRNAs binding[J].Clin Cancer Res,2013,19(21):6044.
[49]Schepeler T,Reinert JT,Ostenfeld MS,et al.Diagnostic and prognostic microRNAs in stage II colon cancer[J].Cancer Res,2008,68(15):6416.
[50]Akcakaya P,Ekelund S,Kolosenko I,et al.miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer[J].Int J Oncol,2011,39(2):311.
[51]Bovell LC,Shanmugam C,Putcha BD,et al.The prognostic value of microRNAs varies with patient race/ethnicity and stage of colorectal cancer[J].Clin Cancer Res,2013,19(14):3955.
[52]Farhana L,Antaki F,Anees MR,et al.Role of cancer stem cells in racial disparity in colorectal cancer[J].Cancer Med,2016,69(18):5315.
1007-4287(2017)08-1471-05
2016-07-26)

